Influence of Migratory Strategy, Group Size, and Environmental Conditions on the Movements of Caribou in Eastern Alaska
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
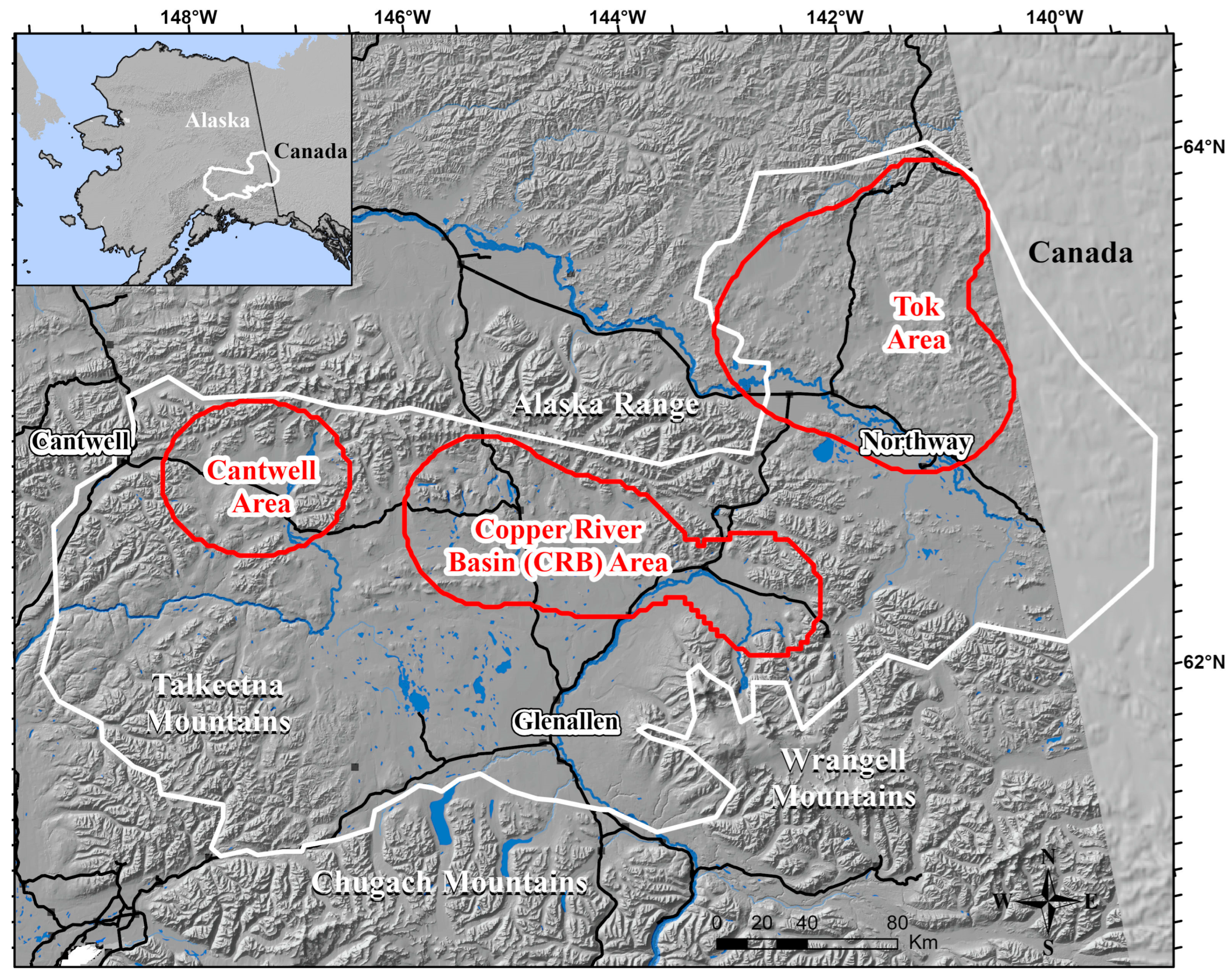
2.1. Study Population and Area
2.2. Data Collection
2.3. Movements
2.4. Survival
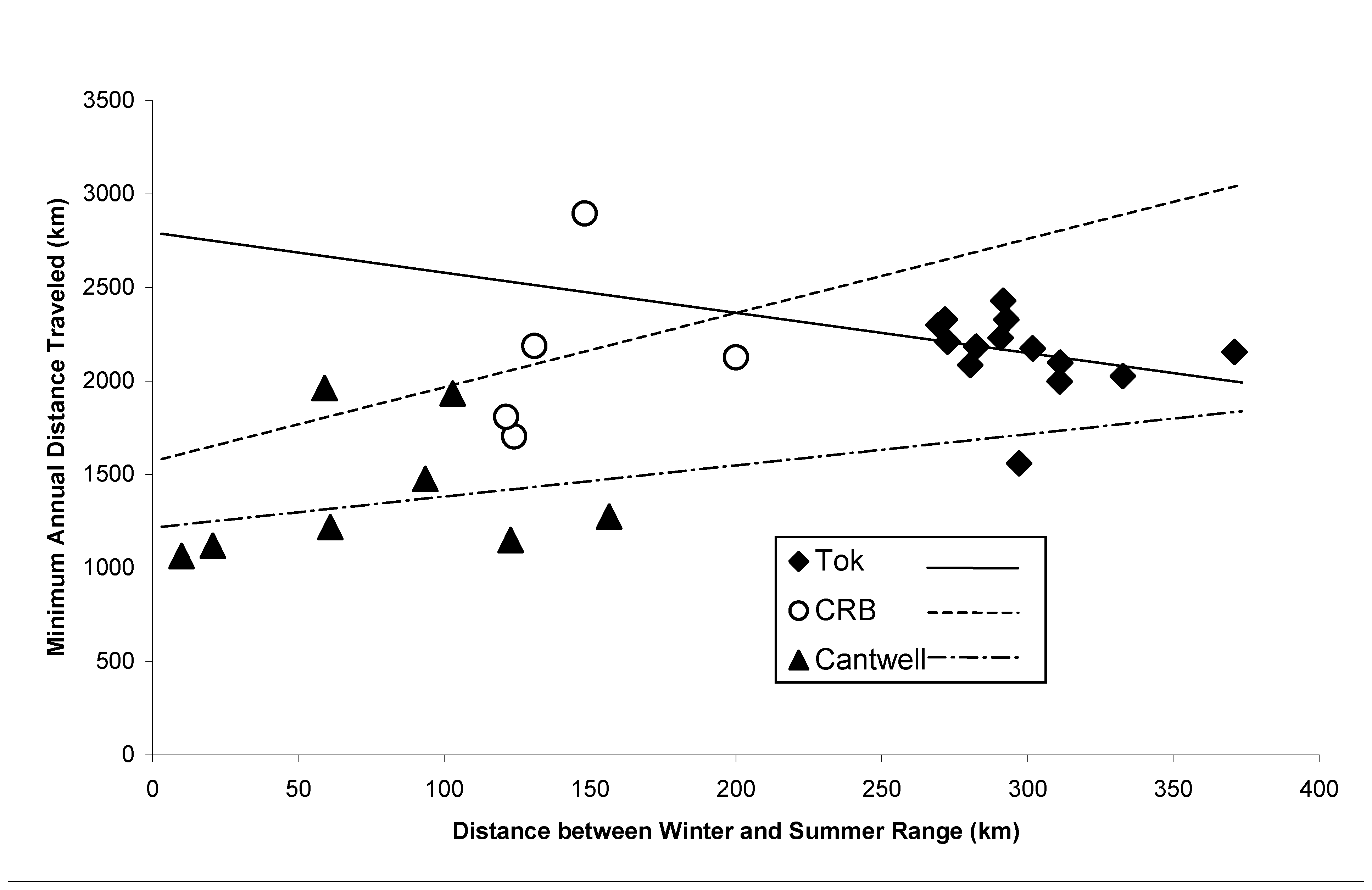
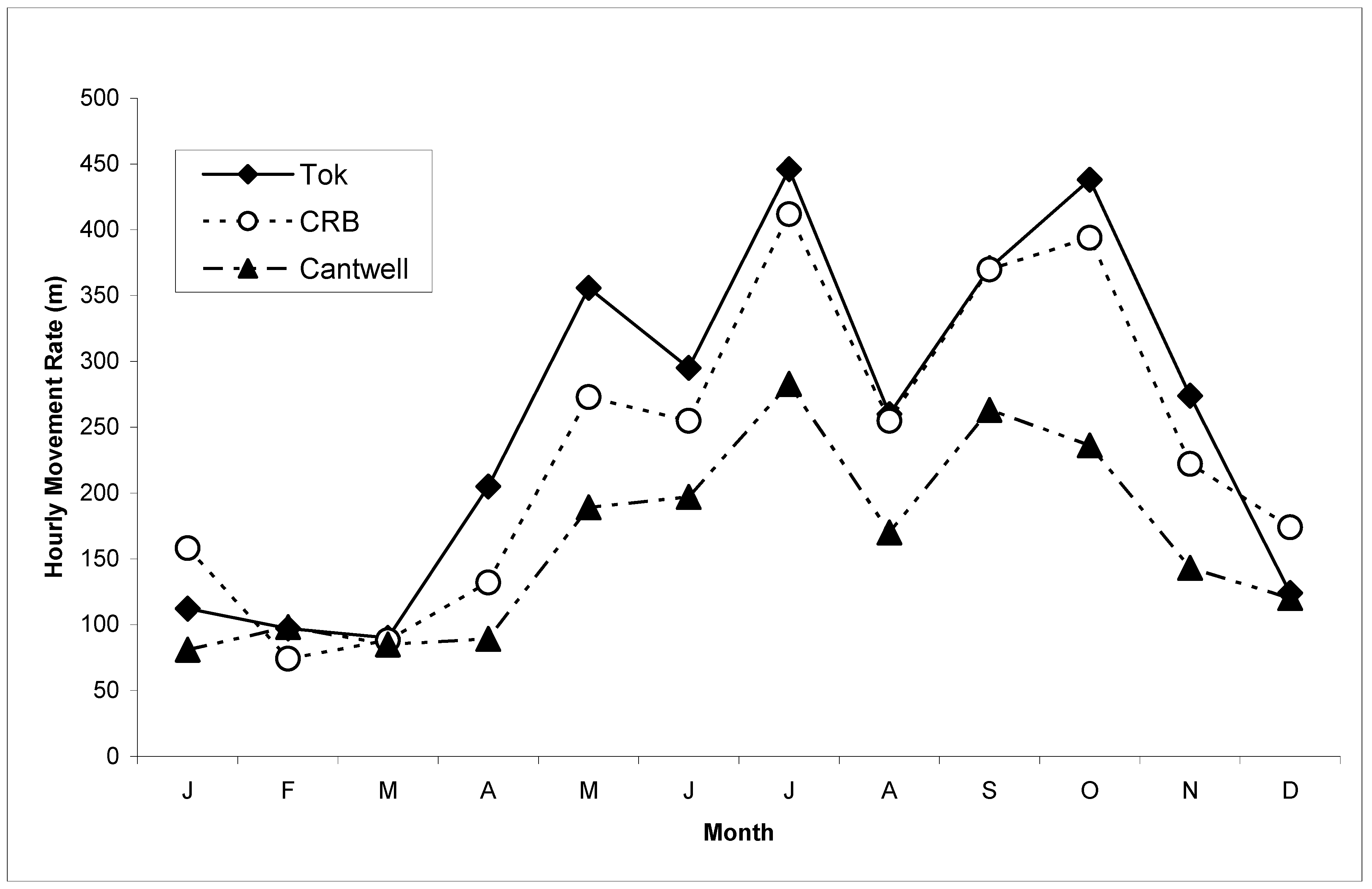
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, J. The last mile: How to sustain long-distance migration in mammals. Conserv. Biol. 2004, 18, 320–331. [Google Scholar] [CrossRef]
- Mahoney, S.P.; Schaefer, J.A. Long-term changes in demography and migration of Newfoundland caribou. J. Mammal. 2002, 83, 957–963. [Google Scholar] [CrossRef]
- Nelson, M.E.; Mech, L.D.; Frame, P.F. Tracking of white-tailed deer migration by Global Positioning System. J. Mammal. 2004, 85, 505–510. [Google Scholar] [CrossRef]
- Fryxell, J.M.; Sinclair, A.R.E. Causes and consequences of migration by large herbivores. Trends Ecol. Evol. 1988, 3, 237–241. [Google Scholar] [CrossRef]
- Avgar, T.; Street, G.; Fryxell, J.M. On the adaptive benefits of mammal migration. Can. J. Zool. 2014, 92, 481–490. [Google Scholar] [CrossRef]
