Effect of Tunicamycin on Viability, Motility, Reactive Oxygen Species, Nitric Oxide, and Lipid Peroxidation in Boar Sperm
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Semen Preparation
2.3. Sperm Motility
2.4. Sperm Viability
2.5. Reactive Oxygen Species
2.6. Nitric Oxide
2.7. Lipid Peroxidation
2.8. Statistical Analysis
3. Results
3.1. Effect of Tunicamycin on Sperm Motility and Viability in Boar Semen
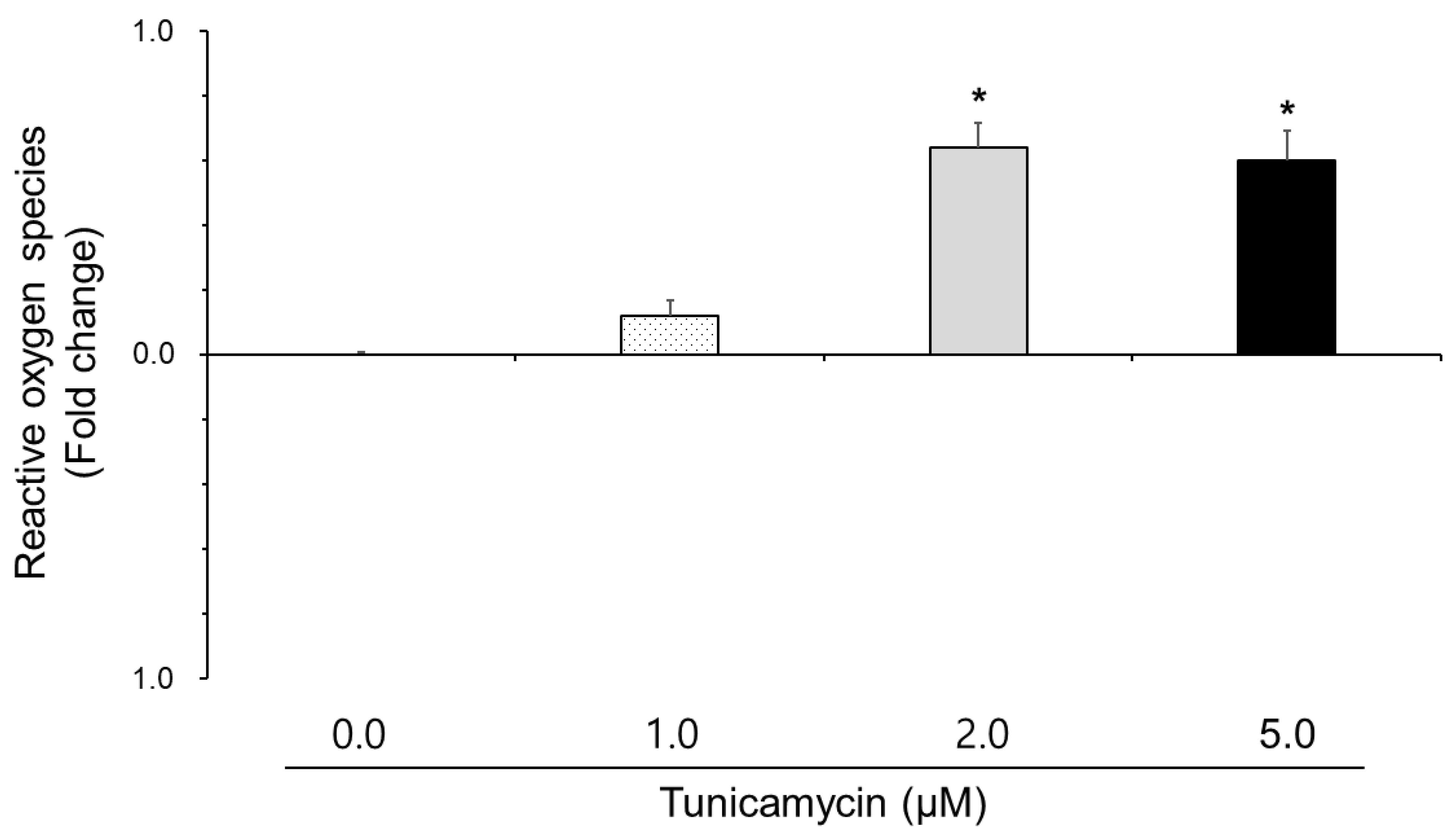
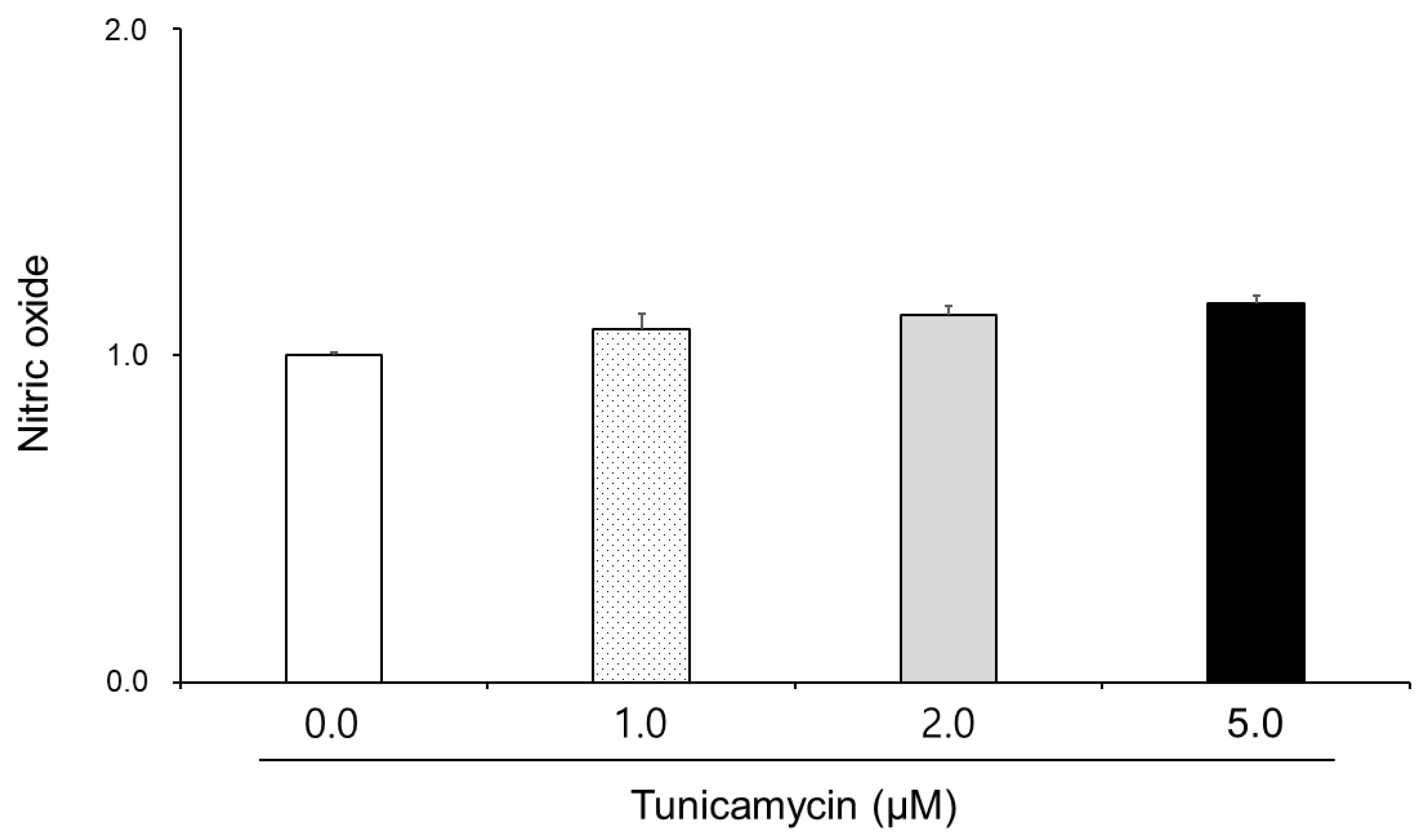
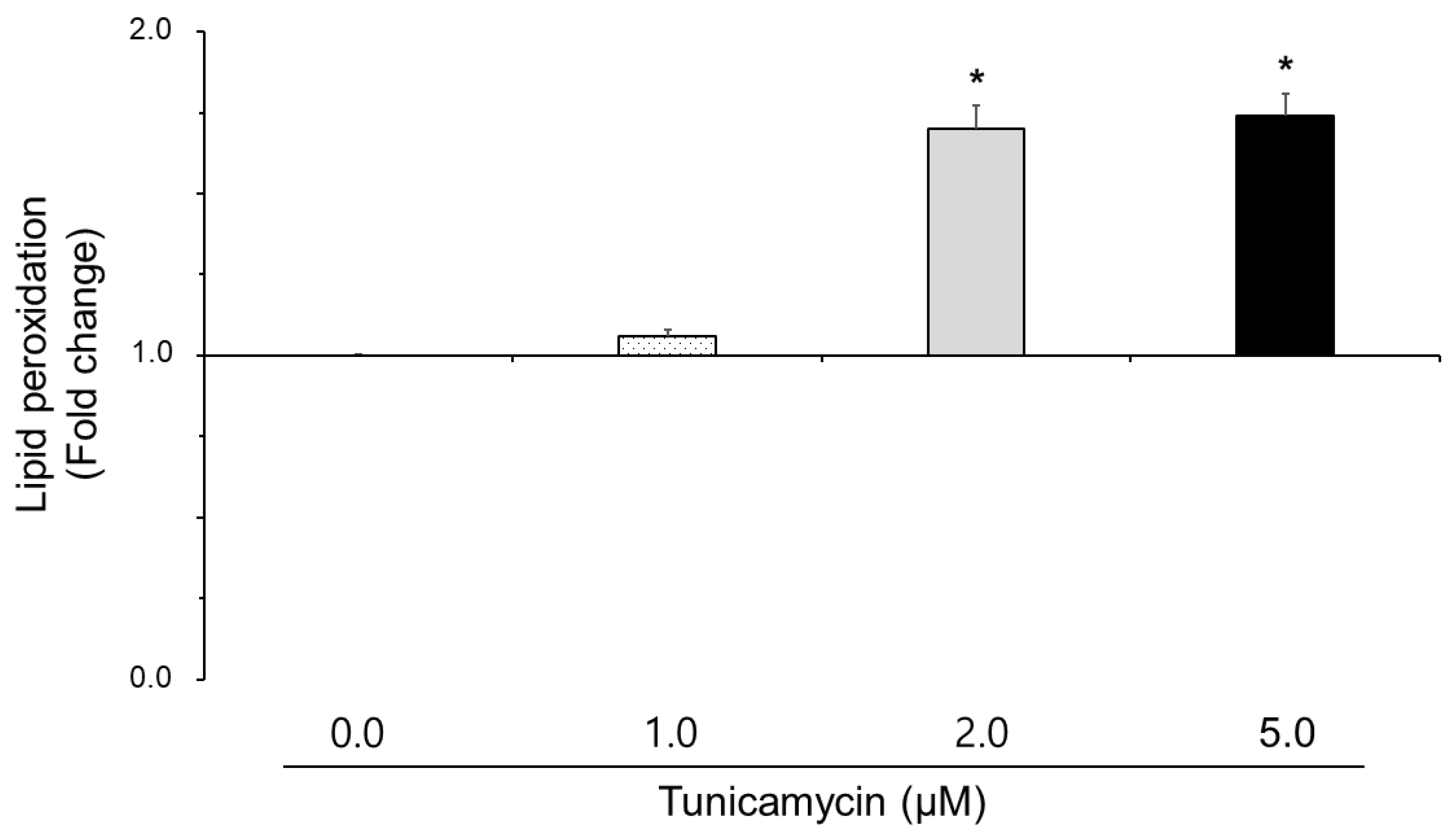
3.2. Effect of Tunicamycin on Reactive Oxygen Species, Nitric Oxide, and Lipid Peroxidation in Boar Semen
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Chen, S.; Liu, H.; Zhang, Z.; Ni, Z.; Chen, J.; Yang, Z.; Nie, Y.; Fan, D. Tunicamycin specifically aggravates ER stress and overcomes chemoresistance in multidrug-resistant gastric cancer cells by inhibiting N-glycosylation. J. Exp. Clin. Cancer Res. 2018, 37, 272. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ichikawa, S. Tunicamycin: Chemical synthesis and biosynthesis. J. Antibiot. 2019, 72, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wang, S.; Ren, B.; Wang, J.; Chen, J.; Lu, J.; Zhan, S.; Fu, Y.; Huang, L.; Tan, J. CHOP favors endoplasmic reticulum stress-induced apoptosis in hepatocellular carcinoma cells via inhibition of autophagy. PLoS ONE 2017, 12, e0183680. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Ge, C.; Sun, H.; Li, H.; Li, J.; Tian, H. Tunicamycin inhibits cell proliferation and migration in hepatocellular carcinoma through suppression of CD44s and the ERK1/2 pathway. Cancer Sci. 2018, 109, 1088–1100. [Google Scholar] [CrossRef]
