Sexual Selection in Mosquitofish: Differences in the Use of Mating Cues Between Sexes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin and Maintenance of Test Fish
2.2. Test 1- Influence of Male Gonopidium Status on Mate Choice in Females
2.2.1. Creation of Male G. affinis Animations
2.2.2. Female Mate Choice Test
2.3. Test 2- Influence of Female Age on Mate Choice in Males
2.3.1. Acquisition of High-Resolution Images of Young and Old Female Fish
2.3.2. Quantify the Relative Gravid Spot Area of Young and Old Female Fish
2.3.3. Creation of Female G. affinis Animations
2.3.4. Male Preference for Females of Different Ages
2.3.5. Male Preference for Females with Different Relative Areas of Gravid Spots
2.3.6. Male Preference for Females with Different Morphological Traits (Spine and Abdomen Morphology)
2.4. Statistical Analyses
3. Results
3.1. Female Preference for Males with Different Gonopodium Status
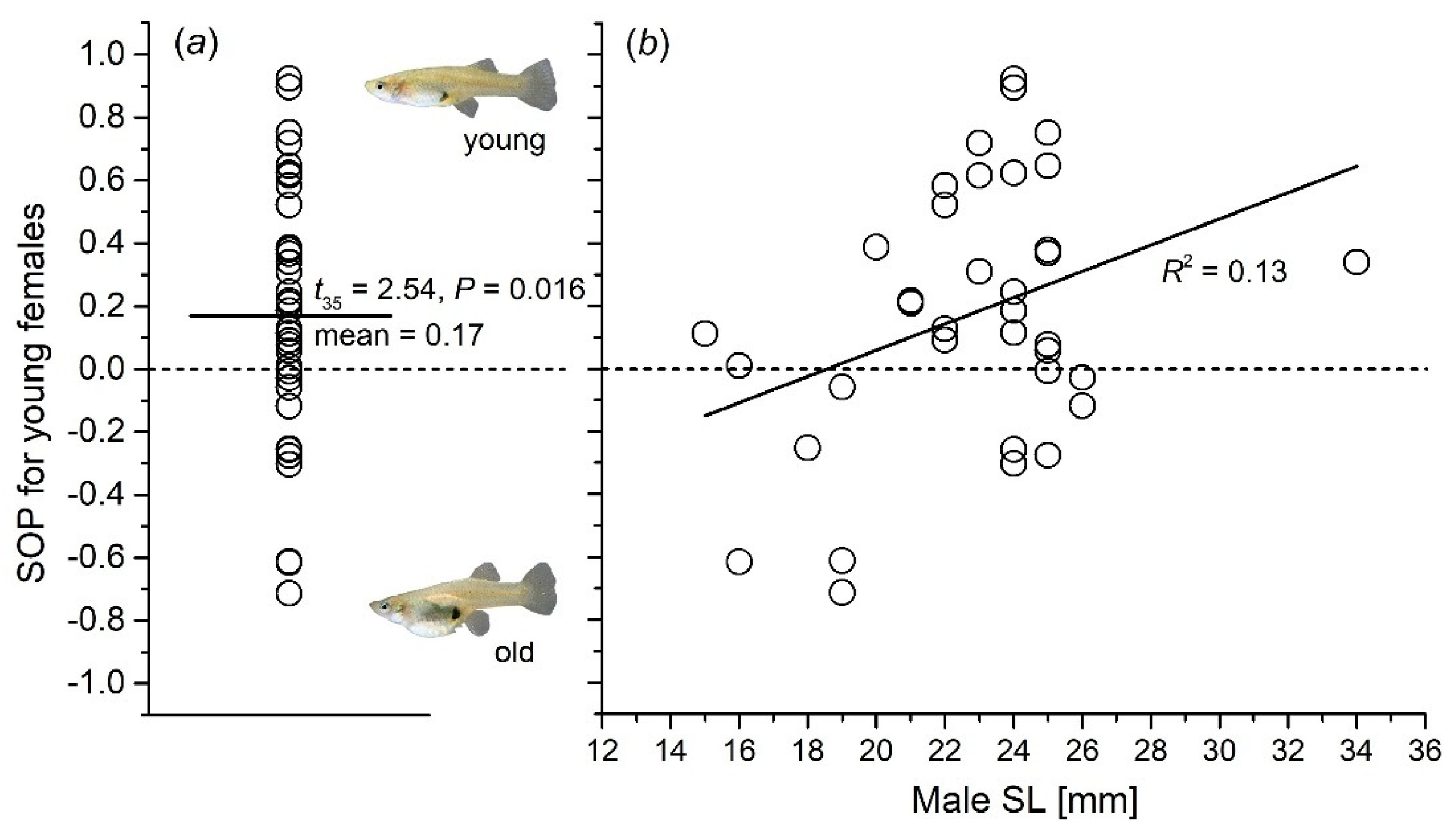