- Joly, K.; Gurarie, E.; Sorum, M.S.; Kaczensky, P.; Cameron, M.D.; Jakes, A.F.; Borg, B.L.; Nandintsetseg, D.; Hopcraft, J.G.C.; Buuveibaatar, B.; et al. Longest terrestrial migrations and movements around the world. Sci. Rep. 2019, 9, 15333. [Google Scholar] [CrossRef]
- Joly, K.; Gurarie, E.; Hansen, D.A.; Cameron, M.D. Seasonal patterns of spatial fidelity and temporal consistency in the distribution and movements of a migratory ungulate. Ecol. Evol. 2021, 11, 8183–8200. [Google Scholar] [CrossRef] [PubMed]
- Faille, G.; Dussault, C.; Ouellet, J.-P.; Fortin, D.; Courtois, R.; St-Laurent, M.-H.; Dussault, C. Range fidelity: The missing link between caribou decline and habitat alteration? Biol. Conserv. 2010, 143, 2840–2850. [Google Scholar] [CrossRef]
- Joly, K.; Cameron, M.D.; White, R.G. Behavioral adaptation to seasonal resource scarcity by Caribou (Rangifer tarandus) and its role in partial migration. J. Mammal. 2025, 106, 96–104. [Google Scholar] [CrossRef]
- Duquette, L.S.; Klein, D.R. Activity budgets and group size of caribou during spring migration. Can. J. Zool. 1987, 65, 164–168. [Google Scholar] [CrossRef]
