A Meta-Analysis of Global Prevalence of Psittacine Beak and Feather Disease Virus Infection and Associated Risk Factors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Inclusion and Exclusion Criteria
2.3. Data Extraction
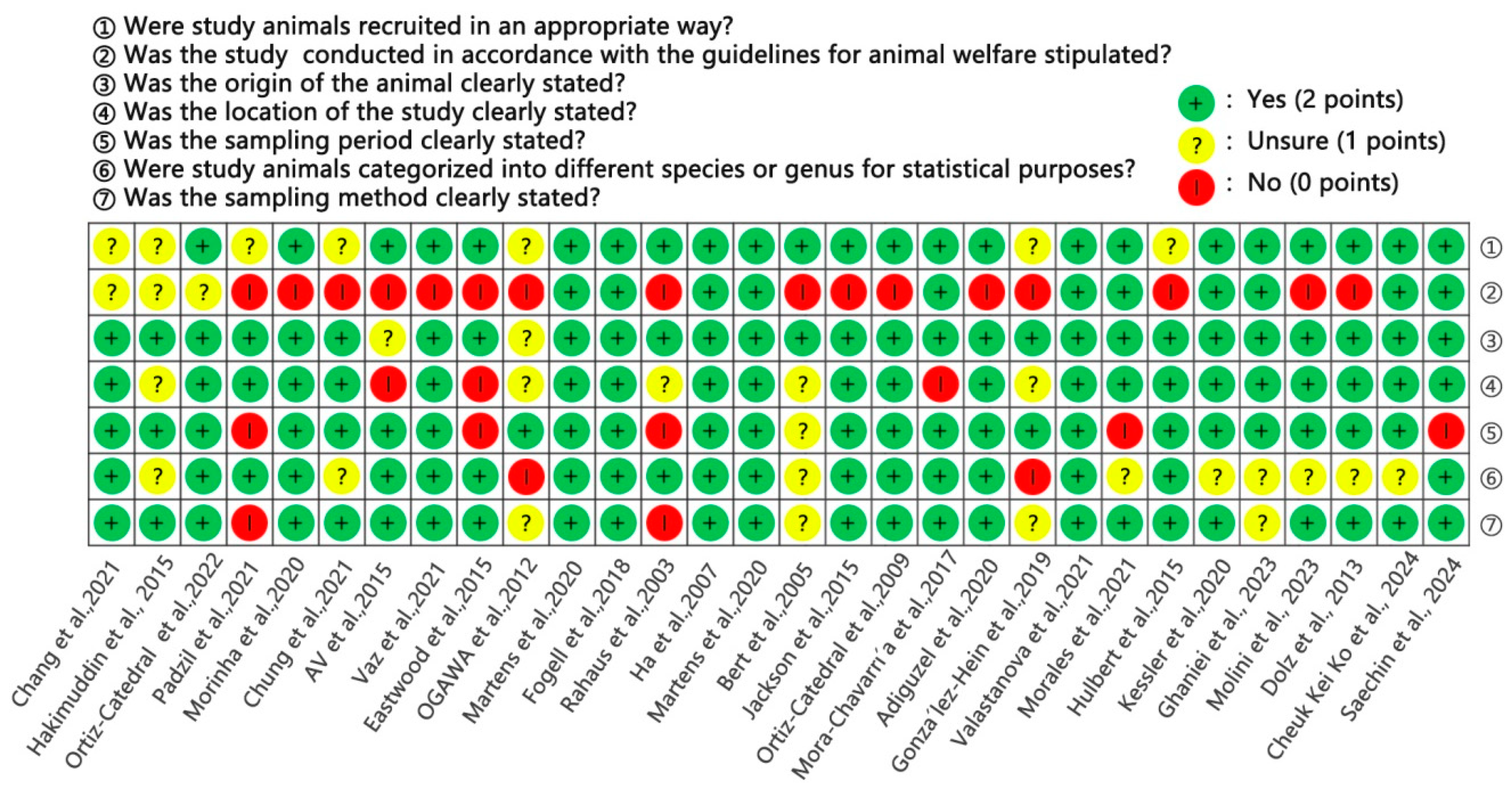
2.4. Literature Quality Assessment
2.5. Statistical Analysis
2.6. Overall Pooled Prevalence of BFDV in Parrots
2.7. Subgroup Meta-Analysis of Heterogeneity
2.8. Bias and Sensitivity Tests
3. Results
3.1. Literature Search and Study Characteristics
3.2. Quality Appraisal of Included Studies
3.3. Pooling and Heterogeneity Analyses
3.4. Subgroup Meta-Analysis for Prevalence Estimation
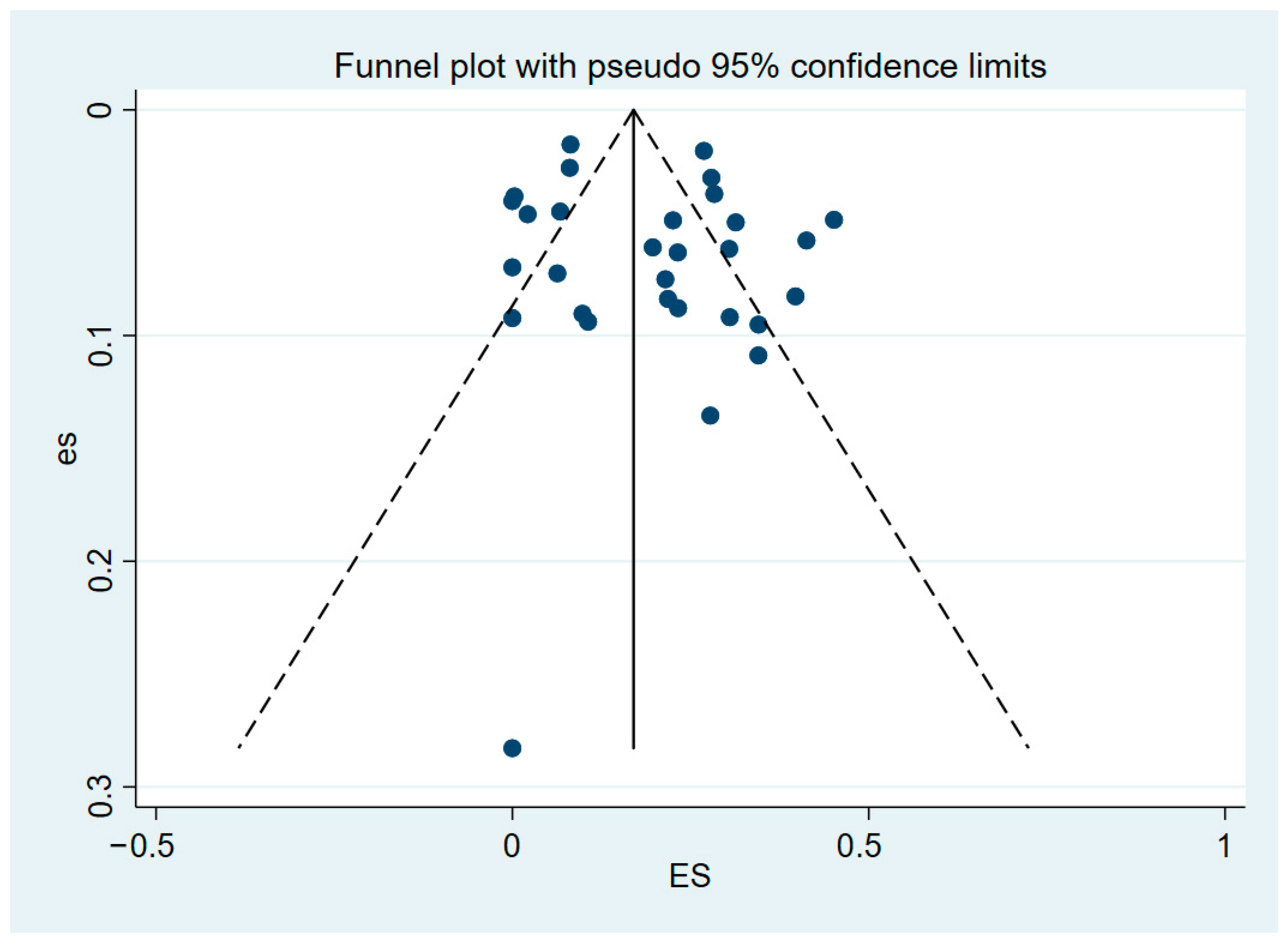
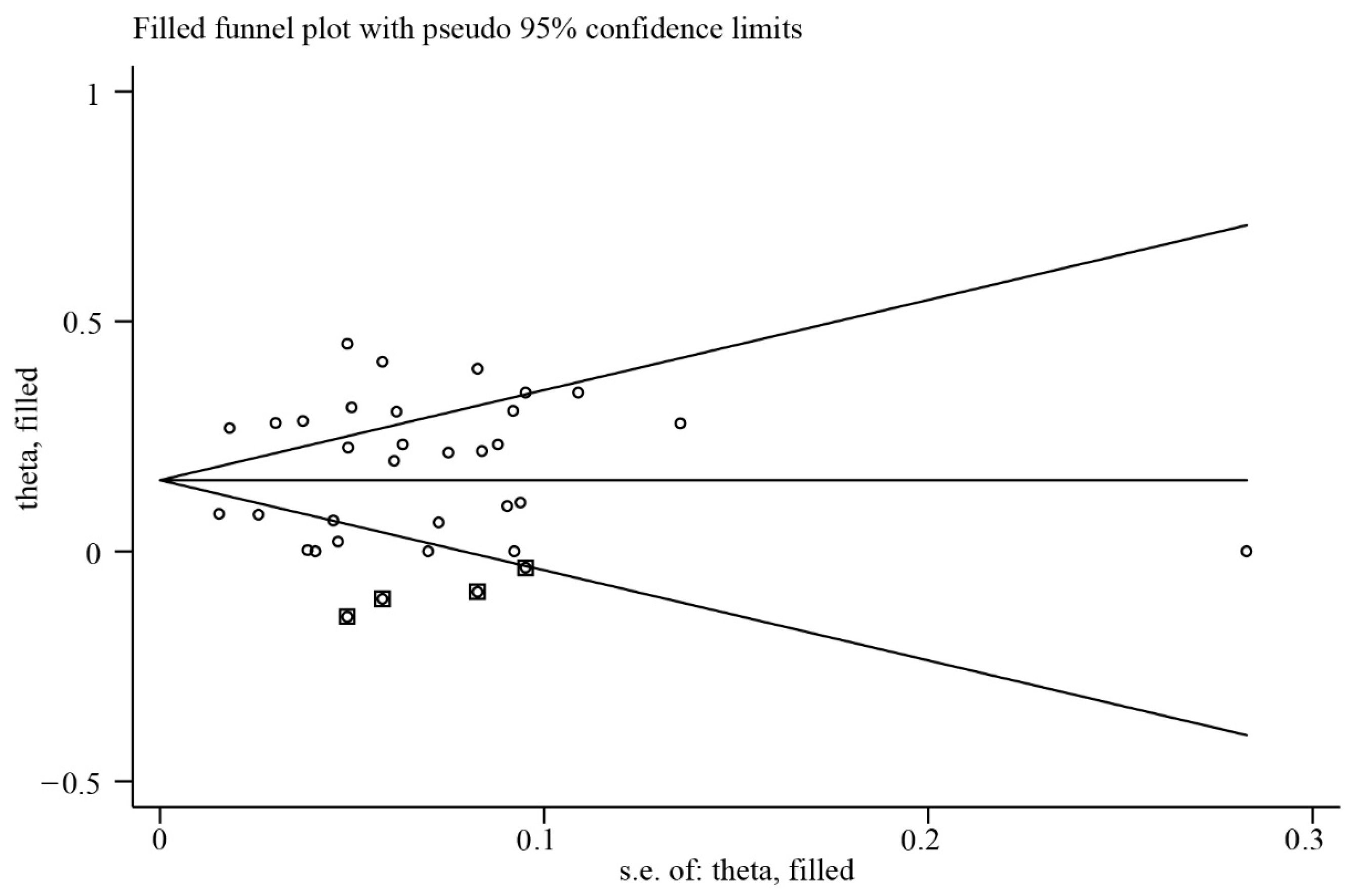
3.5. Publication Bias and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katohi, H.; Ogawa, H.; Ohya, K.; Fukushi, H. A Review of DNA Viral Infections in Psittacine Birds. J. Vet. Med. Sci. 2010, 72, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Hsiao, C.L.; Lo, A.R.; Wang, C.Y. Characterization of the nuclear localization sequence of beak and feather disease virus capsid proteins and their assembly into virus-like particles. Virus Res. 2020, 289, 198144. [Google Scholar] [CrossRef]
- Greenacre, B.C. Viral diseases of companion birds. Vet. Clin. N. Am. Exot. Anim. Pract. 2005, 8, 85–105. [Google Scholar] [CrossRef]
- Kaszab, E.; Lengyel, G.; Marton, S.; Dan, A.; Banyai, K.; Feher, E. Occurrence and genetic diversity of CRESS DNA viruses in wild birds: A Hungarian study. Sci. Rep. 2020, 1, 7036. [Google Scholar] [CrossRef]
- Pass, D.A.; Perry, R.A. The pathology of psittacine beak and feather disease. Aust. Vet. J. 1984, 61, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Rahaus, M.; Desloge, N.; Probst, S.; Loebbert, B.; Lantermann, W.; Wolff, M.H. Detection of beak and feather disease virus DNA in embryonated eggs of psittacine birds. Trop. Anim. Health Prod. 2008, 40, 77–83. [Google Scholar] [CrossRef]
- Amery-Gale, J.; Marenda, M.S.; Owens, J.; Eden, P.A.; Browing, G.F.; Devlin, J.M. A high prevalence of beak and feather disease virus in non-psittacine Australian birds. J. Med. Microbiol. 2017, 66, 1005–1013. [Google Scholar] [CrossRef]
- Heath, L.; Martin, D.P.; Warburton, L.; Perrin, M.; Horsfield, W.; Kingsley, C.; Rybicki, E.P.; Williamson, A.L. Evidence of unique genotypes of Beak and Feather disease virus in southern Africa. J. Virol. 2004, 78, 9277–9284. [Google Scholar] [CrossRef]
- Bush, E.R.; Baker, S.E.; Macdonald, D.W. Global Trade in Exotic Pets 2006–2012. Conserv. Biol. 2014, 28, 663–676. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Jia, Q. Research on the development of the pet industry in the new era. Mod. Bus. Trade Ind. 2021, 42, 17–18. [Google Scholar] [CrossRef]
- Su, S.; Vall-llosera, M.; Cassey, P.; Blackburn, T.M.; Carrete, M.; Tella, J.L. Drivers of alien species composition in bird markets across the world. Ecol. Evol. 2022, 12, e8397. [Google Scholar] [CrossRef]
- Tella, J.L.; Hiraldo, F. Illegal and Legal Parrot Trade Shows a Long-Term, Cross-Cultural Preference for the Most Attractive Species Increasing Their Risk of Extinction. PLoS ONE 2014, 9, e107546. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.P.; Song, Y.; Sun, S.H. Whole-Genome Sequences of Psittacine Beak and Feather Disease Virus Isolated in Shandong Province, China and Preliminary Study on the Pathogenicity of Specific Pathogen Free Chicken Embryo. Chin. J. Wildl. 2019, 40, 670–678. [Google Scholar] [CrossRef]
