Differences in Larval Microhabitat Between Two Cryptic, Sympatric Salamander Species (Desmognathus folkertsi and D. amphileucus) in Northeastern Georgia, USA
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
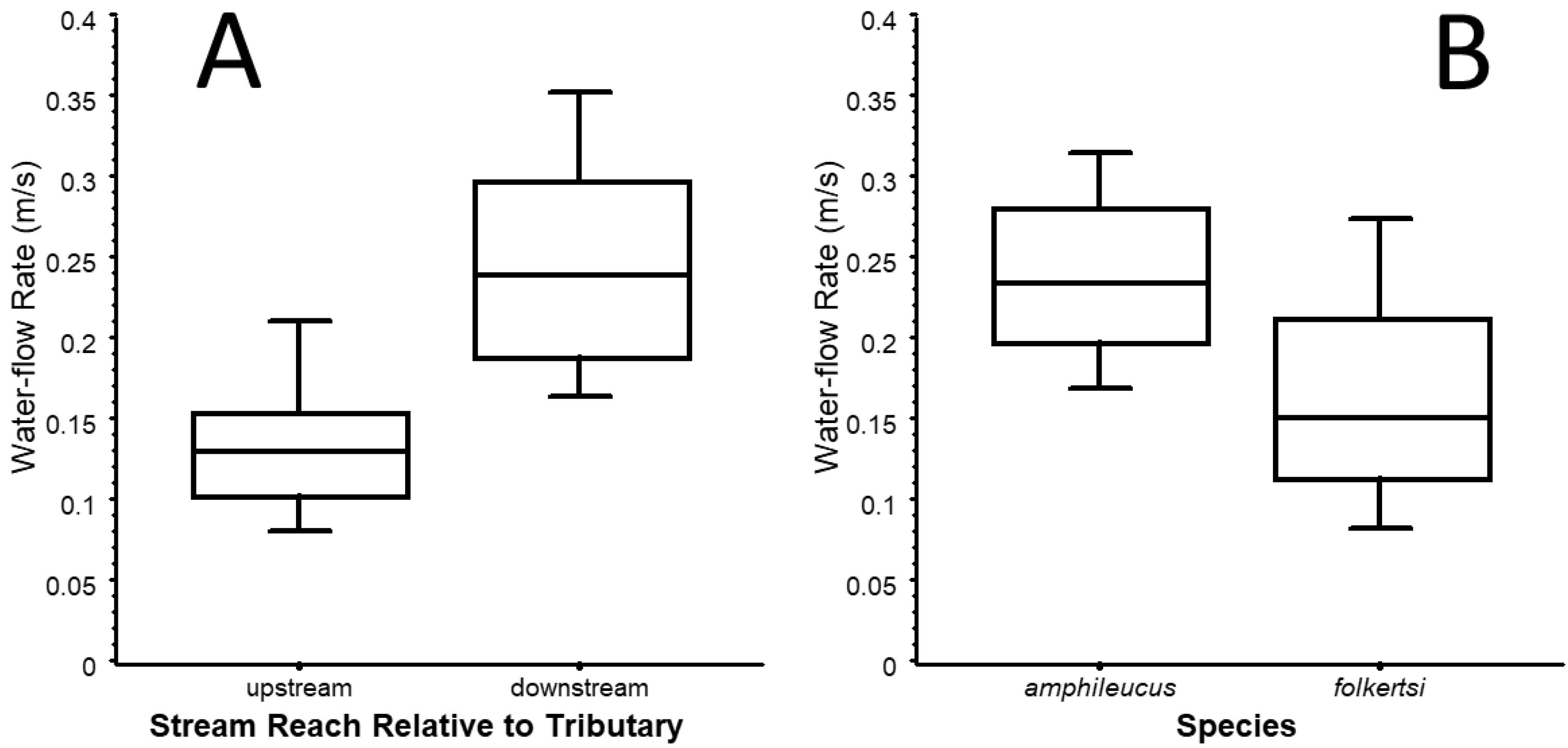
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, R.A.; McGehee, R. Competitive exclusion. Am. Nat. 1980, 115, 151–170. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource partitioning in ecological communities. Science 1974, 185, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, S.J.; Schmitt, R.J. Resource overlap, prey dynamics, and the strength of competition. Ecology 1989, 70, 1943–1953. [Google Scholar] [CrossRef]
- Ziv, Y.; Abramsky, Z.; Kotler, B.P.; Subach, A. Interference competition and temporal and habitat partitioning in two gerbil species. Oikos 1993, 66, 237–246. [Google Scholar] [CrossRef]
- Thiago Gonçalves-Souza, T.; Brescovit, A.D.; Rossa-Feres, D.D.C.; Romero, G.Q. Bromeliads as biodiversity amplifiers and habitat segregation of spider communities in a Neotropical rainforest. J. Arachnol. 2010, 38, 270–279. [Google Scholar] [CrossRef]
- Palomares, F.; Fernández, N.; Roques, S.; Chávez, C.; Silveira, L.; Keller, C.; Adrados, B. Fine-scale habitat segregation between two ecologically similar top predators. PLoS ONE 2016, 11, e0155626. [Google Scholar] [CrossRef]
- Keller, M.L.; Howard, D.R.; Hall, C.L. Spatiotemporal niche partitioning in a specious silphid community (Coleoptera: Silphidae Nicrophorus). Sci. Nat. 2019, 106, 57. [Google Scholar] [CrossRef]
- Weber, M.G.; Strauss, S.Y. Coexistence in close relatives: Beyond competition and reproductive isolation in sister taxa. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 359–381. [Google Scholar] [CrossRef]
- Lingle, S. Coyote predation and habitat segregation of white-tailed deer and mule deer. Ecology 2002, 83, 2037–2048. [Google Scholar] [CrossRef]
- Kaldonski, N.; Lagrue, C.; Motreuil, S.; Rigaud, T.; Bollache, L. Habitat segregation mediates predation by the benthic fish Cottus gobio on the exotic amphipod species Gammarus roeseli. Naturwissenschaften 2008, 95, 839–844. [Google Scholar] [CrossRef]
- Kotler, B.P. Risk of predation and the structure of desert rodent communities. Ecology 1984, 65, 689–701. [Google Scholar] [CrossRef]
- Reinert, H.K. Habitat separation between sympatric snake populations. Ecology 1984, 65, 478–486. [Google Scholar] [CrossRef]
- Howard, D.J.; Harrison, R.G. Habitat segregation in ground crickets: The role of interspecific competition and habitat selection. Ecology 1984, 65, 69–76. [Google Scholar] [CrossRef]
- Dunson, W.A.; Travis, J. The role of abiotic factors in community organization. Am. Nat. 1991, 138, 1067–1091. [Google Scholar] [CrossRef]
- Wei, F.; Feng, Z.; Wang, Z.; Hu, J. Habitat use and separation between the Giant Panda and the Red Panda. J. Mammal. 2000, 81, 448–455. [Google Scholar] [CrossRef]