- Eastland, W.G. Influence of Weather on Movements and Migrations of Caribou. Ph.D. Thesis, University of Alaska, Fairbanks, AK, USA, 1991; 111p. [Google Scholar]
- Walsh, N.E.; Fancy, S.G.; McCabe, T.R.; Pank, L.F. Habitat use by the Porcupine Caribou Herd during predicted insect harassment. J. Wildl. Manag. 1992, 56, 465–473. [Google Scholar] [CrossRef]
- Mörschel, F.M.; Klein, D.R. Effects of weather and parasitic insects on behavior and group dynamics of caribou of the Delta Herd, Alaska. Can. J. Zool. 1997, 75, 1659–1670. [Google Scholar] [CrossRef]
- Joly, K.; Couriot, O.; Cameron, M.D.; Gurarie, E. Behavioral, physiological, demographic and ecological impacts of hematophagous and endoparasitic insects on an arctic ungulate. Toxins 2020, 12, 334. [Google Scholar] [CrossRef]
- Pruitt, W.O. Snow as a factor in the winter ecology of the barren ground caribou (Rangifer tarandus). Arctic 1959, 12, 159–179. [Google Scholar] [CrossRef]
- Fancy, S.G.; White, R.G. Energy expenditures for locomotion by barren-ground caribou. Can. J. Zool. 1987, 65, 122–128. [Google Scholar] [CrossRef]
- Schaeffer, J.A.; Luttich, S.N. Movements and activity of caribou, Rangifer tarandus caribou, of the Torngat Mountains, Northern Labrador and Quebec. Can. Field-Nat. 1998, 112, 486–490. [Google Scholar] [CrossRef]