- Shah, P.T.; Wang, J.; Liu, Y.; Hussain, B.; Ma, Z.H.; Wu, C.X.; Xing, L. The phylogenetic and phylogeographic landscape of the beak and feather disease virus, 1996–2022. Infect. Genet. Evol. 2023, 112, 105442. [Google Scholar] [CrossRef] [PubMed]
- Martens, J.M.; Stokes, H.S.; Berg, M.L.; Walder, K.; Raidal, S.R.; Magrath, M.J.L.; Bennett, A.T.D. Beak and feather disease virus (BFDV) prevalence, load and excretion in seven species of wild caught common Australian parrots. PLoS ONE 2020, 15, e0235406. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 4, reprinted in Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Riitano, D.; Lisy, K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int. J. Health Policy Manag. 2014, 3, 123–128. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef]
- Huedo-Medina, T.B.; Sáchesz-Meca, J.; Marin-Martinez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q. statistic or I² index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef]
- Guido, S.; Gerta, R. Meta-Analysis of Proportions. Methods Mol. Biol. 2022, 2345, 159–172. [Google Scholar] [CrossRef]
- Lin, L.F.; Xu, C. Arcsine-based transformations for meta-analysis of proportions: Pros, cons, and alternatives. Health Sci. Rep. 2020, 3, e178. [Google Scholar] [CrossRef]
- Ye, L.; Zhou, S.; Zhang, H.; Zhang, T.; Yang, D.; Hong, X. A meta-analysis for vaccine protection rate of duck hepatitis A virus in mainland China in 2009–2021. BMC Vet. Res. 2023, 19, 179. [Google Scholar] [CrossRef] [PubMed]
- Julian, P.T.H.; Simon, G.T. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Migliavaca, C.B.; Stein, C.; Colpani, V.; Barker, T.H.; Ziegelmann, P.K.; Munn, Z.; Falavigna, M.; Prevalence Estimates Reviews—Systematic Review Methodology Group (PERSyst). Meta-analysis of prevalence: I2 statistic and how to deal with heterogeneity. Res. Synth. Methods 2022, 13, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Chang, W.; Chen, K.Y.; Yang, S.L.; Yuan, M.; Chen, X.L.; Tang, Y.; Ma, Y.M. Molecular epidemiological investigation of psittacine beak and feather disease in some parts in the Fujian area and prediction of Cap protein antigen epitopes of the virus. Anim. Husb. Vet. Med. 2021, 53, 71–76. [Google Scholar]
- Hakimuddin, F.; Abidi, F.; Jafer, O.; Li, C.; Wernery, U.; Hebel, C.; Khazanehdari, K. Incidence and detection of beak and feather disease virus in psittacine birds in the UAE. Biomol. Detect. Quantif. 2016, 6, 27–32. [Google Scholar] [CrossRef]
- Ortiz-Catedral, L.; Wallace, C.J.; Heinsohn, R.; Krebs, E.A.; Langmore, N.E.; Vukelic, D.; Bucher, E.H.; Varsani, A.; Masello, J.F. A PCR-Based Retrospective Study for Beak and Feather Disease Virus (BFDV) in Five Wild Populations of Parrots from Australia, Argentina and New Zealand. Diversity 2022, 14, 148. [Google Scholar] [CrossRef]
- Padzil, M.F.M.; Halim, N.S.A.; Najihah, N.; Najian, A.B.N.; Abu, J.; Isa, N.M.; Lau, H.Y.; Mariatulqabtiah, A.R. Evaluation of beak and feather disease virus, avian polyomavirus and avian papillomavirus of captives psittacine birds in Seri Kembangan, Selangor, Malaysia. Malays. J. Microbiol. 2021, 17, 338–344. [Google Scholar] [CrossRef]
- Morinha, F.; Carrete, M.; Tella, J.L.; Blanco, G. High Prevalence of Novel Beak and Feather Disease Virus in Sympatric Invasive Parakeets Introduced to Spain From Asia and South America. Diversity 2020, 12, 192. [Google Scholar] [CrossRef]
