Solid Surface Vitrification Is Better than Slow Freezing for the Long-Term Preservation of Testicular Fragments from Prepubertal Collared Peccaries (Pecari tajacu Linnaeus, 1758)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
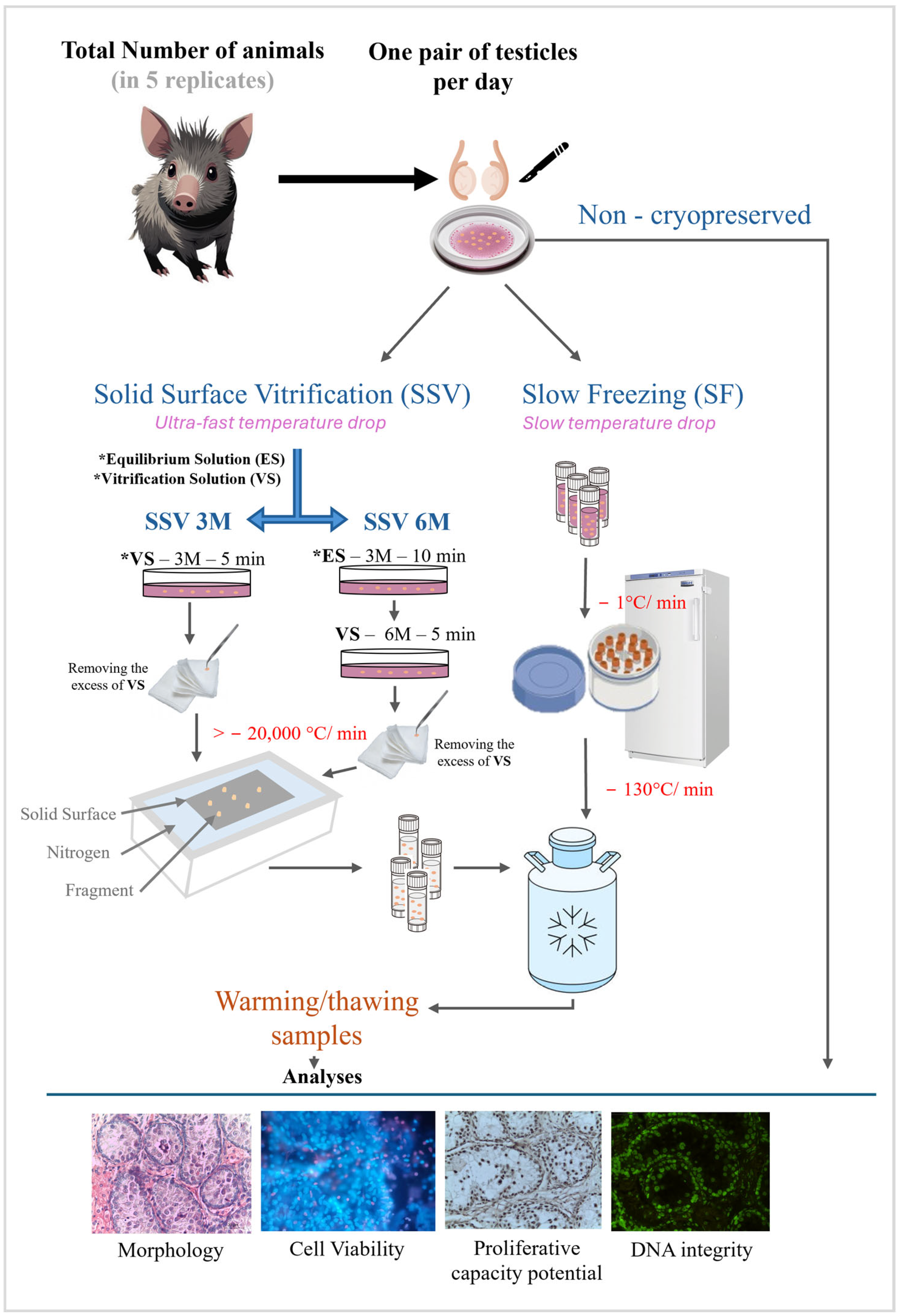
2.2. Testis Collection and Experimental Design
2.3. Cryopreservation and Thawing/Warming
2.4. Testicular Cell Morphology Analysis
2.5. Testicular Cell Viability
2.6. Proliferative Capacity Potential of Testicular Cells
2.7. DNA Integrity
2.8. Statistical Analysis
3. Results
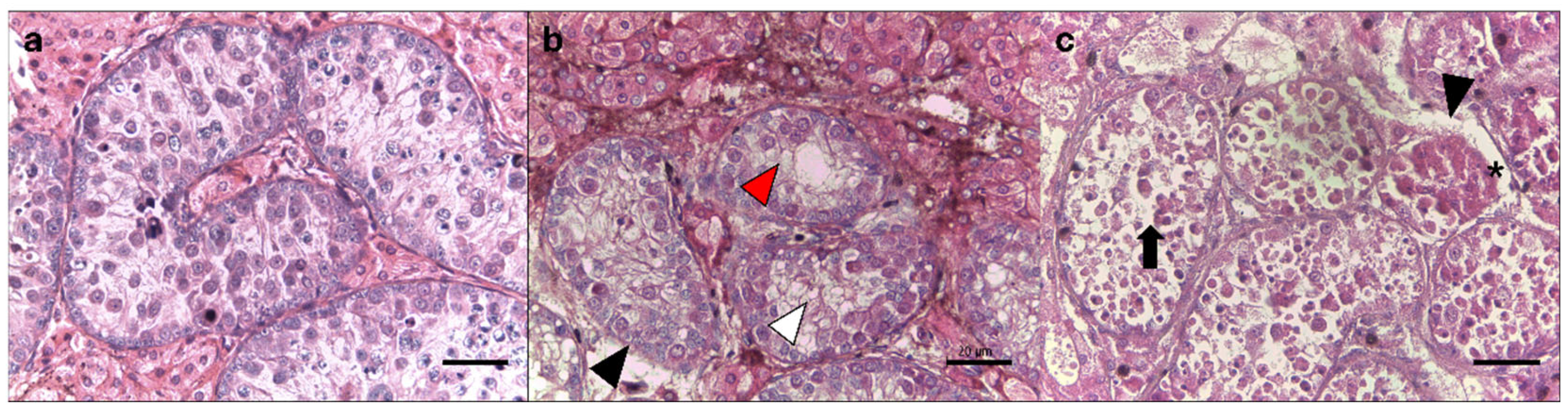
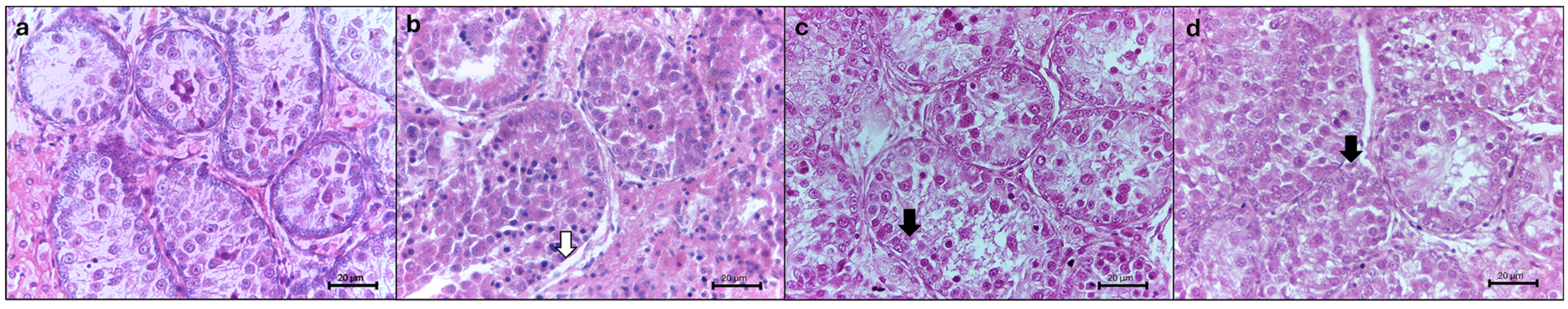
3.1. Histomorphology
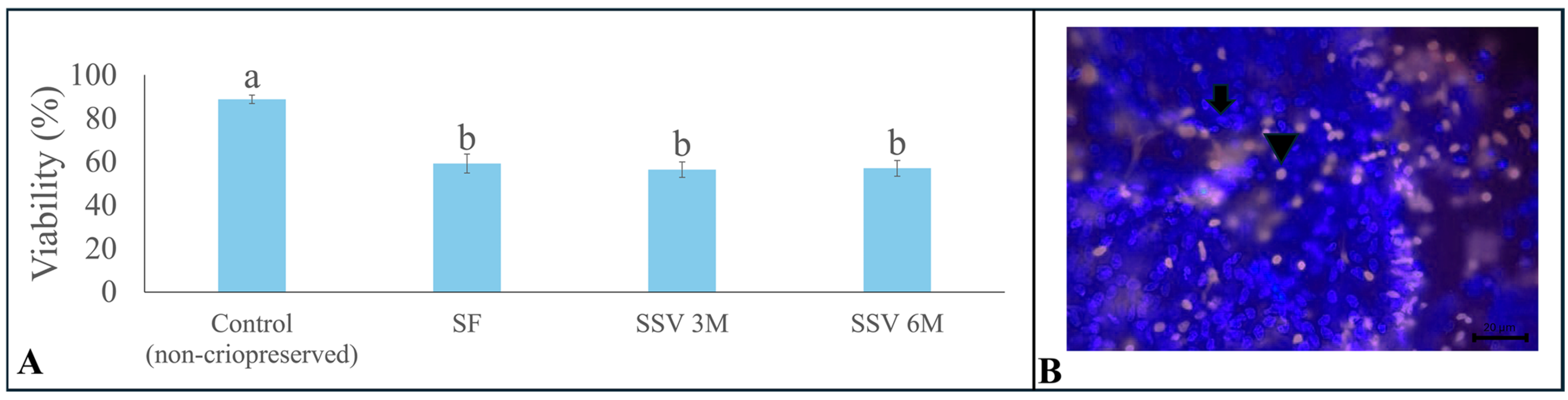
3.2. Cell Viability
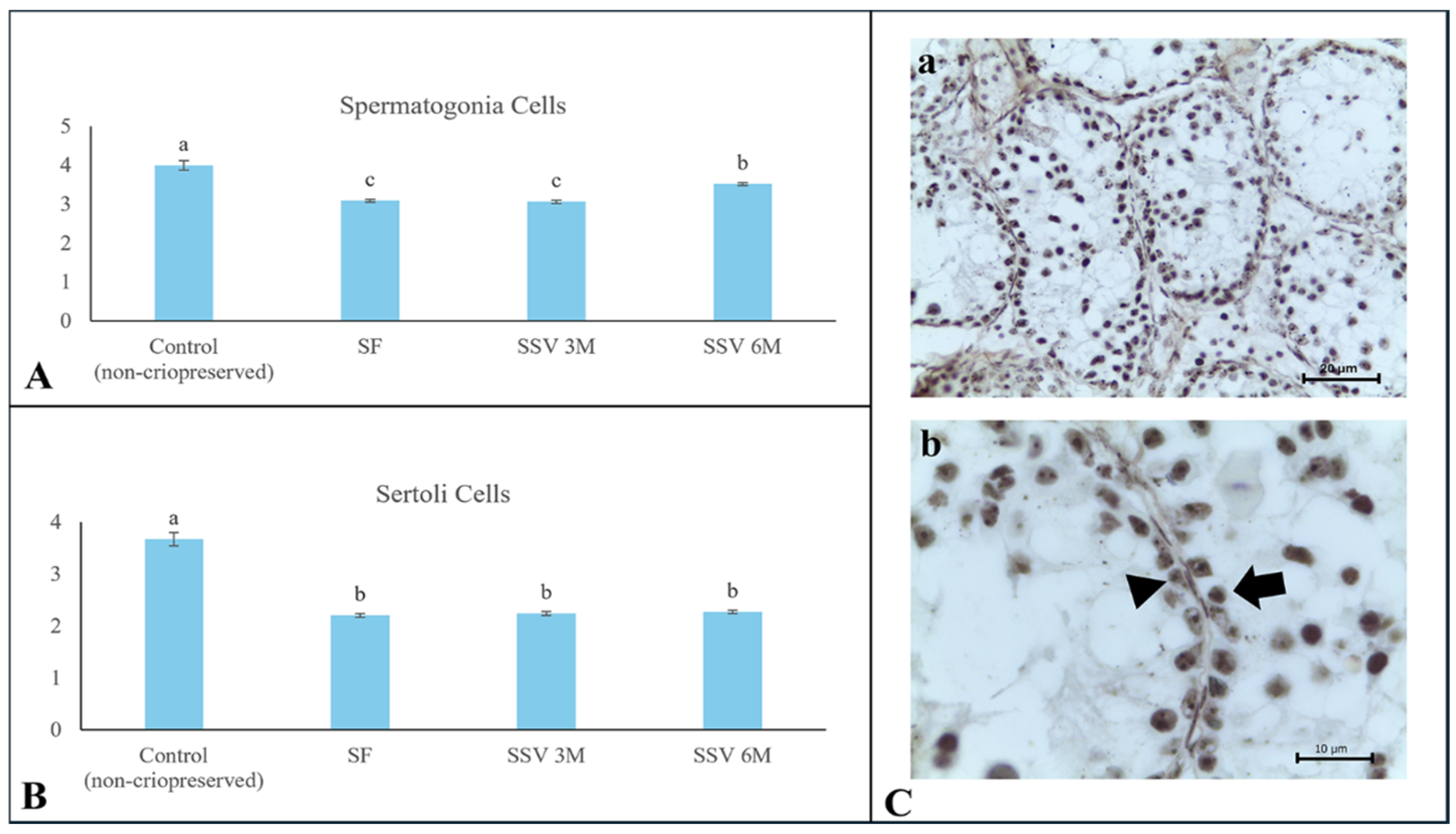
3.3. Proliferative Capacity Potential
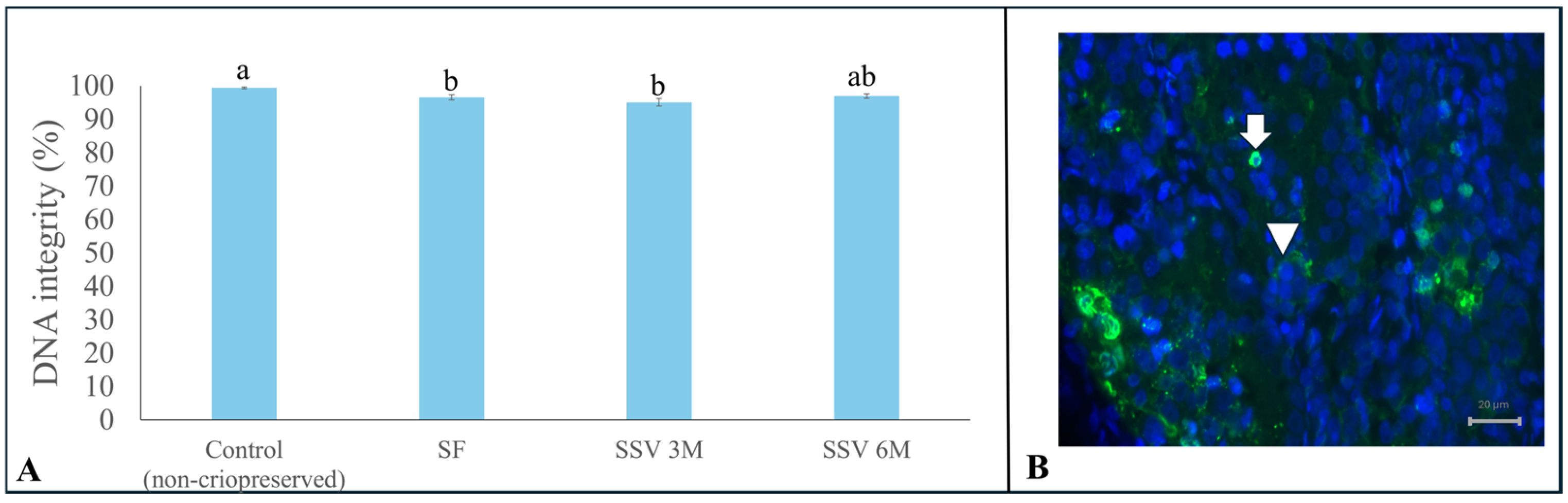
3.4. DNA Integrity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cerda, J.R.; Webb, T.L. Wildlife Conservation and Preserving Biodiversity: Impactful Opportunities for Veterinarians? J. Am. Vet. Med. Assoc. 2023, 261, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.B.; Holtze, S. Advanced Assisted Reproduction Technologies in Endangered Mammalian Species. Reprod. Domest. Anim. 2024, 59, e14700. [Google Scholar] [CrossRef] [PubMed]
- Comizzoli, P. Biobanking Efforts and New Advances in Male Fertility Preservation for Rare and Endangered Species. Asian J. Androl. 2015, 17, 640–645. [Google Scholar] [CrossRef]
- Silva, A.; Lima, G.; Comizzoli, P.; Silva, A. Gonadal Tissue Preservation Technologies and Culture Offer Opportunities to Bridge Knowledge between Wildlife and Humans. FS Rep. 2025, 6, 50–54. [Google Scholar] [CrossRef]
- Sharma, S.; Sandhowe-Klaverkamp, R.; Schlatt, S. Differentiation of Testis Xenografts in the Prepubertal Marmoset Depends on the Sex and Status of the Mouse Host. Front. Endocrinol. 2018, 9, 467. [Google Scholar] [CrossRef]