- Loureiro, S.N.; Reis-Filho, J.A.; Giarrizzo, T. Evidence for habitat-driven segregation of an estuarine fish assemblage. J. Fish Biol. 2016, 89, 804–820. [Google Scholar] [CrossRef]
- Estevo, C.A.; Nagy-Reis, M.B.; Nichols, J.D. When habitat matters: Habitat preferences can modulate co-occurrence patterns of similar sympatric species. PLoS ONE 2017, 12, e0179489. [Google Scholar] [CrossRef]
- Cruz, P.; Iezzi, M.E.; De Angelo, C.; Varela, D.; Di Bitetti, M.S. Landscape use by two opossums is shaped by habitat preferences rather than by competitive interactions. J. Mammal. 2019, 100, 1966–1978. [Google Scholar] [CrossRef]
- Cordero, R.D.; Jackson, D.A. Abiotic factors influence species co-occurrence patterns of lake fishes. J. Anim. Ecol. 2021, 90, 2859–2874. [Google Scholar] [CrossRef]
- Martin, T.E. Abiotic vs. biotic influences on habitat selection of coexisting species: Climate change impacts? Ecology 2001, 82, 175–188. [Google Scholar] [CrossRef]
- Toft, C.A. Resource partitioning in amphibians and reptiles. Copeia 1985, 1985, 1–21. [Google Scholar] [CrossRef]
- Alford, R.A. Habitat use and positional behavior of anuran larvae in a northern Florida temporary Pond. Copeia 1986, 1986, 408–423. [Google Scholar] [CrossRef]
- Eterovick, P.C.; Fernandes, G.W. Tadpole distribution within montane meadow streams at the Serra do Cipó, southeastern Brazil: Ecological or phylogenetic constraints? J. Trop. Ecol. 2001, 17, 683–693. [Google Scholar] [CrossRef]
- Braz, E.; Joly, P. Micro-habitat use, resource partitioning and ecological succession in a size-structured guild of newt larvae (g. Triturus, Caudata, Amphibia). Arch. Hydrobiol. 1994, 131, 129–139. [Google Scholar] [CrossRef]
- Brodman, R. Effects of intraguild interactions on fitness and microhabitat use of larval Ambysyoma salamanders. Copeia 1996, 1996, 372–378. [Google Scholar] [CrossRef]
- Jaeger, R.G. Moisture as a factor influencing the distributions of two species of terrestrial salamanders. Oecologia 1971, 6, 191–207. [Google Scholar] [CrossRef]
- Griffis, M.R.; Jaeger, R.G. Competition leads to an extinction-prone species of salamander: Interspecific territoriality in a metapopulations. Ecology 1998, 79, 2494–2502. [Google Scholar] [CrossRef]
- Bruce, R.C. Intraguild interactions and population regulation in plethodontid salamanders. Herpetol. Monogr. 2008, 22, 31–53. [Google Scholar] [CrossRef]
- Organ, J.A. Studies of the local distribution, life history, and population dynamics of the salamander genus Desmognathus in Virginia. Ecol. Monogr. 1961, 31, 189–220. [Google Scholar] [CrossRef]
- Hairston, N.G. Species packing in the salamander genus Desmognathus: What are the interspecific interactions involved? Am. Nat. 1980, 115, 354–366. [Google Scholar] [CrossRef]