- Boertje, R.D. Seasonal activity of the Denali Caribou Herd, Alaska. Rangifer 1985, 5, 32–42. [Google Scholar] [CrossRef]
- Fancy, S.G. Movements and activity budgets of caribou near oil drilling sites in the Sagavanirktok River floodplain, Alaska. Arctic 1983, 36, 193–197. [Google Scholar] [CrossRef]
- Fancy, S.G.; Pank, L.F.; Whitten, K.R.; Regelin, W.L. Seasonal movements of caribou in arctic Alaska as determined by satellite. Can. J. Zool. 1989, 67, 644–650. [Google Scholar] [CrossRef]
- Bergman, C.M.; Schaefer, J.A.; Luttich, S.N. Caribou movement as a correlated random walk. Oecologia 2000, 123, 364–374. [Google Scholar] [CrossRef]
- Rettie, W.J.; Messier, F. Range use and movement rates of woodland caribou in Saskatchewan. Can. J. Zool. 2001, 79, 1933–1940. [Google Scholar] [CrossRef]
- Johnson, C.J.; Parker, K.L.; Heard, D.C.; Gillingham, M.P. A multiscale behavioral approach to understanding the movements of woodland caribou. Ecol. Appl. 2002, 12, 1840–1860. [Google Scholar] [CrossRef]
- Joly, K. The effects of sampling regime on the analysis of movements of overwintering female caribou in east-central Alaska. Rangifer 2005, 25, 67–74. [Google Scholar] [CrossRef]