- Silva, A.M.; Bezerra, L.G.P.; Praxedes, E.C.G.; Moreira, S.S.J.; Souza, C.M.P.; Oliveira, M.F.; Pereira, A.F.; Comizzoli, P.; Silva, A.R. Combination of Intracellular Cryoprotectants Preserves the Structure and the Cells Proliferative Capacity Potential of Adult Collared Peccary Testicular Tissue Subjected to Solid Surface Vitrification. Cryobiology 2019, 91, 53–60. [Google Scholar] [CrossRef]
- Silva, A.M.; Pereira, A.G.; Brasil, A.V.; Macedo, L.B.; Souza-Júnior, J.B.F.; Bezerra de Moura, C.E.; Pereira, A.F.; Oliveira, M.F.; Comizzoli, P.; Silva, A.R. Influence of Freezing Techniques and Glycerol-Based Cryoprotectant Combinations on the Survival of Testicular Tissues from Adult Collared Peccaries. Theriogenology 2021, 167, 111–119. [Google Scholar] [CrossRef]
- Gongora, J.; Reyna-Hurtado, R.; Beck, H.; Taber, A.; Altrichter, M.; Keuroghlian, A. Pecari tajacu. Available online: https://www.iucnredlist.org/species/41777/10562361 (accessed on 25 July 2019).
- Desbiez, A.; Keuroghlian, A.; Beisiege, B.; Medici, E.; Gatt, A.; Pontes, A.; Campos, C.; Tófoli, C.; Moraes Junior, E.; Azevedo, F.; et al. Avaliação do Risco de Extinção do Cateto-Pecari tajacu Linnaeus, 1758, No Brasil. Biodivers. Brasil. 2012, 2, 74–83. [Google Scholar] [CrossRef]
- Amelkina, O.; da Silva, A.M.; Silva, A.R.; Comizzoli, P. Transcriptome Dynamics in Developing Testes of Domestic Cats and Impact of Age on Tissue Resilience to Cryopreservation. BMC Genom. 2021, 22, 847. [Google Scholar] [CrossRef]
- Unni, S.; Kasiviswanathan, S.; D’Souza, S.; Khavale, S.; Mukherjee, S.; Patwardhan, S.; Bhartiya, D. Efficient Cryopreservation of Testicular Tissue: Effect of Age, Sample State, and Concentration of Cryoprotectant. Fertil. Steril. 2012, 97, 200–208. [Google Scholar] [CrossRef]