- Hairston, N.G. Species packing in Desmognathus salamanders: Experimental demonstration of predation and competition. Am. Nat. 1986, 127, 266–291. [Google Scholar] [CrossRef]
- Bruce, R.C. Community assembly in the salamander genus Desmognathus. Herpetol. Monogr. 2011, 25, 1–24. [Google Scholar] [CrossRef]
- Peterson, W.E.; Truslow, S.C. Density estimation of Eurycea wilderae: A comparison of mark-recapture and depletion sampling. Herpetol. Rev. 2008, 39, 439–442. [Google Scholar]
- Nowakowski, A.J.; Maerz, J.C. Estimation of larval stream salamander densities in three proximate streams in the Georgia Piedmont. J. Herpetol. 2009, 43, 503–509. [Google Scholar] [CrossRef]
- Davic, R.D.; Orr, L.P. The relationship between rock density and salamander density in a mountain stream. Herpetologica 1987, 43, 357–361. [Google Scholar]
- Davic, R.D.; Welsh, H.H., Jr. On the ecological roles of salamanders. Ann. Rev. Ecol. Evol. Syst. 2004, 35, 405–434. [Google Scholar] [CrossRef]
- Davic, R.D. An Investigation of Salamander Guild Predation in a North Carolina Stream: An Experimental Approach (Unpublished). Ph.D. Thesis, Kent State University, Kent, OH, USA, 1983. [Google Scholar]
- Keitzer, S.C.; Goforth, R.R. Salamander diversity alters stream macroinvertebrate community structure. Freshw. Biol. 2013, 58, 2114–2125. [Google Scholar] [CrossRef]
- Ennen, J.R.; Davenport, J.M.; Alford, K.F. Evidence for asymmetric competition among headwater stream vertebrates. Hydrobiologia 2016, 772, 207–213. [Google Scholar] [CrossRef]
- Milanovich, J.R.; Maerz, J.C.; Rosemond, A.D. Stoichiometry and estimates of nutrient standing stocks of larval salamanders in Appalachian headwater streams. Freshw. Biol. 2015, 60, 1340–1353. [Google Scholar] [CrossRef]
- Keitzer, S.C.; Goforth, R.R. Spatial and seasonal variation in the ecological significance of nutrient recycling by larval salamanders in Appalachian headwater streams. Freshw. Sci. 2013, 32, 1136–1147. [Google Scholar] [CrossRef]
- Peterman, W.E.; Crawford, J.A.; Semlitsch, R.D. Productivity and significance of headwater streams: Population structure and biomass of the black-bellied salamander (Desmognathus quadramaculatus). Freshw. Biol. 2008, 53, 347–357. [Google Scholar] [CrossRef]
- Wake, D.B. What salamanders have taught us about evolution. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 333–352. [Google Scholar] [CrossRef]
- Camp, C.D.; Wooten, J.A. Hidden in plain sight: Cryptic diversity in the Plethodontidae. Copeia 2016, 104, 111–117. [Google Scholar] [CrossRef]
- Pierson, T.W.; Fitzpatrick, B.M.; Camp, C.D. Genetic data reveal fine-scale ecological segregation between larval plethodontid salamanders in replicate contact zones. Evol. Ecol. 2021, 35, 309–322. [Google Scholar] [CrossRef]
- Pyron, R.A.; Beamer, D.A. Nomenclatural solutions for diagnosing ‘cryptic’ species using molecular and morphological data facilitate a taxonomic revision of the Black-bellied Salamanders (Urodela, Desmognathus ‘quadramaculatus’) from the southern Appalachian Mountains. Bionomina 2022, 27, 1–43. [Google Scholar] [CrossRef]