- Chung, S.C.; Chang, C.D.; Shen, P.; Lin, W.; Ting, C.H.; Wu, H.Y. Investigation of Avian polyomavirus and Psittacine beak and Feather disease virus in parrots in Taiwan. Thai J. Vet. Med. 2021, 51, 239–245. [Google Scholar] [CrossRef]
- Araujo, A.V.; Andery, D.A.; Ferreira, F.C., Jr.; Ortiz, M.C.; Marques, M.V.R.; Marin, S.Y.; Vilela, D.A.R.; Resende, J.S.; Resende, M.; Donatti, R.V.; et al. Molecular Diagnosis of Beak and Feather Disease in Native Brazilian Psittacines. Braz. J. Poult. Sci. 2015, 17, 451–458. [Google Scholar] [CrossRef]
- Vaz, F.F.; Sipinski, E.A.B.; Seixas, G.H.F.; Prestes, N.P.; Martinez, J.; Raso, T.F. Molecular Survey of Pathogens in Wild Amazon Parrot Nestlings: Implications for Conservation. Diversity 2021, 13, 272. [Google Scholar] [CrossRef]
- Eastwood, J.R.; Berg, M.L.; Spolding, B.; Buchanan, K.L.; Bennett, A.T.D.; Walder, K. Prevalence of beak and feather disease virus in wild Platycercus elegans: Comparison of three tissue types using a probe-based real-time qPCR test. Aust. J. Zool. 2015, 63, 1–8. [Google Scholar] [CrossRef]
- Ogawa, H.; Chahota, R.; Ohya, K.; Yamaguchi, T.; Fukushi, H. Relatedness between Host Species and Genotype of Beak and Feather Disease Virus Suggesting Possible Interspecies Cross Infection during Bird Trade. J. Vet. Med. Sci. 2013, 75, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Martens, J.M.; Stokes, H.S.; Berg, M.L.; Walder, K.; Bennett, A.T.D. Seasonal fluctuation of beak and feather disease virus (BFDV) infection in wild Crimson Rosellas (Platycercus elegans). Sci. Rep. 2020, 10, 7894. [Google Scholar] [CrossRef]
- Fogell, D.J.; Martin, R.O.; Bunbury, N.; Lawson, B.; Sells, J.; Mckeand, A.M.; Tatayah, V.; Trung, C.T.; Groombridge, J.J. Trade and conservation implications of new beak and feather disease virus detection in native and introduced parrots. Conserv. Biol. 2018, 32, 1325–1335. [Google Scholar] [CrossRef]
- Rahaus, M.; Wolff, M.H. Psittacine beak and feather disease: A first survey of the distribution of beak and feather disease virus inside the population of captive psittacine birds in Germany. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Health 2003, 50, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.J.; Anderson, I.L.; Alley, M.R.; Springett, B.P.; Gartrell, S.D. The prevalence of beak and feather disease virus infection in wild populations of parrots and cockatoos in New Zealand. N. Z. Vet. J. 2007, 55, 235–238. [Google Scholar] [CrossRef]
- Bert, E.; Tomassone, L.; Peccati, C.; Navarrete, M.G.; Sola, S.C. Detection of beak and feather disease virus (BFDV) and avian polyomavirus (APV) DNA in psittacine birds in Italy. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Health 2005, 52, 64–68. [Google Scholar] [CrossRef]
- Jackson, B.; Varsani, A.; Holyoake, C.; Jakob-Hoff, R.; Robertson, I.; Mclnnes, K.; Empson, R.; Gray, R.; Nakagawa, K.; Warren, K. Emerging infectious disease or evidence of endemicity? A multi-season study of beak and feather disease virus in wild red-crowned parakeets (Cyanoramphus novaezelandiae). Arch. Virol. 2015, 160, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Catedral, L.; McInnes, K.; Hauber, M.E.; Brunton, D.H. First report of beak and feather disease virus (BFDV) in wild Red-fronted Parakeets (Cyanoramphus novaezelandiae) in New Zealand. Emu—Austral Ornithol. 2009, 109, 244–247. [Google Scholar] [CrossRef]