- Ibtisham, H.; Cham, T.; Fayaz, M.A. Long-Term In Vitro Maintenance of Piglet Testicular Tissue: Effects of Tissue Fragment Size, Preparation Method, and Serum Source. Animals 2023, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In Vitro Production of Functional Sperm in Cultured Neonatal Mouse Testes. Nature 2011, 471, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Khaydukova, I.V.; Ivannikova, V.M.; Zhidkov, D.A.; Belikov, N.V.; Peshkova, M.A.; Timashev, P.S.; Tsiganov, D.I.; Pushkarev, A.V. Current State and Challenges of Tissue and Organ Cryopreservation in Biobanking. Int. J. Mol. Sci. 2024, 25, 11124. [Google Scholar] [CrossRef]
- Mukaida, T.; Oka, C. Vitrification of Oocytes, Embryos and Blastocysts. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 789–803. [Google Scholar] [CrossRef]
- Abrishami, M.; Anzar, M.; Yang, Y.; Honaramooz, A. Cryopreservation of Immature Porcine Testis Tissue to Maintain Its Developmental Potential after Xenografting into Recipient Mice. Theriogenology 2010, 73, 86–96. [Google Scholar] [CrossRef]
- Lima, D.; Silva, T.; Morais, G.; Aquino-Cortez, A.; Evangelista, J.; Xavier Júnior, F.; Viana, D.; Silva, L. Different Associations of Cryoprotectants for Testicular Tissue of Prepubertal Cats Submitted to Vitrification. Reprod. Domest. Anim. 2017, 52, 235–241. [Google Scholar] [CrossRef]
- Teixeira, D.O.; da Silva Oliveira, E.; de Souza Fernandes, J.; Palomino, G.J.Q.; de Alemida Tabosa, B.E.; Barbosa, H.T.S.; Pinheiro, B.Q.; da Silva, L.D.M. Avaliação Histológica Dos Testículos de Cães Pré-Púberes Submetidos à Vitrificação com Diferentes Associações de Crioprotetores. Res. Soc. Develop. 2021, 10, e348101623864. [Google Scholar] [CrossRef]
- Singh, R.P.; Escobar, E.; Wildt, D.; Patel, S.; Costa, G.M.J.; Pukazhenthi, B. Effect of Sphingosine-1-Phosphate on Cryopreserved Sheep Testicular Explants Cultured In Vitro. Theriogenology 2019, 128, 184–192. [Google Scholar] [CrossRef]
- Fernandes, J.S.; Tabosa, B.E.A.; Brito, B.F.; Silva, H.V.R.; Lima, D.B.C.; da Silva, L.D.M. Influence of Different Warming Temperatures on the Vitrified Testicular Fragments from Pre-Pubertal Cat. Reprod. Domest. Anim. 2021, 56, 1342–1348. [Google Scholar] [CrossRef]