- Camp, C.D.; Seymour, Z.L.; Wooten, J.A. Morphological variation in the cryptic species Desmognathus quadramaculatus (Black-bellied Salamander) and Desmognathus folkertsi (Dwarf Blackbellied Salamander). J. Herpetol. 2013, 47, 471–479. [Google Scholar] [CrossRef]
- Camp, C.; Barbour, M.; Wooten, J. Morphological differentiation between the larval forms of two cryptic species of dusky salamander (Desmognathus). Amph. Rept. 2014, 35, 117–122. [Google Scholar] [CrossRef]
- Camp, C.D.; Wooten, J.A.; Corbet, C.M.; Dulka, E.A.; Mitchem, J.A.; Krieger, T.J. Ecological interactions between two broadly sympatric, cryptic species of dusky salamander (genus Desmognathus). Copeia 2013, 2013, 499–506. [Google Scholar] [CrossRef]
- Beachy, C.K. Community Ecology of Larval Plethodontid Salamanders: The Effect of Predation on Age and Size at Metamorphosis (Unpublished). Ph.D. Thesis, University of Southwestern Louisiana, Lafayette, LA, USA, 1992. [Google Scholar]
- Petranka, J.W. Salamanders of the United States and Canada; Smithsonian Institution Press: Washington, DC, USA, 1998; pp. 206–211. [Google Scholar]
- Trice, A.; Rosemond, A.D.; Maerz, J.C. Diet composition of two larval headwater stream salamanders and spatial distribution of prey. Freshw. Biol. 2015, 60, 2424–2434. [Google Scholar] [CrossRef]
- Dunn, E.R. The salamanders of the genera Desmognathus and Leurognathus. Proc. U. S. Natl. Mus. 1917, 63, 393–433. [Google Scholar] [CrossRef]
- Eaton, T.H., Jr. Larvae of some Appalachian plethodontid salamanders. Herpetologica 1956, 12, 303–311. [Google Scholar]
- Feder, M.E.; Pinder, A.W. Ventilation and its effect on “infinite pool” exchangers. Am. Zool. 1988, 28, 973–998. [Google Scholar] [CrossRef]
- Booth, D.T.; Feder, M.E. Formation of hypoxic boundary layers and their biological implications in a skin-breathing aquatic salamander, Desmognathus quadramaculatus. Physiol. Zool. 1991, 64, 1307–1321. [Google Scholar] [CrossRef]
- Feder, M.W.; Booth, D.T. Hypoxic boundary layers surrounding skin-breathing aquatic amphibians: Occurrence, consequences and organismal responses. J. Exp. Biol. 1992, 166, 237–251. [Google Scholar] [CrossRef]
- Darwin, C. The Origin of Species by Means of Natural Selection: Or, the Preservation of Favored Races in the Struggle for Life; John Murray: London, UK, 1859. [Google Scholar]
- Edwards, L.; Ambrose, J.; Kirkman, L.K. The Natural Communities of Georgia. University of Georgia Press: Athens, GA, USA, 2013. [Google Scholar]
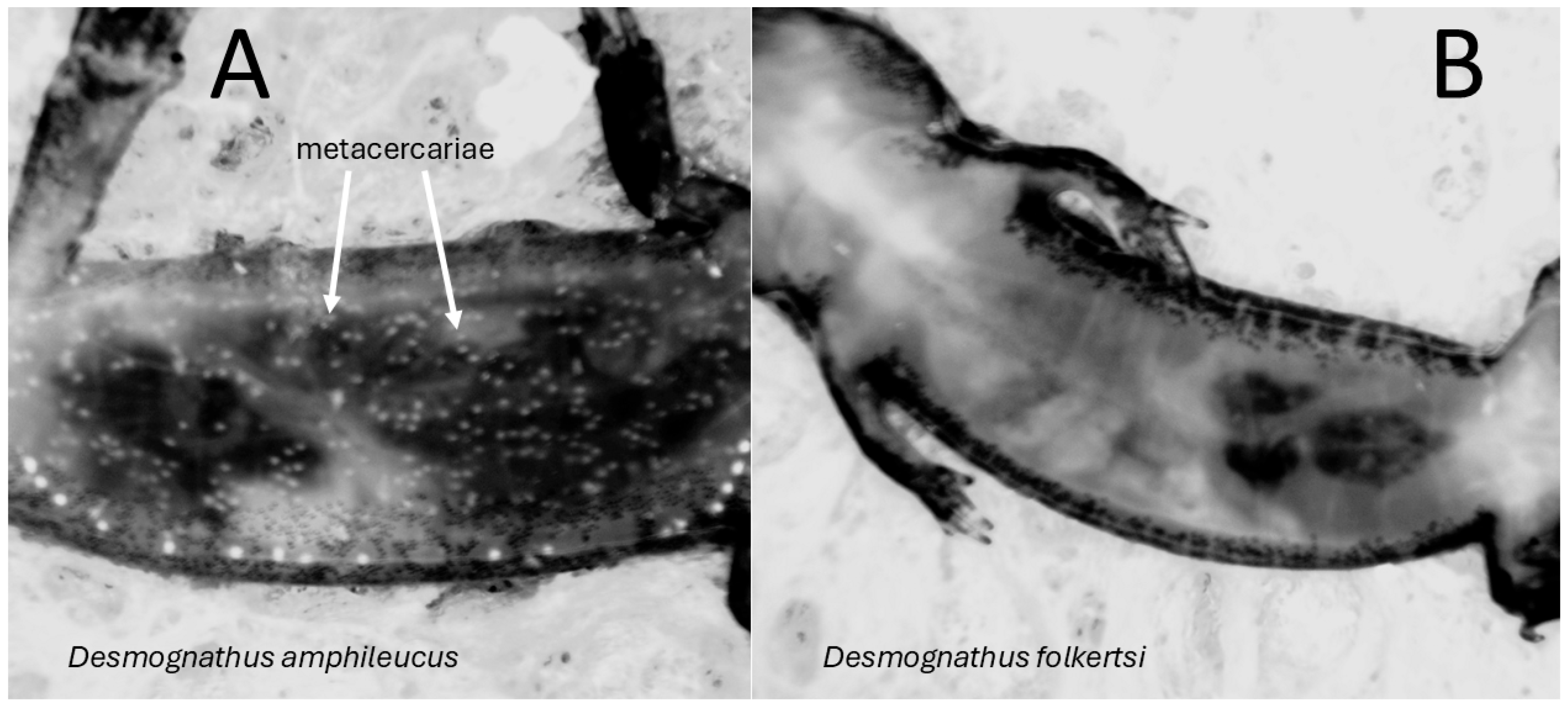
- Camp, C.D.; Jones, D.; Phillips, J.; Brock, T.L.; Wooten, J.A. Differential infection of two sympatric, cryptic species of Appalachian salamander (genus Desmognathus) by the trematode Metagonimoides oregonensis. Comp. Parasitol. 2021, 88, 183–186. [Google Scholar] [CrossRef]