3.2. Male Preference for Females of Different Ages
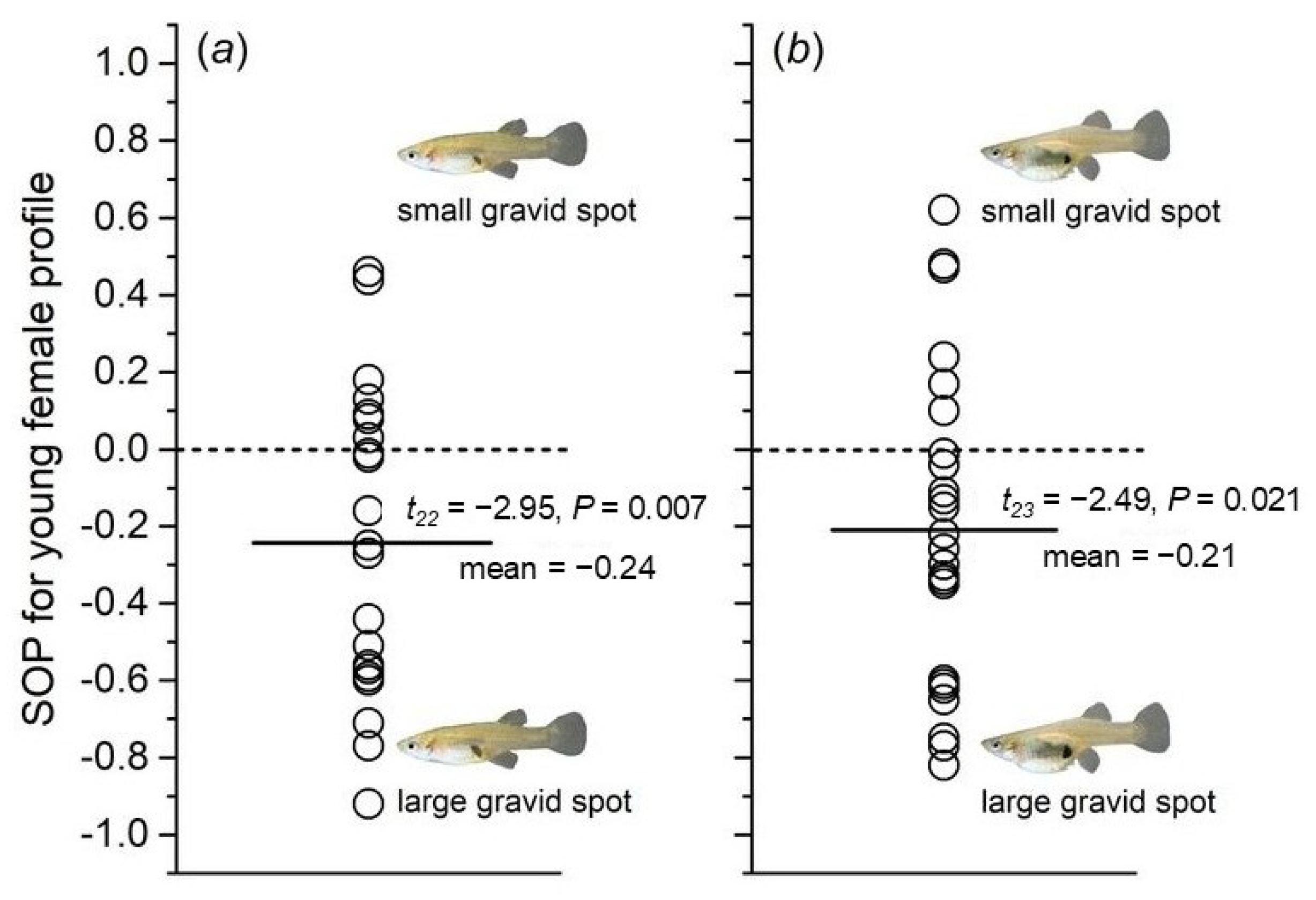
3.3. Male Preference for Females with Different Relative Areas of Gravid Spots
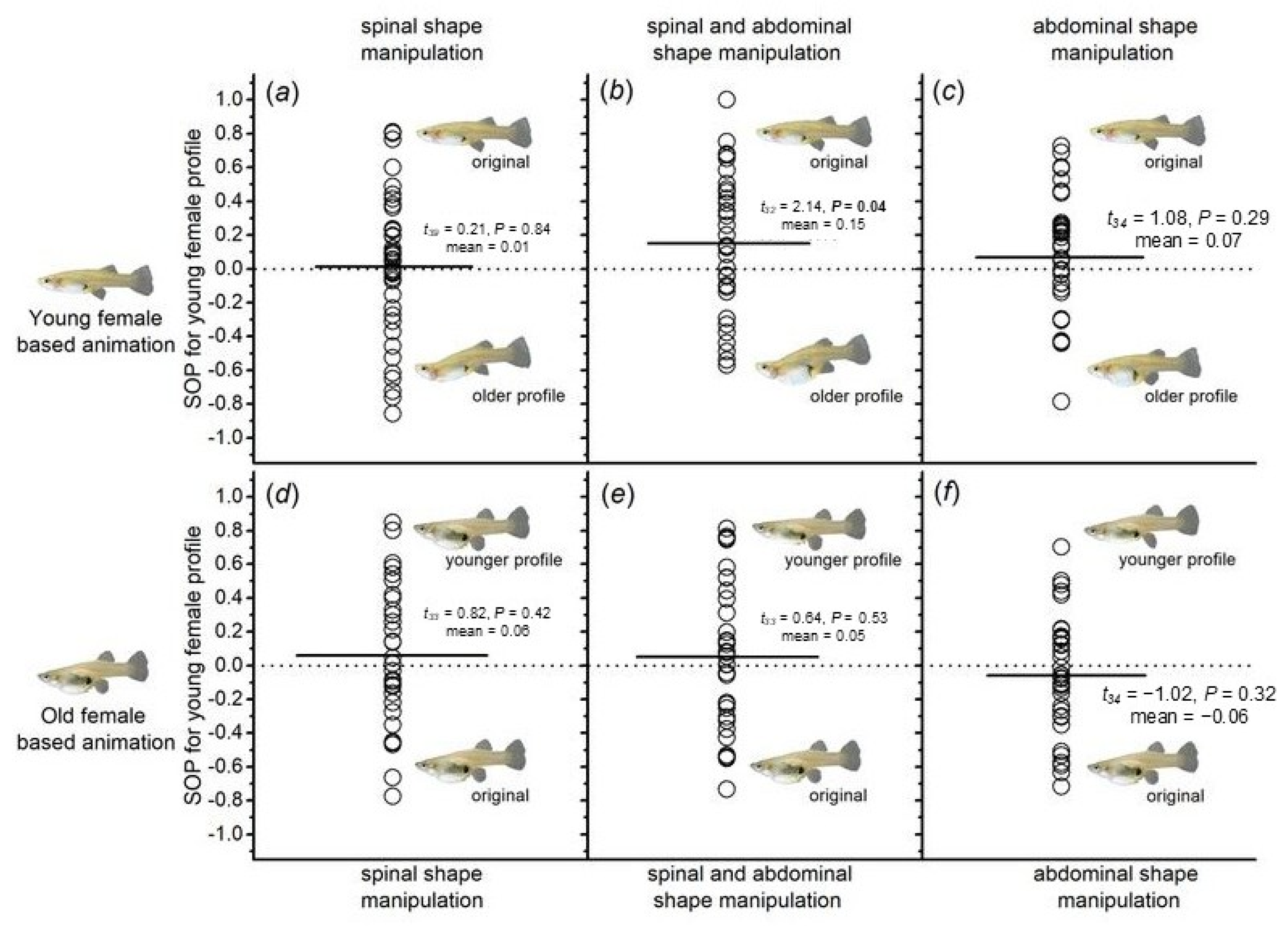
3.4. Male Preference for Females with Different Morphological Traits (Spine and Abdomen Morphology)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darwin, C. The Descent of Man, and Selection in Relation to Sex; D. Appleton: New York, NY, USA, 1872; Volume 2. [Google Scholar]
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Andersson, M.; Simmons, L.W. Sexual selection and mate choice. Trends Ecol. Evol. 2006, 21, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, G.G. Mate Choice: The Evolution of Sexual Decision Making from Microbes to Humans; Princeton University Press: Princeton, NJ, USA, 2017. [Google Scholar]