- Chacur, M.G.M.; Ibrahim, D.B.; Arrebola, T.A.H.; Sanches, O.C.; Giuffrida, R.; Oba, E.; Ramos, A.A. Avaliação da Técnica de Coloração AgNOR em Testículos de Ovinos. Arq. Bras. De Med. Veterinária E Zootec. 2015, 67, 447–454. [Google Scholar] [CrossRef][Green Version]
- Costa, G.M.J.; Leal, M.C.; Silva, J.V.; Cassia, A.; Ferreira, S.; Guimaraes, D.A.; Franca, L.R. Spermatogenic Cycle Length and Sperm Production in a Feral Pig Species (Collared Peccary, Tayassu tajacu). J. Androl. 2010, 31, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.J.; Huang, Y.L.; Liu, Y.L.; Chen, B.S.; Lin, B.Z.; Chen, C.H. Tracking Immature Testicular Tissue after Vitrification In Vitro and In Vivo for Pre-Pubertal Fertility Preservation: A Translational Transgenic Mouse Model. Int. J. Mol. Sci. 2022, 23, 8425. [Google Scholar] [CrossRef]
- Carvalho, J.V.G.; Soares, A.R.B.; Leão, D.L.; Reis, A.N.; Santos, R.R.; Rodrigues, A.P.R.; Domingues, S.F.S. Effect of Different Vitrification Techniques on Viability and Apoptotic Index of Domestic Cat Testicular Tissue Cells. Animals 2023, 13, 2768. [Google Scholar] [CrossRef]
- Lima, D.B.C.; Silva, L.D.M.; Comizzoli, P. Influence of Warming and Reanimation Conditions on Seminiferous Tubule Morphology, Mitochondrial Activity, and Cell Composition of Vitrified Testicular Tissues in the Domestic Cat Model. PLoS ONE 2018, 13, e0207317. [Google Scholar] [CrossRef]
- Picazo, C.M.; Castaño, C.; Bóveda, P.; Toledano-Díaz, A.; Velázquez, R.; Pequeño, B.; Esteso, M.C.; Gadea, J.; Villaverde-Morcillo, S.; Cerdeira, J.; et al. Cryopreservation of Testicular Tissue from the Dog (Canis Familiaris) and Wild Boar (Sus Scrofa) by Slow Freezing and Vitrification: Differences in Cryoresistance According to Cell Type. Theriogenology 2022, 190, 65–72. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Benito-Blanco, J.; Martínez-Nevado, E.; Toledano-Díaz, A.; Castaño, C.; Velázquez, R.; Pequeño, B.; Martinez-Madrid, B.; Esteso, M.; Santiago-Moreno, J. DNA Integrity and Viability of Testicular Cells from Diverse Wild Species after Slow Freezing or Vitrification. Front. Vet. Sci. 2023, 9, 1114695. [Google Scholar] [CrossRef]