- Goater, T.M.; Esch, G.W.; Bush, A.O. Helminth parasites of sympatric salamanders: Ecological concepts at infracommunity, component and compound community levels. Am. Midl. Nat. 1987, 118, 289–300. [Google Scholar] [CrossRef]
- Belden, L.K.; Peterman, W.E.; Smith, S.A.; Brooks, L.R.; Benfield, E.F.; Black, W.P.; Yang, Z.; Wojdak, J.M. Metagonimoides oregonensis (Heterophyidae: Digenea) infection in pleurocerid snails and Desmognathus quadramaculatus salamander larvae in southern Appalachian streams. J. Parasitol. 2012, 98, 760–767. [Google Scholar] [CrossRef]
- Clebsch, E.C.; Busing, R.T. Secondary succession, gap dynamics, and community structure in a southern Appalachian cove forest. Ecology 1989, 70, 728–735. [Google Scholar] [CrossRef]
- Elliott, K.J.; Boring, L.R.; Swank, W.T. Changes in vegetation structure and diversity after grass-to-forest succession in a southern Appalachian watershed. Am. Midl. Nat. 1998, 140, 219–232. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Zutter, B.R.; Gjerstad, D.H.; Glover, G.R.; Wood, C.W. Competition among secondary-successional pine communities: A field study of effects and responses. Ecology 1999, 80, 857–872. [Google Scholar] [CrossRef]
- Kilpatrick, F.A.; Wilson, J.F.; Hubbard, E.F. Measurement of Time of Travel in Streams by Dye Tracing; Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 1989; pp. 1–27. [Google Scholar]
- Di Bella, B.; Khatamifa, M.; Lin, W. Experimental study of flow visualisation using fluorescent dye. Flow Meas. Instrum. 2022, 87, 102231. [Google Scholar] [CrossRef]
- Skjolding, L.M.; Jørgensen, L.v.G.; Dyhr, K.S.; Köppl, C.J.; McKnight, U.S.; Bauer-Gottwein, P.; Mayer, P.; Bjerg, P.L.; Baun, A. Assessing the aquatic toxicity and environmental safety of tracer compounds Rhodamine B and Rhodamine WT. Water Res. 2021, 197, 117109. [Google Scholar] [CrossRef] [PubMed]
- Field, M.S.; Wilhelm, R.G.; Quinlan, J.F.; Aley, T.J. An assessment of the potential adverse properties of fluorescent tracer dyes used for groundwater tracing. Environ. Monit. Assess. 1995, 38, 75–96. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 97, 676–682. [Google Scholar] [CrossRef]
- Austin, R.M., Jr.; Camp, C.D. Larval development of Black-Bellied Salamanders, Desmognathus quadramaculatus, in northeastern Georgia. Herpetologica 1992, 48, 313–317. [Google Scholar]
- Davic, R.D. Ontogenetic shift in diet of Desmognathus quadamaculatus. J. Herpetol. 1991, 25, 108–111. [Google Scholar] [CrossRef]
- Real, R.; Vargas, J.M. The probabilistic basis of Jaccard’s Index of Similarity. Syst. Biol. 1996, 45, 380–385. [Google Scholar] [CrossRef]
- Barr, G.E.; Babbitt, K.J. Trout affect the density, activity and feeding of a larval plethodontid salamander. Freshw. Biol. 2007, 52, 1239–1248. [Google Scholar] [CrossRef]