- Mora-Chavarria, E.; Umana-Castro, R.; Abou-Madi, N.; Solano-Gonzalez, S.; Retamosa-Izaguirre, M.; Jimenez-Soto, M.; Blanco-Pena, K. Health assessment of captive psittacine species in prerelease programs at costa rican rescue centers. J. Zoo Wildl. Med. 2017, 48, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Adiguzel, M.C.; Timurkan, M.O.; Cengiz, S. Investigation and sequence analysis of avian polyomavirus and psittacine beak and feather disease virus from companion birds in eastern Turkey. J. Vet. Res. 2020, 64, 495–501. [Google Scholar] [CrossRef]
- Gonzalez-Hein, G.; Aguirre, G.I.; Sanchez, R.; Huaracan, B. Prevalence of Aves Polyomavirus 1 and Beak and Feather Disease Virus From Exotic Captive Psittacine Birds in Chile. J. Avian Med. Surg. 2019, 33, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Valastanova, M.; Petrikova, M.; Kulikova, L.; Knotek, Z. Psittacine beak and feather disease virus and avian polyomavirus detection rate in clinically healthy captive birds in the Czech Republic. Vet. Med. 2021, 66, 72–75. [Google Scholar] [CrossRef]
- Morales, A.; Sibrian, X.; Porras, D. Survey of Beak and Feather Disease Virus (BFDV) in Guatemalan Neotropical Psittacine Birds. J. Avian Med. Surg. 2021, 35, 325–332. [Google Scholar] [CrossRef]
- Hulbert, C.L.; Chamings, A.; Hewson, K.A.; Steer, P.A.; Gosbell, M.; Noormohammadi, A.H. Survey of captive parrot populations around Port Phillip Bay, Victoria, Australia, for psittacine beak and feather disease virus, avian polyomavirus and psittacine adenovirus. Aust. Vet. J. 2015, 93, 287–292. [Google Scholar] [CrossRef]
- Kessler, S.; Heenemann, K.; Krause, T.; Twietmeyer, S.; Fuchs, J.; Lierz, M.; Corman, V.M.; Vahlenkamp, T.M.; Rubbenstroth, D. Monitoring of free-ranging and captive Psittacula populations in Western Europe for avian bornaviruses, circoviruses and polyomaviruses. Avian Pathol. 2020, 49, 119–130. [Google Scholar] [CrossRef]
- Ghaniei, A.; Tohidi, E. Fluctuation of the prevalence of beak and feather disease virus in captive psittacines in Iran. Arch. Virol. 2023, 168, 274. [Google Scholar] [CrossRef]
- Molini, U.; Villiers, M.D.; Villiers, L.D.; Coetzee, L.M.; Hoebes, E.; Khaiseb, S.; Cattoli, G.; Dundon, W.G.; Franzo, G. Investigation and sequence analysis of psittacine beak and feather disease virus and avian polyomavirus from companion birds in Windhoek, Namibia. Acta Trop. 2023, 238, 106739. [Google Scholar] [CrossRef]
- Dolz, G.; Sheleby-Elías, J.; Romero-Zuñiga, J.J.; Vargas-Leitón, B.; Gutiérrez-Espeleta, G.; Madriz-Ordeñana, K. Prevalence of Psittacine Beak and Feather Disease Virus and Avian Polyomavirus in Captivity Psittacines from Costa Rica. Open J. Vet. Med. 2013, 3, 240–245. [Google Scholar] [CrossRef]
- Ko, J.C.K.; Yannes, W.Y.C.; Emily, S.K.P.; Nicole, W.; Simon, Y.W.S. Prevalence, genotypes, and infection risk factors of psittacine beak and feather disease virus and budgerigar fledgling disease virus in captive birds in Hong Kong. Arch. Virol. 2024, 169, 91. [Google Scholar] [CrossRef] [PubMed]
- Saechin, A.; Suksai, P.; Sariya, L.; Mongkolphan, C.; Tangsudjai, S. Species and seasonality can affect recent trends in beak and feather disease virus prevalence in captive psittacine birds. Acta Trop. 2024, 249, 107071. [Google Scholar] [CrossRef]