- Pépin, D.; Adrados, C.; Mann, C.; Janeau, G. Assessing real daily distance traveled by ungulates using differential GPS locations. J. Mammal. 2004, 85, 774–780. [Google Scholar] [CrossRef]
- Schwanke, R.A.; Robbins, W.F. Units 13 and 14B Caribou Management Report. In Caribou Management Report of Survey and Inventory Activities 1 July 2010–30 June 2012; Harper, P., Ed.; Alaska Department of Fish and Game, Species Management: Juneau, AK, USA, 2013; Report ADF&G/DWC/SMR-2013-3; pp. 104–124. [Google Scholar]
- Joly, K. Year-round group sizes of a barren-ground Caribou (Rangifer tarandus) herd in east-central Alaska. J. Zool. 2025. in submission. [Google Scholar]
- Joly, K.; Dale, B.W.; Collins, W.B.; Adams, L.G. Winter habitat use by female caribou in relation to wildland fires in interior Alaska. Can. J. Zool. 2003, 81, 1192–1201. [Google Scholar] [CrossRef]
- Valkenburg, P.; Keech, M.A.; Sellers, R.A.; Tobey, R.W.; Dale, B.W. Investigations of Regulating and Limiting Factors in the Delta Caribou Herd; Alaska Department of Fish and Game: Juneau, AK, USA, 2002; Research Final Report; Federal Aid in Wildlife Restoration, Projects W-24-5, W-27-1-5, Study 3.42.