- Sih, A.; Kats, L.B.; Moore, R.D. Effects of predatory sunfish on the density, drift, and refuge use of stream salamander larvae. Ecology 1992, 73, 1418–1430. [Google Scholar] [CrossRef]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.; Ly, A.; Gronau, Q.F.; Šmíra, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- Haefner, J.D.; Wallace, J.B. Shifts in aquatic insect populations in a first-order southern Appalachian stream following a decade of old field succession. Can. J. Fish. Aquat. Sci. 1981, 38, 353–359. [Google Scholar] [CrossRef]
- Flebbe, P.A.; Dolloff, C.A. Trout use of woody debris and habitat in Appalachian wilderness streams of North Carolina. N. Am. J. Fish. Manag. 1995, 15, 579–590. [Google Scholar] [CrossRef]
- Wallace, J.B.; Webster, J.R.; Meyer, J.L. Influence of log additions on physical and biotic characteristics of a mountain stream. Can. J. Fish. Aquat. Sci. 1995, 52, 2120–2137. [Google Scholar] [CrossRef]
- Dewson, Z.S.; James, A.B.W.; Death, R.G. A review of the consequences of decreased flow for instream habitat and macroinvertebrates. J. N. Am. Benthol. Soc. 2007, 26, 401–415. [Google Scholar] [CrossRef]
- Mathers, K.L.; White, J.C.; Fornaroli, R.; Chadd, R. Flow regimes control the establishment of invasive crayfish and alter their effects on lotic macroinvertebrate communities. J. Appl. Ecol. 2020, 57, 886–902. [Google Scholar] [CrossRef]
- Crowl, T.A.; Schnell, G.D. Factors determining population density and size distribution of a freshwater snail in streams: Effects of spatial scale. Oikos 1990, 59, 359–367. [Google Scholar] [CrossRef]
- Wellnitz, T.; Frase, D.; Gapinski, M.; Haggerty, H.E. Does stream current modify crayfish impacts on a benthic community? J. Freshw. Ecol. 2019, 34, 633–647. [Google Scholar] [CrossRef]
- Del Signore, A.; Lenders, H.J.R.; Hendriks, A.J.; Vonk, J.A.; Mulder, C.; Leuven, R.W.E.W. Size-mediated effects of water-flow velocity on riverine fish species. River Res. Applic. 2016, 32, 390–398. [Google Scholar] [CrossRef]
- Donaldson, J.A.; Ebner, B.C.; Fulton, C.J. Flow velocity underpins microhabitat selection by gobies of the Australian Wet Tropics. Freshw. Biol. 2013, 58, 1038–1051. [Google Scholar] [CrossRef]
- Ellingson, J.M. Agonistic Behavior in the Black-Bellied Salamander Desmognathus quadramaculatus (Unpublished). Master’s Thesis, University of Texas at Arlington, Arlington, TX, USA, 1988. [Google Scholar]
- Camp, C.D.; Lee, T.P. Intraspecific spacing and interaction within a population of Desmognathus quadramaculatus. Copeia 1996, 1996, 78–84. [Google Scholar] [CrossRef]
- Kework, C.A.; (Kennesaw State University Athletics, Kennesaw, GE, USA). Personal communication, 2022.
- Bruce, R.C. Larval periods, population structure and the effects of stream drift in larvae of the salamanders Desmognathus quadramaculatus and Leurognathus marmoratus in a southern Appalachian Stream. Copeia 1985, 1985, 847–854. [Google Scholar] [CrossRef]