- Anto, E.M.; Sruthi, C.R.; Krishnan, L.; Raghu, K.G.; Purushothaman, J. Tangeretin alleviates Tunicamycin-induced endoplasmic reticulum stress and associated complications in skeletal muscle cells. Cell Stress Chaperones 2023, 28, 151–165. [Google Scholar] [CrossRef]
- Lim, E.J.; Heo, J.; Kim, Y.H. Tunicamycin promotes apoptosis in leukemia cells through ROS generation and downregulation of survivin expression. Apoptosis 2015, 20, 1087–1098. [Google Scholar] [CrossRef]
- Kim, S.H.; Seo, H.; Kwon, D.; Yuk, D.Y.; Jung, Y.S. Taurine Ameliorates Tunicamycin-Induced Liver Injury by Disrupting the Vicious Cycle between Oxidative Stress and Endoplasmic Reticulum Stress. Life 2022, 12, 354. [Google Scholar] [CrossRef]
- Chen, F.; Ge, Z.; Li, N.; Yu, Z.; Wu, R.; Zhao, Y.; He, X.; Cai, G. TUDCA protects against tunicamycin-induced apoptosis of dorsal root ganglion neurons by suppressing activation of ER stress. Exp. Ther. Med. 2022, 24, 509. [Google Scholar] [CrossRef]
- Shi, J.; Chen, L.; Wang, X.; Ma, X. SIRT6 inhibits endoplasmic reticulum stress-mediated ferroptosis by activating Nrf2/HO-1 signaling to alleviate osteoarthritis. Inflamm. Res. 2025, 74, 35. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Kim, M.; Liu, H.; Yang, F.; Chen, K.; Shi, H. Atractylenolide partially alleviates tunicamycin-induced damage in porcine oocytes during in vitro maturation by reducing oxidative stress. Anim. Reprod. Sci. 2025, 273, 107761. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.H.; Li, J.; Ding, P.J.; Wang, Y. Effects of Escitalopram on Endoplasmic Reticulum Stress and Oxidative Stress Induced by Tunicamycin. Front. Neurosci. 2021, 15, 737509. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, Y.M.; Cheong, H.T.; Park, C.K.; Lee, S.H. Effect of magnetized freezing extender on membrane damages, motility, and fertility of boar sperm following cryopreservation. Animals 2023, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, S.; Lee, S.H.; Yang, B.K.; Park, C.K. Effect of cholesterol-loaded cyclodextrin on sperm viability and acrosome reaction in boar semen cryopreservation. Anim. Reprod. Sci. 2015, 159, 124–130. [Google Scholar] [CrossRef]
- Sung, H.J.; Jeong, Y.J.; Kim, J.; Jung, E.; Jun, J.H. Soybean peptides induce apoptosis in HeLa cells by increasing oxidative stress. Biomed. Sci. Lett. 2015, 21, 77–83. [Google Scholar] [CrossRef]
- Kojima, H.; Nakatsubo, N.; Kikuchi, K.; Kawajara, S.; Kirino, Y.; Nagoshi, H.; Hirata, Y.; Nagano, T. Detection and imaging of nitric oxide with novel fluorescent indicators: Diaminofluoresceins. Anal. Chem. 1998, 70, 2446–2453. [Google Scholar] [CrossRef]
- Singh, M.; Mollier, R.T.; Pongener, N.; Bordoloi, L.J.; Kumar, R.; Chaudhary, J.K.; Katiyar, R.; Khan, M.H.; Rajkhowa, D.J.; Mishra, V.K. Linseed oil in boar’s diet during high temperature humidity index (THI) period improves sperm quality characteristics, antioxidant status and fatty acid composition of sperm under hot humid sub-tropical climate. Theriogenology 2022, 189, 127–136. [Google Scholar] [CrossRef]
- Uribe, P.; Merino, J.; Matus, C.E.; Schulz, M.; Zambrano, F.; Villegas, J.V.; Conejeros, I.; Taubert, A.; Hermosilla, C.; Sanchez, R. Autophagy is activated in human spermatozoa subjected to oxidative stress and its inhibition impairs sperm quality and promotes cell death. Hum. Reprod. 2022, 37, 680–695. [Google Scholar] [CrossRef]
- Sharma, P.; Kaushal, N.; Saleth, L.R.; Ghavami, S.; Dhingra, S.; Kaur, P. Oxidative stress-induced apoptosis and autophagy: Balancing the contrary forces in spermatogenesis. Biochim. Biophys. Acta Mol. Basis. Dis. 2023, 1869, 166742. [Google Scholar] [CrossRef]