- Sarker, S.; Forwood, J.K.; Ghorashi, S.A.; Peters, A.; Raidal, S.R. Beak and feather disease virus genotypes in Australian parrots reveal flexible host-switching. Aust. Vet. J. 2015, 93, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Fogell, D.J.; Martin, R.O.; Groombridge, J.J. Beak and feather disease virus in wild and captive parrots: An analysis of geographic and taxonomic distribution and methodological trends. Arch. Virol. 2016, 161, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Patterson, E.I.; Baker, B.G.; Holdsworth, M.; Saker, S.; Ghorashi, S.A.; Raidal, S.R. Evidence of psittacine beak and feather disease virus spillover into wild critically endangered Orange-bellied Parrots (Neophema chrysogaster). J. Wildl. Dis. 2014, 50, 288–296. [Google Scholar] [CrossRef]
- Kasimov, V.; Wille, M.; Sarker, S.; Dong, Y.; Shao, R.; Hall, C.; Potvin, D.; Conroy, G.; Valenza, L.; Gillett, A.; et al. Unexpected Pathogen Diversity Detected in Australian Avifauna Highlights Potential Biosecurity Challenges. Viruses 2023, 15, 146. [Google Scholar] [CrossRef]
- Sarker, S.; Moylan, K.G.; Ghorashi, S.A.; Forwood, J.K.; Peters, A.; Raidal, S.R. Evidence of a deep viral host switch event with beak and feather disease virus infection in rainbow bee-eaters (Merops omatus). Sci. Rep. 2015, 5, 14511. [Google Scholar] [CrossRef]
- Shabani, H.; Talazadeh, F.; Kaydani, G.A.; Seifi, M.R. Sequencing of pigeon circovirus and the first report of identification of beak and feather disease virus in clinical specimens of domestic pigeons. Vet. Res. Forum 2024, 15, 151–158. [Google Scholar] [CrossRef]
- Sarker, S.; Athukorala, A.; Phalen, D.N. Genome Sequence of a Beak and Feather Disease Virus from an Unusual Novel Host, Australian Boobook Owl (Ninox boobook). Microbiol. Resour. Announce. 2022, 11, e0017222. [Google Scholar] [CrossRef] [PubMed]
- MacColl, C.; Watson, J.E.M.; Leseberg, N.P.; Seaton, R.; Das, T.; Das, S.; Raidal, S.R. Beak and feather disease virus detected in the endangered Red Goshawk (Erythrotriorchis radiatus). Sci. Rep. 2024, 14, 10263. [Google Scholar] [CrossRef]
- Ning, S.Y.; Xiao, Y.Q.; Qian, Y.C.; Feng, Z.H.; Dai, Z.Y.; Zhang, W.; Wang, H.; Tang, Y.J. Viromic analysis of feces from laboratory rabbits reveals a new Circovirus. Virus Res. 2022, 319, 198861. [Google Scholar] [CrossRef] [PubMed]
- Bassami, M.R.; Ypelaar, I.; Berryman, D.; Wilcox, G.E.; Raidal, S.R. Genetic diversity of beak and feather disease virus detected in psittacine species in Australia. Virology 2001, 279, 392–400. [Google Scholar] [CrossRef]
- McLeod, A.; Rushton, J.; Riviere-Cinnamond, A.; Brandenburg, B.; Hinrichs, J.; Loth, L. Economic issues in vaccination against highly pathogenic avian influenza in developing countries. Dev. Biol. 2007, 130, 63–72. [Google Scholar]
- Todd, D. Avian circovirus and polyomavirus diseases. Vet. Res. 2000, 31, 277–286. [Google Scholar] [CrossRef]
- Bourne, A.R.; Cunningham, S.J.; Spottiswoode, C.N.; Ridley, A.R. High temperatures drive offspring mortality in a cooperatively breeding bird. Pro. Biol. Sci. 2020, 287, 20201140. [Google Scholar] [CrossRef]
- McDermott, M.E.; DeGroote, L.W. Long-term climate impacts on breeding bird phenology in Pennsylvania, USA. Global Change Biol. 2016, 22, 3304–3319. [Google Scholar] [CrossRef]
- Wille, M.; Shi, M.; Hurt, A.C.; Klaassen, M.; Holmes, E.C. RNA virome abundance and diversity is associated with host age in a bird species. Virology. 2021, 561, 98–106. [Google Scholar] [CrossRef]