- Cornuau, J.H.; Rat, M.; Schmeller, D.S.; Loyau, A. Multiple signals in the palmate newt: Ornaments help when courting. Behav. Ecol. Sociobiol. 2012, 66, 1045–1055. [Google Scholar] [CrossRef]
- Herdman, E.J.; Kelly, C.D.; Godin, J.G.J. Male mate choice in the guppy (Poecilia reticulata): Do males prefer larger females as mates? Ethology 2004, 110, 97–111. [Google Scholar] [CrossRef]
- Sommer-Trembo, C.; Plath, M.; Gismann, J.; Helfrich, C.; Bierbach, D. Context-dependent female mate choice maintains variation in male sexual activity. R. Soc. Open Sci. 2017, 4, 170303. [Google Scholar] [CrossRef]
- Ryan, M.J.; Rand, A.S. The sensory basis of sexual selection for complex calls in the túngara frog, Physalaemus pustulosus (sexual selection for sensory exploitation). Evolution 1990, 44, 305–314. [Google Scholar]
- Heuschele, J.; Mannerla, M.; Gienapp, P.; Candolin, U. Environment-dependent use of mate choice cues in sticklebacks. Behav. Ecol. 2009, 20, 1223–1227. [Google Scholar] [CrossRef]
- Candolin, U.; Salesto, T.; Evers, M. Changed environmental conditions weaken sexual selection in sticklebacks. J. Evol. Biol. 2007, 20, 233–239. [Google Scholar] [CrossRef]
- Järvenpää, M.; Lindström, K. Water turbidity by algal blooms causes mating system breakdown in a shallow-water fish, the sand goby Pomatoschistus minutus. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 271, 2361–2365. [Google Scholar] [CrossRef]
- Amundsen, T.; Forsgren, E. Male mate choice selects for female coloration in a fish. Proc. Natl. Acad. Sci. USA 2001, 98, 13155–13160. [Google Scholar] [CrossRef]
- López, S. Parasitized female guppies do not prefer showy males. Anim. Behav. 1999, 57, 1129–1134. [Google Scholar] [CrossRef][Green Version]
- Harmison, J.; Ogborn, M.; Pett, J.; Tolman, D. Female Mate Choice in Guppies Based on Color Contrast and Sensory Exploitation. Biology Poster 2013. Available online: https://digitalcommons.usu.edu/biology_posters/98 (accessed on 18 May 2025).