- Krause, E.T.; Caspers, B.A. The influence of a water current on the larval deposition pattern of females of a diverging fire salamander population (Salamandra salamandra). Salamandra 2015, 51, 156–160. [Google Scholar]
- Bruce, R.C. Life history variation in the salamander Desmognathus quadramaculatus. Herpetologica 1988, 44, 218–227. [Google Scholar]
- Camp, C.D.; Marshall, J.L. Reproductive life history of Desmognathus folkertsi (Dwarf Black-bellied Salamander). Southeast. Nat. 2006, 5, 669–684. [Google Scholar] [CrossRef]
- Carter, R.E., Jr.; Myers, J.; Shelburne, V.B.; Jones, S.M. Ecological land classification in the high rainfall belt of the southern Appalachian Mountains. Castanea 2000, 65, 258–272. [Google Scholar]
- Hursh, C.R.; Haasis, F.W. Effects of 1925 summer drought on southern Appalachian hardwoods. Ecology 1931, 12, 380–386. [Google Scholar] [CrossRef]
- Olano, J.M.; Palmer, M.W. Stand dynamics of an Appalachian old-growth forest during a severe drought episode. For. Ecol. Manag. 2003, 174, 139–148. [Google Scholar] [CrossRef]
- Pederson, N.; Bell, A.R.; Knight, T.A.; Leland, C.; Malcomb, N.; Anchukaitis, K.J.; Tackett, K.; Scheff, J.; Brice, A.; Catron, B.; et al. A long-term perspective on a modern drought in the American Southeast. Environ. Res. Lett. 2012, 7, 014034. [Google Scholar] [CrossRef]
- Dyer, J.; Mercer, A.; Raczyński, K. Identifying spatial patterns of hydrologic drought over the southeast US using retrospective national water model simulations. Water 2022, 14, 1525. [Google Scholar] [CrossRef]
- Fritz, K.M.; Johnson, B.R.; Walters, D.M. Physical indicators of hydrologic permanence in forested headwater streams. J. N. Am. Benthol. Soc. 2008, 27, 690–704. [Google Scholar] [CrossRef]
- Malone, E.W.; Perkin, J.S.; Gibbs, W.K.; Padgett, M.; Kulp, M.; Moore, S.E. High and dry in days gone by: Life-history theory predicts Appalachian Mountain stream fish assemblage transformation during historical drought. Ecol. Freshw. Fish 2021, 31, 29–44. [Google Scholar] [CrossRef]
- Hitt, N.P.; Landsman, A.P.; Raesly, R.L. Life history strategies of stream fishes linked to predictors of hydrologic stability. Ecol. Evol. 2022, 12, e8861. [Google Scholar] [CrossRef] [PubMed]
- Lowe, W.H.; Swartza, L.K.; Addisa, B.R.; Likens, G.E. Hydrologic variability contributes to reduced survival through metamorphosis in a stream salamander. Proc. Natl. Acad. Sci. USA 2019, 116, 19563–19570. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, M.M.; Addis, B.R.; Lowe, W.H. Stage-specific demographic effects of hydrologic variation in a stream salamander. Am. Nat. 2024, 203, E175–E187. [Google Scholar] [CrossRef]
- Lowe, W.H.; Martin, T.E.; Skelly, D.K.; Woods, H.A. Metamorphosis in an era of increasing climate variability. Trends Ecol. Evol. 2020, 36, 360–375. [Google Scholar] [CrossRef]