- Gurtovenko, A.A.; Anwar, J. Modulating the Structure and Properties of Cell Membranes: The Molecular Mechanism of Action of Dimethyl Sulfoxide. J. Phys. Chem. B 2007, 111, 10453–10460. [Google Scholar] [CrossRef]
- Chaytor, J.L.; Tokarew, J.M.; Wu, L.K.; Leclere, M.; Tam, R.Y.; Capicciotti, C.J.; Guolla, L.; von Moos, E.; Findlay, C.S.; Allan, D.S.; et al. Inhibiting Ice Recrystallization and Optimization of Cell Viability after Cryopreservation. Glycobiology 2012, 22, 123–133. [Google Scholar] [CrossRef]
- Weng, L.; Chen, C.; Zuo, J.; Li, W. Molecular Dynamics Study of Effects of Temperature and Concentration on Hydrogen-Bond Abilities of Ethylene Glycol and Glycerol: Implications for Cryopreservation. J. Phys. Chem. A 2011, 115, 4729–4737. [Google Scholar] [CrossRef]
- Chi, H.J.; Koo, J.J.; Kim, M.Y.; Joo, J.Y.; Chang, S.S.; Chung, K.-S. Cryopreservation of Human Embryos Using Ethylene Glycol in Controlled Slow Freezing. Hum. Reprod. 2002, 17, 2146–2151. [Google Scholar] [CrossRef]
- Wowk, B. Thermodynamic Aspects of Vitrification. Cryobiology 2010, 60, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Song, Y.C.; Brockbank, K.G.M. Vitrification in Tissue Preservation: New Developments. In Life in the Frozen State; Tayl Lane N, F.B., Ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Mruk, D.D.; Cheng, C.Y. Sertoli-Sertoli and Sertoli-Germ Cell Interactions and Their Significance in Germ Cell Movement in the Seminiferous Epithelium during Spermatogenesis. Endocr. Rev. 2004, 25, 747–806. [Google Scholar] [PubMed]
- Su, J.; Song, Y.; Zhu, Z.; Huang, X.; Fan, J.; Qiao, J.; Mao, F. Cell–Cell Communication: New Insights and Clinical Implications. Signal. Transduct. Target Ther. 2024, 7, 9–196. [Google Scholar] [CrossRef]