- Aspbury, A.S.; Basolo, A.L. Repeatable female preferences, mating order and mating success in the poeciliid fish, Heterandria formosa. Behav. Ecol. Sociobiol. 2002, 51, 238–244. [Google Scholar] [CrossRef]
- Ala-Honkola, O.; Säilä, L.; Lindström, K. Males prefer small females in a dichotomous choice test in the poeciliid fish Heterandria formosa. Ethology 2010, 116, 736–743. [Google Scholar] [CrossRef]
- Clutton-Brock, T. Reproductive competition and sexual selection. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160310. [Google Scholar] [CrossRef]
- Parker, G.A. Sexual conflict over mating and fertilization: An overview. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Bisazza, A.; Vaccari, G.; Pilastro, A. Female mate choice in a mating system dominated by male sexual coercion. Behav. Ecol. 2001, 12, 59–64. [Google Scholar] [CrossRef]
- Pyke, G.H. A review of the biology of Gambusia affinis and G. holbrooki. Rev. Fish Biol. Fish. 2005, 15, 339–365. [Google Scholar] [CrossRef]
- Hughes, A.L. Male size, mating success, and mating strategy in the mosquitofish Gambusia affinis (Poeciliidae). Behav. Ecol. Sociobiol. 1985, 17, 271–278. [Google Scholar] [CrossRef]
- Wang, S.; Cummings, M.; Kirkpatrick, M. Coevolution of male courtship and sexual conflict characters in mosquitofish. Behav. Ecol. 2015, 26, 1013–1020. [Google Scholar] [CrossRef]
- Martinez-Rivera, N.; Serrano-Velez, J.L.; Torres-Vazquez, I.I.; Langerhans, R.B.; Rosa-Molinar, E. Are superficial neuromasts proprioceptors underlying fast copulatory behavior? Front. Neural Circuits 2022, 16, 921568. [Google Scholar] [CrossRef]
- Simmons, L.W. Sexual selection and genital evolution. Austral Entomol. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Gonzalez-Voyer, A.; Fitzpatrick, J.L.; Kolm, N. Sexual selection determines parental care patterns in cichlid fishes. Evolution 2008, 62, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Jennions, M.D.; Petrie, M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2000, 75, 21–64. [Google Scholar] [CrossRef] [PubMed]
- Grayson, K.L.; De Lisle, S.P.; Jackson, J.E.; Black, S.J.; Crespi, E.J. Behavioral and physiological female responses to male sex ratio bias in a pond-breeding amphibian. Front. Zool. 2012, 9, 24. [Google Scholar] [CrossRef]
- Mühlhäuser, C.; Blanckenhorn, W.U. The costs of avoiding matings in the dung fly Sepsis cynipsea. Behav. Ecol. 2002, 13, 359–365. [Google Scholar] [CrossRef]
- Magurran, A.E.; Seghers, B.H. A cost of sexual harassment in the guppy, Poecilia reticulata. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1994, 258, 89–92. [Google Scholar]
- Swierk, L.; Myers, A.; Langkilde, T. Male mate preference is influenced by both female behaviour and morphology. Anim. Behav. 2013, 85, 1451–1457. [Google Scholar] [CrossRef]
- Edward, D.A.; Chapman, T. The evolution and significance of male mate choice. Trends Ecol. Evol. 2011, 26, 647–654. [Google Scholar] [CrossRef]