- Blanch-Lázaro, B.; Chamings, A.; Ribot, R.F.H.; Bhatta, T.R.; Berg, M.L.; Alexandersen, S.; Bennett, A.T.D. Beak and feather disease virus (BFDV) persists in tissues of asymptomatic wild Crimson Rosellas. Commun. Biol. 2024, 7, 1017. [Google Scholar] [CrossRef]
- Dijk, J.G.B.V.; Hoye, B.J.; Verhagen, J.H.; Nolet, B.A.; Fouchier, R.A.M.; Klaassen, M. Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus. J. Anim. Ecol. 2014, 83, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Xu, X.Y.; Zhang, K.; Liu, W.L. China’s parrot resources and its market development. Chin. Poult. 2004, 21, 51–53. [Google Scholar] [CrossRef]
- Robino, P.; Grego, E.; Rossi, G.; Bert, E.; Tramuta, C.; Stella, M.C.; Bertoni, P.; Nebbia, P. Molecular analysis and associated pathology of beak and feather disease virus isolated in Italy from young Congo African grey parrots (Psittacus erithacus) with an “atypical peracute form” of the disease. Avian Pathol. 2014, 43, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.; Scope, A.; Heincz, U. Comparitive sensitivity of polymerase chain reaction diagnosis of psittacine beak and feather disease on feather samples, cloacal swabs and blood from budgerigars (Melopsittacus undulates, Shaw 18005). Avian Pathol. 2004, 33, 477–481. [Google Scholar] [CrossRef]
- Khalesi, B.; Bonne, N.; Stewart, M.; Sharp, M.; Raidal, S. A comparison of haemagglutination, haemagglutination inhibition and PCR for the detection of psittacine beak and feather disease virus infection and a comparison of isolates obtained from loriids. J. Gen. Virol. 2005, 86, 3039–3046. [Google Scholar] [CrossRef]
- Lephart, P.R.; Bachman, M.A.; LeBar, W.; McClellan, S.; Barron, K.; Schroeder, L.; Newton, D.W. Comparative study of four SARS-CoV-2 Nucleic Acid Amplification Test (NAAT) platforms demonstrates that ID NOW performance is impaired substantially by patient and specimen type. Diagn. Microbiol. Infect. Dis. 2021, 99, 115200. [Google Scholar] [CrossRef] [PubMed]
- Cernikova, L.; Vitaskova, E.; Nagy, A. Development and evaluation of TaqMan real-time PCR assay for detection of beak and feather disease virus. J. Virol. Methods 2017, 244, 55–60. [Google Scholar] [CrossRef]
- Chae, H.G.; Lim, D.R.; Kim, H.R.; Park, M.J.; Park, C.K. An advanced loop-mediated isothermal amplification assay for the rapid detection of beak and feather disease virus in psittacine birds. J. Virol. Methods 2020, 277, 113819. [Google Scholar] [CrossRef]



| No | Authors | Study Years | Nation | Diagnostic Techniques | Sample Time | Sample Type | Sample Size | No. Positive |
|---|---|---|---|---|---|---|---|---|
| 1 | Chang et al., 2021 [26] | 2021 | Mainland China | PCR | 2017–2018 | ③ | 298 | 123 |
| 2 | Hakimuddin et al., 2016 [27] | 2016 | UAE | PCR | 2009–2014 | ①/② | 421 | 190 |
| 3 | Ortiz-Catedral et al., 2022 [28] | 2022 | Australia | PCR | 1997–2007, 1993–1995 , 1999, 2000, 2014 | ① | 612 | 0 |
| 4 | Padzil et al., 2021 [29] | 2021 | Malaysia | PCR | / | ③ | 12 | 0 |
| 5 | Morinha et al., 2020 [30] | 2020 | Spain | PCR | 2015–2018 | ① | 110 | 38 |
| 6 | Chung et al., 2021 [31] | 2021 | Taiwan | PCR | 2019 | ① | 720 | 204 |
| 7 | Araujo et al., 2015 [32] | 2015 | Brazilian | PCR | 2009–2010 | ①/④ | 190 | 12 |
| 8 | Vaz et al., 2021 [33] | 2021 | Brazil | PCR | 2013–2018 | ①/④/⑤ | 205 | 0 |
| 9 | Eastwood et al., 2015 [34] | 2015 | / | PCR | / | ①/②/⑤ | 84 | 29 |