- Skoog, R.O. Ecology of Caribou (Rangifer tarandus granti) in Alaska. Ph.D. Thesis, University of California-Berkeley, Berkeley, CA, USA, 1968; 699p. [Google Scholar]
- Adams, L.G.; Dale, B.W.; Mech, L.D. Wolf Predation on Caribou Calves in Denali National Park, Alaska. In Ecology and Conservation of Wolves in a Changing World; Carbyn, L.N., Fritts, S.H., Seip, D.R., Eds.; Canadian Circumpolar Institute Occasional Publication: Edmonton, AB, Canada, 1995; Volume 35, pp. 245–260. [Google Scholar]
- Adams, L.G.; Valkenburg, P.; Davis, J.L. Efficacy of carfentanil citrate and naloxone for field immobilization of Alaskan caribou. Proc. N. Am. Caribou Workshop 1987, 3, 167–168. [Google Scholar]
- Worton, B.J. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Seaman, D.E.; Powell, R.A. An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology 1996, 77, 2075–2085. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1984; 718p. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information Theoretic Approach; Springer: New York, NY, USA, 1998; 353p. [Google Scholar]
- White, G.C.; Burnham, K.P. Program MARK: Survival estimation from populations of marked animals. Bird Study 1999, 46, 120–138. [Google Scholar] [CrossRef]
- Lack, D. The Natural Regulation of Animal Numbers; Oxford University Press: London, UK, 1954; 343p. [Google Scholar]
- Swingland, I.R. Intraspecific Differences in Movement. In The Ecology of Animal Movement; Swingland, I.R., Greenwood, P.J., Eds.; Clarendon Press: Oxford, UK, 1983; pp. 102–115. [Google Scholar]
- Talbot, L.M.; Talbot, M.H. The wildebeest in western Masailand, East Africa. Wildl. Monogr. 1963, 12, 1–88. [Google Scholar]
- Dingle, H. Migration: The Biology of Life on the Move; Oxford University Press: New York, NY, USA, 1996; 474p. [Google Scholar]
- Joly, K.; Craig, T.; Cameron, M.D.; Gall, A.E.; Sorum, M.S. Lying in wait: Limiting factors on a low-density ungulate population and the latent traits that can facilitate escape from them. Acta Oecologica 2017, 85, 174–183. [Google Scholar] [CrossRef]
- Hinkes, M.T.; Collins, G.H.; Van Daele, L.J.; Kovach, S.D.; Aderman, A.R.; Woolington, J.D.; Seavoy, R.J. Influence of population growth on caribou herd identity, calving ground fidelity, and behavior. J. Wildl. Manag. 2005, 69, 1147–1162. [Google Scholar] [CrossRef]
- Russell, D.E.; Martell, A.M.; Nixon, W.A.C. Range ecology of the Porcupine Caribou Herd in Canada. Rangifer Spec. Issue 1993, 8, 1–168. [Google Scholar] [CrossRef]
- Kelsall, J.P. The Migratory Barren-Ground Caribou of Canada; Canadian Wildlife Service Monograph 1968, No. 3; Queen’s Printer: Ottawa, ON, Canada, 1968; 339p. [Google Scholar]
- Messier, F.; Huot, J.; Le Hénaff, D.; Luttich, S. Demography of the George River caribou herd: Evidence of population regulation by forage exploitation and range expansion. Arctic 1988, 41, 279–287. [Google Scholar] [CrossRef]
- Bergerud, A.T. Caribou. In Ecology and Management of Large Mammals in North America; Demarais, S., Krausman, P.R., Eds.; Prentice-Hall: Englewood Cliffs, NJ, USA, 2000; pp. 658–693. [Google Scholar]
- Seip, D.R. Predation and caribou populations. Rangifer 1991, 7, 46–52. [Google Scholar] [CrossRef]
- Bergerud, A.T.; Jakimchuk, R.D.; Carruthers, D.R. The buffalo of the north: Caribou (Rangifer tarandus) and human development. Arctic 1984, 37, 7–22. [Google Scholar] [CrossRef]