- Rosenqvist, G. Male mate choice and female-female competition for mates in the pipefish Nerophis ophidion. Anim. Behav. 1990, 39, 1110–1115. [Google Scholar] [CrossRef]
- Morina, D.L.; Demarais, S.; Strickland, B.K.; Larson, J.E. While males fight, females choose: Male phenotypic quality informs female mate choice in mammals. Anim. Behav. 2018, 138, 69–74. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U.; Mühlhäuser, C.; Morf, C.; Reusch, T.; Reuter, M. Female choice, female reluctance to mate and sexual selection on body size in the dung fly Sepsis cynipsea. Ethology 2000, 106, 577–593. [Google Scholar] [CrossRef]
- Schlupp, I.; McKnab, R.; Ryan, M. Sexual harassment as a cost for molly females: Bigger males cost less. Behaviour 2001, 138, 277–286. [Google Scholar] [CrossRef]
- Wong, R.Y.; So, P.; Cummings, M.E. How female size and male displays influence mate preference in a swordtail. Anim. Behav. 2011, 82, 691–697. [Google Scholar] [CrossRef]
- Dosen, L.D.; Montgomerie, R. Female size influences mate preferences of male guppies. Ethology 2004, 110, 245–255. [Google Scholar] [CrossRef]
- Bonduriansky, R. The evolution of male mate choice in insects: A synthesis of ideas and evidence. Biol. Rev. 2001, 76, 305–339. [Google Scholar] [CrossRef]
- Pilastro, A.; Mandelli, M.; Gasparini, C.; Dadda, M.; Bisazza, A. Copulation duration, insemination efficiency and male attractiveness in guppies. Anim. Behav. 2007, 74, 321–328. [Google Scholar] [CrossRef]
- Wong, B.B.; McCarthy, M. Prudent male mate choice under perceived sperm competition risk in the eastern mosquito fish. Behav. Ecol. 2009, 20, 278–282. [Google Scholar] [CrossRef]
- Deaton, R. Factors influencing male mating behaviour in Gambusia affinis (Baird & Girard) with a coercive mating system. J. Fish Biol. 2008, 72, 1607–1622. [Google Scholar]
- Reznick, D.; Hrbek, T.; Caura, S.; De Greef, J.; Roff, D. Life history of Xenodexia ctenolepis: Implications for life history evolution in the family Poeciliidae. Biol. J. Linn. Soc. 2007, 92, 77–85. [Google Scholar] [CrossRef]
- Fleuren, M.; Quicazan-Rubio, E.M.; van Leeuwen, J.L.; Pollux, B.J. Why do placentas evolve? Evidence for a morphological advantage during pregnancy in live-bearing fish. PLoS ONE 2018, 13, e0195976. [Google Scholar] [CrossRef]
- Norazmi-Lokman, N.H.; Purser, G.; Patil, J.G. Gravid spot predicts developmental progress and reproductive output in a livebearing fish, Gambusia holbrooki. PLoS ONE 2016, 11, e0147711. [Google Scholar] [CrossRef]
- Kodama, I.; Yamanaka, A.; Endo, K.; Koya, Y. Role of the yellow spot around the urogenital opening of female mosquitofish (Gambusia affinis) as a cue for copulation. Zool. Sci. 2008, 25, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Bisazza, A.; Marconato, A.; Marin, G. Male mate preferences in the mosquitofish Gambusia holbrooki. Ethology 1989, 83, 335–343. [Google Scholar] [CrossRef]
- Winkler, P. Thermal preference of Gambusia affinis affinis as determined under field and laboratory conditions. Copeia 1979, 1979, 60–64. [Google Scholar] [CrossRef]