- Wallig, M.A.; Janovitz, E.B. Morphologic Manifestations of Toxic Cell Injury. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 77–105. ISBN 9780124157590. [Google Scholar]
- Pukazhenthi, B.S.; Nagashima, J.; Travis, A.J.; Costa, G.M.; Escobar, E.N.; França, L.R.; Wildt, D.E. Slow Freezing, but Not Vitrification Supports Complete Spermatogenesis in Cryopreserved, Neonatal Sheep Testicular Xenografts. PLoS ONE 2015, 10, e0123957. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, K.; Kojima, K.; Komeya, M.; Yao, M.; Ogawa, T. In Vitro Spermatogenesis in Explanted Adult Mouse Testis Tissues. PLoS ONE 2015, 10, e0130171. [Google Scholar] [CrossRef]
- Miller, M.A.; Zachary, J.F. Mechanisms and Morphology of Cellular Injury, Adaptation, and Death. In Pathologic Basis of Veterinary Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 2–43.e19. [Google Scholar]
- Brar, G.A.; Weissman, J.S. Ribosome Profiling Reveals the What, When, Where and How of Protein Synthesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 651–664. [Google Scholar] [CrossRef]
- Gossler, V.S.A.; Santos, F.A.G.; de Azevedo, A.R.; Gonçalves, P.C.; Rigolo, H.A.; Trevisan, C.; Masseno, A.P.B.; Cardoso, A.P.M.M.; Papa, P.C.; Castilho, C.; et al. Evaluation of Cell Proliferation and Endometrial Thickness of Bitches in Different Periods of Diestrus. An. Acad. Bras. Cienc. 2017, 89, 1719–1727. [Google Scholar] [CrossRef][Green Version]
- Ribas-Maynou, J.; Garcia-Bonavila, E.; Bonet, S.; Catalán, J.; Salas-Huetos, A.; Yeste, M. The TUNEL Assay Underestimates the Incidence of DNA Damage in Pig Sperm Due to Chromatin Condensation: The TUNEL Assay Underestimates Sperm DNA Fragmentation. Theriogenology 2021, 174, 94–101. [Google Scholar] [CrossRef]