| 10 | Ogawa et al., 2013 [35] | 2013 | Guyana, Indonesia, Japan, Singapore, South Africa, U.S.A. | PCR | 2003–2004 | ①/② | 402 | 126 |
| 11 | Martens et al., 2020 [36] | 2020 | Australia | qPCR | 2016–2018 | ①/④ | 142 | 31 |
| 12 | Fogell et al., 2018 [37] | 2018 | Bangladesh, Gambia, Germany, Japan, Mauritius, Nigeria, Pakistan, Senegal, Seychelles, South Africa, United Kingdom, Vietnam, Western Africa | PCR | 2013, 2014, 2015, 2013–2015, 2007–2010, 2015, 2009–2011, 1994–2016, 2009–2012 | ①/②/⑤ | 1103 | 308 |
| 13 | Rahaus et al., 2003 [38] | 2003 | Germany | PCR | / | ② | 146 | 58 |
| 14 | Ha et al., 2007 [39] | 2007 | New Zealand | PCR | 2001–2004, 2004–2006 | ② | 417 | 94 |
| 15 | Martens et al., 2020 [15] | 2020 | Australia | PCR | 2017–2018 | ①/④/⑤ | 263 | 80 |
| 16 | Bert et al., 2005 [40] | 2005 | Italy | PCR | 2000–2004 | ① | 1516 | 122 |
| 17 | Jackson et al., 2015 [41] | 2015 | New Zealand | PCR | 2011–2013 | ①/② | 467 | 10 |
| 18 | Ortiz-Catedral et al., 2009 [42] | 2009 | New Zealand | PCR | 2008 | ② | 54 | 15 |
| 19 | Mora-Chavarría et al., 2017 [43] | 2017 | Costa Rica | PCR | 2011–2012 | ①/② | 122 | 12 |
| 20 | Adiguzel et al., 2020 [44] | 2020 | Turkey | PCR | 2016–2020 | ③ | 113 | 12 |
| 21 | González-Hein et al., 2019 [45] | 2019 | Chile | PCR | 2013–2016 | ② | 250 | 58 |
| 22 | Valastanova et al., 2021 [46] | 2021 | Czech Republic | nested PCR | / | ② | 177 | 38 |
| 23 | Morales et al., 2021 [47] | 2021 | Guatemala | real-time PCR | / | ① | 117 | 0 |
| 24 | Hulbert et al., 2015 [48] | 2015 | Australia | PCR | / | ③ | 118 | 36 |
| 25 | Kessler et al., 2020 [49] | 2020 | Germany, France | PCR | 2012–2017 | ①/④/⑤ | 680 | 2 |
| 26 | Ghaniei et al., 2023 [50] | 2023 | Iran | PCR | 2019–2021 | ② | 3029 | 814 |
| 27 | Molini et al., 2023 [51] | 2023 | Namibia | PCR | 2021–2022 | ④ | 129 | 30 |
| 28 | Dolz et al., 2013 [52] | 2013 | Costa Rica | PCR | 2005–2009 | ③ | 269 | 53 |
| 29 | Ko et al., 2024 [53] | 2024 | Taiwan, China | PCR | 2019–2022 | ③ | 492 | 33 |
| 30 | Saechin et al., 2024 [54] | 2024 | Thailand | PCR | 2012–2017 | ① | 4243 | 346 |
| Group | No. Studies | Total No. of Parrots | No. of Positive Parrots | Prevalence | Heterogeneity | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Estimates | (95% CI) | Q (x2) | PQ | I2 (%) | |||||
| Overall | 30 | 16,901 | 2874 | 16.30% | 11.40–22.00% | 2279.81 | <0.01 | 98.73% | |
| Continentsa | |||||||||
| Asia | 10 | 9518 | 1813 | 25.30% | 15.40–36.60% | 953.62 | <0.01 | 99.06% | <0.01 |
| North America | 2 | 120 | 1 | 0.00% | 0.00–0.00% | NA | NA | NA | |
| Oceania | 7 | 1949 | 221 | 15.00% | 4.10–30.80% | 424.02 | <0.01 | 98.59% | |
| Africa | 3 | 1389 | 365 | 26.20% | 23.90–28.50% | 1.09 | 0.58 | 0.00% | |
| South America | 4 | 647 | 70 | 2.80% | 0.00–22.30% | 99.93 | <0.01 | 97.00% | |
| Europe | 10 | 3163 | 335 | 11.90% | 4.40–22.00% | 348.33 | <0.01 | 97.42% | |
| Age | |||||||||
| Nestling | 2 | 578 | 128 | 10.30% | 7.90–13.00% | NA | NA | NA | 0.04 |
| Young | 5 | 548 | 108 | 22.50% | 8.80–39.60% | 42.60 | <0.01 | 90.61% | |
| Adult | 7 | 959 | 77 | 4.50% | 0.10–12.90% | 104.38 | <0.01 | 94.25% | |
| Sex | |||||||||
| Male | 4 | 243 | 50 | 21.40% | 5.70–43.00% | 36.75 | <0.01 | 91.84% | 0.96 |
| Female | 4 | 215 | 54 | 20.90% | 6.00–41.00% | 30.27 | <0.01 | 90.09% | |
| Season | |||||||||
| Spring | 2 | 273 | 80 | 22.60% | 17.80–27.80% | NA | NA | NA | <0.01 |