- Vargas, M.; De Sostoa, A. Life history of Gambusia holbrooki (Pisces, Poeciliidae) in the Ebro delta (NE Iberian peninsula). Hydrobiologia 1996, 341, 215–224. [Google Scholar] [CrossRef]
- Chen, B.-j.; Liu, K.; Zhou, L.-j.; Gomes-Silva, G.; Sommer-Trembo, C.; Plath, M. Personality differentially affects individual mate choice decisions in female and male Western mosquitofish (Gambusia affinis). PLoS ONE 2018, 13, e0197197. [Google Scholar] [CrossRef]
- Swenton, D.M.; Kodric-Brown, A. Habitat and life history differences between two species of Gambusia. Environ. Biol. Fishes 2012, 94, 669–680. [Google Scholar] [CrossRef]
- Pyke, G.H. Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 171–191. [Google Scholar] [CrossRef]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Ouyang, X.; Gao, J.; Xie, M.; Liu, B.; Zhou, L.; Chen, B.; Jourdan, J.; Riesch, R.; Plath, M. Natural and sexual selection drive multivariate phenotypic divergence along climatic gradients in an invasive fish. Sci. Rep. 2018, 8, 11164. [Google Scholar] [CrossRef]
- McKinney, F.; Evarts, S. Sexual coercion in waterfowl and other birds. Ornithol. Monogr. 1998, 163–195. [Google Scholar] [CrossRef]
- Réale, D.; Boussès, P.; Chapuis, J.-L. Female-biased mortality induced by male sexual harassment in a feral sheep population. Can. J. Zool. 1996, 74, 1812–1818. [Google Scholar] [CrossRef]
- Bisazza, A. Male competition, female mate choice and sexual size dimorphism in poeciliid fishes. Mar. Freshw. Behav. Physiol. 1993, 23, 257–286. [Google Scholar] [CrossRef]
- Plath, M.; Makowicz, A.M.; Schlupp, I.; Tobler, M. Sexual harassment in live-bearing fishes (Poeciliidae): Comparing courting and noncourting species. Behav. Ecol. 2007, 18, 680–688. [Google Scholar] [CrossRef]
- Peters, G.; Mäder, B. Morphologische Veränderungen der Gonadenausführgänge sich fortpflanzender Schwertträgerweibchen (Xiphophorus helleri Heckel). Zool. Anz. 1964, 173, 243–257. [Google Scholar]
- Pilastro, A.; Benetton, S.; Bisazza, A. Female aggregation and male competition reduce costs of sexual harassment in the mosquitofish Gambusia holbrooki. Anim. Behav. 2003, 65, 1161–1167. [Google Scholar] [CrossRef]
- Pocklington, R.; Dill, L.M. Predation on females or males: Who pays for bright male traits? Anim. Behav. 1995, 49, 1122–1124. [Google Scholar] [CrossRef]
- Watkins, C.D. Mate assessment based on physical characteristics: A review and reflection. Biol. Rev. 2025, 100, 113–130. [Google Scholar] [CrossRef]
- Kenrick, D.T.; Keefe, R.C. Age preferences in mates reflect sex differences in human reproductive strategies. Behav. Brain Sci. 1992, 15, 75–91. [Google Scholar] [CrossRef]
- Sutter, D.A.; Suski, C.D.; Philipp, D.P.; Klefoth, T.; Wahl, D.H.; Kersten, P.; Cooke, S.J.; Arlinghaus, R. Recreational fishing selectively captures individuals with the highest fitness potential. Proc. Natl. Acad. Sci. USA 2012, 109, 20960–20965. [Google Scholar] [CrossRef]