- Lowe, W.H.; Bolger, D.T. Local and landscape-scale predictors of salamander abundance in New Hampshire headwater streams. Conserv. Biol. 2002, 16, 183–193. [Google Scholar] [CrossRef]
- Kroll, A.J.; Risenhoover, K.; McBride, T.; Beach, E.; Kernohan, B.J.; Light, J.; Bach, J. Factors influencing stream occupancy and detection probability parameters of stream-associated amphibians in commercial forests of Oregon and Washington, USA. For. Ecol. Manag. 2008, 255, 3726–3735. [Google Scholar] [CrossRef]
- Campbell Grant, E.H.; Wiewel, A.N.M.; Rice, K.C. Stream-water temperature limits occupancy of salamanders in mid-Atlantic protected areas. J. Herpetol. 2014, 48, 45–50. [Google Scholar] [CrossRef]
- Cecala, K.K.; Maerz, J.C.; Halstead, B.J.; Frisch, J.R.; Gragson, T.L.; Hepinstall-Cymerman, J.; Leigh, D.S.; Jackson, C.R.; Peterson, J.T.; Pringle, C.M. Multiple drivers, scales, and interactions influence southern Appalachian stream salamander occupancy. Ecosphere 2018, 9, e02150. [Google Scholar] [CrossRef]
- Weaver, N.; Barrett, K. In-stream habitat predicts salamander occupancy and abundance better than landscape-scale factors within exurban watersheds in a global diversity hotspot. Urban Ecosyst. 2018, 21, 97–105. [Google Scholar] [CrossRef]
| Downstream | Upstream | |||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| Temperature (°C) | 15.0 | --- | 15.0 | --- |
| pH | 7.7 | --- | 7.7 | --- |
| DO (mg/L) | 7.05 | 7.0–7.1 | 7.3 | |
| Depth (cm) | 10.0 | 2.5–22.0 | 10.4 | 5.1–25.4 |
| Flow Rate | Riffle Length | Mean Particle Diameter | Streambed Width | |
|---|---|---|---|---|
| Flow rate | --- | 0.0292 * | 0.8863 | 0.0012 * |
| Riffle length | −0.328 | --- | 0.6404 | 0.2136 |
| Mean particle diameter | 0.022 | 0.073 | --- | 0.8220 |
| Streambed width | 0.495 | −0.190 | −0.034 | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camp, C.D.; Fortner, E.M. Differences in Larval Microhabitat Between Two Cryptic, Sympatric Salamander Species (Desmognathus folkertsi and D. amphileucus) in Northeastern Georgia, USA. Animals 2025, 15, 1479. https://doi.org/10.3390/ani15101479
Camp CD, Fortner EM. Differences in Larval Microhabitat Between Two Cryptic, Sympatric Salamander Species (Desmognathus folkertsi and D. amphileucus) in Northeastern Georgia, USA. Animals. 2025; 15(10):1479. https://doi.org/10.3390/ani15101479
Chicago/Turabian StyleCamp, Carlos D., and Erick M. Fortner. 2025. "Differences in Larval Microhabitat Between Two Cryptic, Sympatric Salamander Species (Desmognathus folkertsi and D. amphileucus) in Northeastern Georgia, USA" Animals 15, no. 10: 1479. https://doi.org/10.3390/ani15101479
APA StyleCamp, C. D., & Fortner, E. M. (2025). Differences in Larval Microhabitat Between Two Cryptic, Sympatric Salamander Species (Desmognathus folkertsi and D. amphileucus) in Northeastern Georgia, USA. Animals, 15(10), 1479. https://doi.org/10.3390/ani15101479