- Dcunha, R.; Aravind, A.; Bhaskar, S.; Mutalik, S.; Mutalik, S.; Kalthur, S.G.; Kumar, A.; Hegde, P.; Adiga, S.K.; Zhao, Y.; et al. Enhanced Cell Survival in Prepubertal Testicular Tissue Cryopreserved with Membrane Lipids and Antioxidants Rich Cryopreservation Medium. Cell Tissue. Res. 2025, 399, 97–117. [Google Scholar] [CrossRef]
- Nikmahzar, A.; Khadivi, F.; Abbasi, M.; Mahdavinezhad, F.; Abbasi, Y.; Daneshi, E. Testicular Tissue Vitrification: A Promising Strategy for Male Fertility Preservation. Reprod. Sci. 2023, 30, 1687–1700. [Google Scholar] [CrossRef]
- Silva, L.D.M. Canine and Feline Testicular Preservation. Animals 2022, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Kikuchi, K.; Men, N.T.; Nakai, M.; Noguchi, J.; Kashiwazaki, N.; Ito, J. Production of Sperm from Porcine Fetal Testicular Tissue after Cryopreservation and Grafting into Nude Mice. Theriogenology 2017, 91, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Pereira, A.G.; Bezerra, L.G.P.; Brasil, A.V.; Pereira, A.F.; de Oliveira, M.F.; Rodrigues, A.P.R.; Ñaupas, L.V.S.; Comizzoli, P.; Silva, A.R. Synergistic Effects of Glial Cell Line-Derived Neurotrophic Factor and Base-Medium on in Vitro Culture of Testicular Tissue Derived from Prepubertal Collared Peccary. Cell Biol. Int. 2024, 48, 1364–1377. [Google Scholar] [CrossRef]
| Parameter | Score | ||
|---|---|---|---|
| #3 | #2 | #1 | |
| Tubular cell swelling | No swelling | >50% cells without swelling | >50% cells with swelling |
| Tubular cell loss | No cell loss | <75% cell types lost | >75% cell types lost |
| Rupture from basal membrane | No rupture | <50% partly ruptured | >50% mostly ruptured |
| Shrinkage from basal membrane | No shrinkage | <50% partly shrunken | >50% mostly shrunken |
| Tubular structure | Structure intact | All cell types present, although with slightly disordered structure | Random distribution of remaining cells |
| Control (Non-Cryopreserved) | SF | SSV 3 M | SSV 6 M | |
|---|---|---|---|---|
| Tubular cell swelling | 2.69 ± 0.05 a | 2.16 ± 0.06 b | 1.92 ± 0.06 c | 2.13 ± 0.04 b |