- Guevara-Fiore, P.; Skinner, A.; Watt, P. Do male guppies distinguish virgin females from recently mated ones? Anim. Behav. 2009, 77, 425–431. [Google Scholar] [CrossRef]
- Edwards, T.M.; Guillette, L.J., Jr. Reproductive characteristics of male mosquitofish (Gambusia holbrooki) from nitrate-contaminated springs in Florida. Aquat. Toxicol. 2007, 85, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hebets, E.A.; Papaj, D.R. Complex signal function: Developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 2005, 57, 197–214. [Google Scholar] [CrossRef]

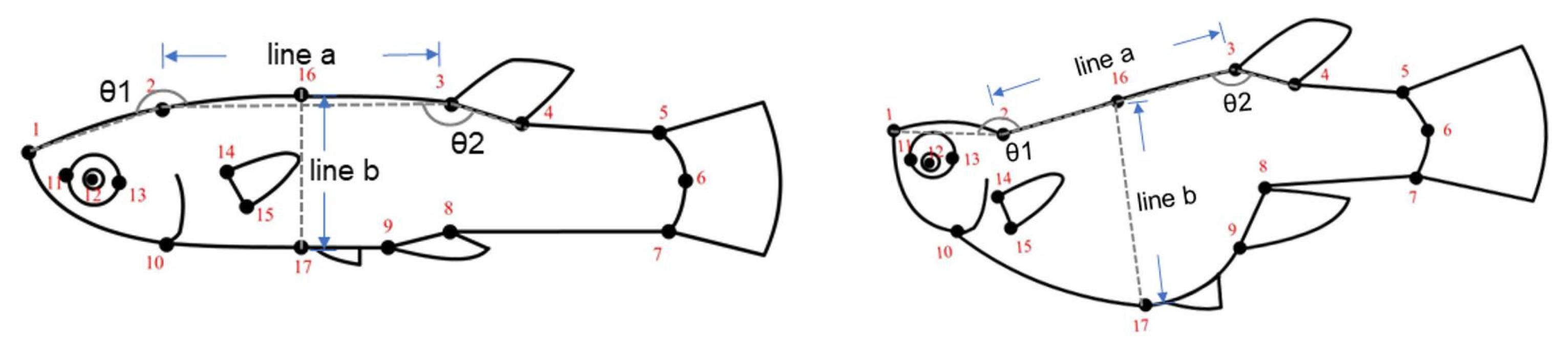

| Relative Warps (RW) | Eigen Value | Variance Explained (%) |
|---|---|---|
| RW1 | 1.91 × 10−3 | 60.90 |
| RW2 | 2.65 × 10−4 | 8.46 |
| RW3 | 2.34 × 10−4 | 7.46 |
| RW4 | 1.13 × 10−4 | 3.59 |
| RW5 | 1.06 × 10−4 | 3.39 |
| RW6 | 8.74 × 10−5 | 2.79 |
| RW7 | 7.95 × 10−5 | 2.54 |
| RW8 | 5.90 × 10−5 | 1.88 |
| RW9 | 5.51 × 10−5 | 1.76 |
| RW10 | 3.95 × 10−5 | 1.26 |
| Source | df | F | p | Wilks’ Partial ηp2 |
|---|---|---|---|---|
| Animation ID | 20 | 1.37 | 0.28 | 0.66 |
| SL | 1 | 0.13 | 0.72 | 0.009 |
| Error | 30 |
| Factor | df | F | p | Wilks’ Partial ηp2 |
|---|---|---|---|---|
| Animation type | 0 | - | - | - |
| Animation ID | 18 | 0.92 | 0.57 | 0.40 |
| Male body size | 1 | 0.55 | 0.47 | 0.022 |
| Animation type × male body size | 1 | 4.21 | 0.051 | 0.14 |
| Error | 25 |
| Source | df | F | p | Wilks’ Partial ηp2 |
|---|---|---|---|---|
| (a) Both animation types combined | ||||
| Animation type | 1 | 0.20 | 0.66 | 0.001 |
| Spinal shape | 1 | 0.05 | 0.83 | 0.0002 |
| Abdominal shape | 1 | 1.38 | 0.24 | 0.01 |
| Male body size | 1 | 1.88 | 0.17 | 0.01 |
| Error | 206 | |||
| (b) Animations derived from young females | ||||
| Spinal shape | 1 | 0.81 | 0.37 | 0.01 |
| Abdominal shape | 1 | 2.21 | 0.14 | 0.02 |
| Male body size | 1 | 0.11 | 0.74 | 0.001 |
| Error | 104 | |||
| (c) Animations derived from old females | ||||
| Spinal shape | 1 | 1.78 | 0.19 | 0.02 |
| Abdominal shape | 1 | 0.03 | 0.86 | 0.0003 |
| Male body size | 1 | 3.05 | 0.084 | 0.03 |
| Error | 99 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Feng, B.; Dong, C.; Chen, B.; Liu, K. Sexual Selection in Mosquitofish: Differences in the Use of Mating Cues Between Sexes. Animals 2025, 15, 1489. https://doi.org/10.3390/ani15101489
Wei J, Feng B, Dong C, Chen B, Liu K. Sexual Selection in Mosquitofish: Differences in the Use of Mating Cues Between Sexes. Animals. 2025; 15(10):1489. https://doi.org/10.3390/ani15101489
Chicago/Turabian StyleWei, Jiefei, Bowen Feng, Chenglong Dong, Bojian Chen, and Kai Liu. 2025. "Sexual Selection in Mosquitofish: Differences in the Use of Mating Cues Between Sexes" Animals 15, no. 10: 1489. https://doi.org/10.3390/ani15101489
APA StyleWei, J., Feng, B., Dong, C., Chen, B., & Liu, K. (2025). Sexual Selection in Mosquitofish: Differences in the Use of Mating Cues Between Sexes. Animals, 15(10), 1489. https://doi.org/10.3390/ani15101489