| Summer | 4 | 1796 | 118 | 3.80% | 0.10–11.10% | 43.67 | <0.01 | 93.13% | |
| Autumn | 4 | 420 | 87 | 12.10% | 0.00–39.70% | 126.39 | <0.01 | 97.63% | |
| Winter | 3 | 1360 | 116 | 9.00% | 4.60–14.60% | 8.22 | 0.02 | 75.67% | |
| Sampling years | |||||||||
| 1993–2008 | 9 | 3495 | 424 | 11.60% | 4.00–22.20% | 440.11 | <0.01 | 98.18% | 0.31 |
| 2009–2022 | 16 | 7560 | 1691 | 18.70% | 10.70–28.20% | 1260.64 | <0.01 | 98.81% | |
| Sampling methods | |||||||||
| blood | 9 | 6911 | 821 | 11.20% | 4.40–20.70% | 826.50 | <0.01 | 99.03% | <0.01 |
| feather | 9 | 5398 | 1225 | 19.30% | 13.20–26.30% | 192.61 | <0.01 | 95.85% | |
| fecal | 6 | 1302 | 257 | 16.80% | 6.00–31.20% | 160.67 | <0.01 | 96.89% | |
| cloacal swabs | 3 | 373 | 115 | 30.80% | 23.10–39.00% | 5.69 | 0.06 | 64.85% | |
| Genus | |||||||||
| Agapornis | 9 | 234 | 43 | 26.60% | 9.80–46.50% | 33.77 | <0.01 | 76.31% | <0.01 |
| Amazona | 12 | 931 | 53 | 8.00% | 2.80–15.00% | 88.13 | <0.01 | 87.52% | |
| Ara | 14 | 2081 | 100 | 4.20% | 0.10–11.70% | 80.89 | <0.01 | 83.93% | |
| Aratinga | 13 | 512 | 77 | 2.10% | 0.00–12.30% | 56.84 | <0.01 | 78.89% | |
| Cacatua | 10 | 1574 | 280 | 18.90% | 9.30–30.30% | 113.51 | <0.01 | 92.07% | |
| Diopsittaca | 5 | 69 | 6 | 1.20% | 0.00–9.00% | 2.23 | 0.69 | 92.07% | |
| Eclectus | 8 | 589 | 45 | 4.60% | 0.00–20.50% | 98.48 | <0.01 | 92.89% | |
| Eolophus | 7 | 89 | 12 | 17.80% | 0.90–43.70% | 26.26 | <0.01 | 77.16% | |
| Melopsittacus | 10 | 372 | 78 | 22.60% | 10.80–36.60% | 59.37 | <0.01 | 84.84% | |
| Myiopsitta | 7 | 317 | 32 | 7.10% | 0.00–25.20% | 51.72 | <0.01 | 88.40% | |
| Nymphicus | 10 | 1496 | 590 | 10.10% | 0.00–34.60% | 207.20 | <0.01 | 95.66% | |
| Pionites | 6 | 276 | 13 | 8.30% | 0.00–29.30% | 30.61 | <0.01 | 83.67% | |
| Pionus | 8 | 56 | 4 | 0.40% | 0.00–12.70% | 9.59 | 0.21 | 27.04% | |
| Platycercus | 11 | 594 | 128 | 12.90% | 2.70–26.90% | 77.22 | <0.01 | 87.05% | |
| Poicephalus | 6 | 199 | 55 | 21.40% | 0.60–54.10% | 73.94 | <0.01 | 93.24% | |
| Psittacula | 13 | 2375 | 530 | 20.20% | 7.40–36.50% | 639.72 | <0.01 | 98.12% | |
| Psittacus | 11 | 1361 | 267 | 15.00% | 3.00–31.70% | 347.38 | <0.01 | 97.12% | |
| Pyrrhura | 7 | 458 | 36 | 7.70% | 0.00–26.00% | 45.06 | <0.01 | 86.68% | |
| Trichoglossus | 7 | 155 | 32 | 22.60% | 6.10–43.80% | 25.88 | <0.01 | 76.82% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, H.; Shi, J.; Zhou, H.; Lin, X.; Zhang, H.; Zhang, T. A Meta-Analysis of Global Prevalence of Psittacine Beak and Feather Disease Virus Infection and Associated Risk Factors. Animals 2025, 15, 1473. https://doi.org/10.3390/ani15101473
Zhang X, Liu H, Shi J, Zhou H, Lin X, Zhang H, Zhang T. A Meta-Analysis of Global Prevalence of Psittacine Beak and Feather Disease Virus Infection and Associated Risk Factors. Animals. 2025; 15(10):1473. https://doi.org/10.3390/ani15101473
Chicago/Turabian StyleZhang, Xueping, Hongxiang Liu, Jiayu Shi, Hongyu Zhou, Xinyi Lin, Huiling Zhang, and Tangjie Zhang. 2025. "A Meta-Analysis of Global Prevalence of Psittacine Beak and Feather Disease Virus Infection and Associated Risk Factors" Animals 15, no. 10: 1473. https://doi.org/10.3390/ani15101473
APA StyleZhang, X., Liu, H., Shi, J., Zhou, H., Lin, X., Zhang, H., & Zhang, T. (2025). A Meta-Analysis of Global Prevalence of Psittacine Beak and Feather Disease Virus Infection and Associated Risk Factors. Animals, 15(10), 1473. https://doi.org/10.3390/ani15101473