- Guha, P.; Kaptan, E.; Gade, P.; Kalvakolanu, D.V.; Ahmed, H. Tunicamycin induced endoplasmic reticulum stress promotes apoptosis of prostate cancer cells by activating mTORC1. Oncotarget 2017, 8, 68191–68207. [Google Scholar] [CrossRef]
- Kim, Y.J.; Han, J.; Han, S. The interplay between endoplasmic reticulum stress and oxidative stress in chondrocyte catabolism. Sage J. 2024, 10, 1177. [Google Scholar]
- Malhotra, J.D.; Kaufman, R.J. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 2007, 18, 716–731. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kwon, D.Y.; Kwak, J.H.; Lee, S.; Lee, Y.H.; Yun, J.; Son, T.G.; Jung, Y.S. Tunicamycin-Induced ER Stress is Accompanied with Oxidative Stress via Abrogation of Sulfur Amino Acids Metabolism in the Liver. Int. J. Mol. Sci. 2018, 19, 4114. [Google Scholar] [CrossRef] [PubMed]
- Beavers, W.N.; Monteith, A.J.; Amarnath, V.; Mernaugh, R.L.; Roberts, L.J.; Chazin, W.J.; Davies, S.S.; Skaar, E.P. Arachidonic Acid Kills Staphylococcus aureus through a Lipid Peroxidation Mechanism. mBio 2019, 10, e101333. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Prabakaran, S. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. Indian J. Exp. Biol. 2005, 43, 963–974. [Google Scholar]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2010, 2011, 686137. [Google Scholar] [CrossRef]
- Hosoi, T.; Noguchi, J.; Takakuwa, M.; Honda, M.; Okuma, Y.; Nomura, Y.; Ozawa, K. Inhibition of inducible nitric oxide synthase and interleukin-1beta expression by tunicamycin in cultured glial cells exposed to lipopolysaccharide. Brain Res. 2014, 1558, 11–17. [Google Scholar] [CrossRef]