| Tubular cell loss | 2.81 ± 0.04 a | 2.47 ± 0.07 bc | 2.33 ± 0.07 c | 2.60 ± 0.06 b |
| Rupture from basal membrane | 2.74 ± 0.05 a | 2.33 ± 0.07 b | 2.08 ± 0.07 b | 2.30 ± 0.07 b |
| Shrinkage from basal membrane | 2.98 ± 0.02 a | 2.66 ± 0.07 b | 2.57 ± 0.07 b | 2.79 ± 0.04 b |
| Tubular structure | 2.80 ± 0.04 a | 2.08 ± 0.05 b | 1.87 ± 0.05 c | 1.93 ± 0.05 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.M.; Pereira, A.G.; Bezerra, G.S.C.; Matos, Y.G.; Bezerra, L.G.P.; Pereira, A.F.; Oliveira, M.F.; Comizzoli, P.; Silva, A.R. Solid Surface Vitrification Is Better than Slow Freezing for the Long-Term Preservation of Testicular Fragments from Prepubertal Collared Peccaries (Pecari tajacu Linnaeus, 1758). Animals 2025, 15, 1488. https://doi.org/10.3390/ani15101488
Silva AM, Pereira AG, Bezerra GSC, Matos YG, Bezerra LGP, Pereira AF, Oliveira MF, Comizzoli P, Silva AR. Solid Surface Vitrification Is Better than Slow Freezing for the Long-Term Preservation of Testicular Fragments from Prepubertal Collared Peccaries (Pecari tajacu Linnaeus, 1758). Animals. 2025; 15(10):1488. https://doi.org/10.3390/ani15101488
Chicago/Turabian StyleSilva, Andréia M., Ana G. Pereira, Gabriel S. C. Bezerra, Yuri G. Matos, Luana G. P. Bezerra, Alexsandra F. Pereira, Moacir F. Oliveira, Pierre Comizzoli, and Alexandre R. Silva. 2025. "Solid Surface Vitrification Is Better than Slow Freezing for the Long-Term Preservation of Testicular Fragments from Prepubertal Collared Peccaries (Pecari tajacu Linnaeus, 1758)" Animals 15, no. 10: 1488. https://doi.org/10.3390/ani15101488
APA StyleSilva, A. M., Pereira, A. G., Bezerra, G. S. C., Matos, Y. G., Bezerra, L. G. P., Pereira, A. F., Oliveira, M. F., Comizzoli, P., & Silva, A. R. (2025). Solid Surface Vitrification Is Better than Slow Freezing for the Long-Term Preservation of Testicular Fragments from Prepubertal Collared Peccaries (Pecari tajacu Linnaeus, 1758). Animals, 15(10), 1488. https://doi.org/10.3390/ani15101488