- Kucuksayan, E.; Konuk, E.K.; Demir, N.; Mutus, B.; Aslan, M. Neutral sphingomyelinase inhibition decreases ER stress-mediated apoptosis and inducible nitric oxide synthase in retinal pigment epithelial cells. Free Radic. Biol. Med. 2014, 72, 113–123. [Google Scholar] [CrossRef]
- Ohta, S.; Hattori, Y.; Nakanishi, N.; Sugimoto, H.; Kasai, K. Differential modulation of immunostimulant-triggered NO production by endoplasmic reticulum stress inducers in vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2011, 57, 434–438. [Google Scholar] [CrossRef]
| Tunicamycin (μM) | |||||
|---|---|---|---|---|---|
| 0.0 | 1.0 | 2.0 | 5.0 | 10.0 | |
| Motility | 79.68 ± 1.88 | 79.82 ± 1.77 | 73.28 ± 0.99 * | 71.48 ± 0.48 * | 54.48 ± 1.35 * |
| Viability | 65.08 ± 1.07 | 65.72 ± 0.77 | 55.44 ± 1.11 * | 53.20 ± 0.78 * | 40.00 ± 1.15 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S. Effect of Tunicamycin on Viability, Motility, Reactive Oxygen Species, Nitric Oxide, and Lipid Peroxidation in Boar Sperm. Animals 2025, 15, 1422. https://doi.org/10.3390/ani15101422
Lee S. Effect of Tunicamycin on Viability, Motility, Reactive Oxygen Species, Nitric Oxide, and Lipid Peroxidation in Boar Sperm. Animals. 2025; 15(10):1422. https://doi.org/10.3390/ani15101422
Chicago/Turabian StyleLee, Seunghyung. 2025. "Effect of Tunicamycin on Viability, Motility, Reactive Oxygen Species, Nitric Oxide, and Lipid Peroxidation in Boar Sperm" Animals 15, no. 10: 1422. https://doi.org/10.3390/ani15101422
APA StyleLee, S. (2025). Effect of Tunicamycin on Viability, Motility, Reactive Oxygen Species, Nitric Oxide, and Lipid Peroxidation in Boar Sperm. Animals, 15(10), 1422. https://doi.org/10.3390/ani15101422