- Hughes, M.M.; Bourbon, C.; Milanesi, P.; Veitch, J.S.M.; Deakin, S.; Schwantje, H.; Thacker, C.; Pelletier, A.; Polfus, J.; Neuhaus, P.; et al. Integrating movement behaviours for intra-specific conservation: The caribou case. Biol. Conserv. 2025, 302, 10933. [Google Scholar] [CrossRef]
- Colman, J.E.; Pedersen, C.; Hjermann, D.O.; Holand, O.; Moe, S.R.; Reimers, E. Do wild reindeer exhibit grazing compensation during insect harassment? J. Wildl. Manag. 2003, 67, 11–19. [Google Scholar] [CrossRef]
- Folstad, I.; Nilssen, A.C.; Halvorsen, O.; Andersen, J. Parasite avoidance: The cause of post-calving migrations in Rangifer? Can. J. Zool. 1991, 69, 2423–2429. [Google Scholar] [CrossRef]
- Telfer, E.S.; Kelsall, J.P. Adaptation of some large North American mammals for survival in snow. Ecology 1984, 65, 1828–1834. [Google Scholar] [CrossRef]
- Cameron, M.D.; Eisaguirre, J.M.; Breed, G.A.; Joly, K.; Kielland, K. Mechanistic movement models identify continuously updated autumn migration cues in Arctic caribou. Mov. Ecol. 2021, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Middleton, A.D.; Kauffman, M.J.; McWhirter, D.E.; Cook, J.G.; Cook, R.C.; Nelson, A.A.; Jimenez, M.D.; Klaver, R.W. Animal migration amid shifting patterns of phenology and predation: Lessons from a Yellowstone elk herd. Ecology 2013, 94, 1245–1256. [Google Scholar] [CrossRef]
- Nicholson, M.C.; Bowyer, R.T.; Kie, J.G. Habitat selection and survival of mule deer: Tradeoffs associated with migration. J. Mammal. 1997, 78, 483–504. [Google Scholar] [CrossRef]
- Adams, L.G.; Dale, B.W. Reproductive performance of female Alaskan caribou. J. Wildl. Manag. 1998, 62, 1184–1195. [Google Scholar] [CrossRef]
- Gerhart, K.L.; Russell, D.E.; Van DeWetering, D.; White, R.G.; Cameron, R.D. Pregnancy of adult caribou (Rangifer tarandus): Evidence of lactational infertility. J. Zool. 1997, 242, 17–30. [Google Scholar] [CrossRef]
- Arnemo, J.M.; Ahlqvist, P.; Andersen, R.; Berntsen, F.; Ericsson, G.; Odden, J.; Brunberg, S.; Segerström, P.; Swenson, J.E. Risk of capture-related mortality in large free-ranging mammals: Experiences from Scandinavia. Wildl. Biol. 2006, 12, 109–113. [Google Scholar] [CrossRef]
- Cattet, M.; Boulanger, J.; Stenhouse, G.; Powell, R.A.; Reynolds-Hogland, M.J. An evaluation of long-term capture effects in ursids: Implications for wildlife welfare and research. J. Mammal. 2008, 89, 973–990. [Google Scholar] [CrossRef]
- Dau, J. Caribou survey-inventory management report. In Caribou. Federal Aid in Wildlife Restoration Survey-Inventory Activities, 1 July 1994–30 June 1996; Hicks, M.V., Ed.; Alaska Department of Fish and Game: Juneau, AK, USA, 1997; pp. 158–185. [Google Scholar]
- Miller, D.R. Caribou response to human activity: Research and management. Rangifer Spec. Issue 2003, 14, 89–93. [Google Scholar] [CrossRef][Green Version]
- Brooks, C.; Bonyongo, C.; Harris, S. Effects of Global Positioning System collar weight on zebra behavior and location error. J. Wildl. Manag. 2008, 72, 527–534. [Google Scholar] [CrossRef]
- Kauffman, M.J.; Cagnacci, F.; Chamaillé-Jammes, S.; Hebblewhite, M.; Hopcraft, J.G.C.; Merkle, J.A.; Mueller, T.; Mysterud, A.; Peters, W.; Roettger, C.; et al. Mapping out a future for ungulate migrations. Science 2021, 372, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Collins, W.B.; Dale, B.W.; Adams, L.G.; McElwain, D.; Joly, K. Fire, grazing history, lichen abundance, and winter distribution of caribou in Alaska’s taiga. J. Wildl. Manag. 2011, 75, 369–377. [Google Scholar] [CrossRef]
- Gannon, W.L.; Sikes, R.S.; the Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2007, 88, 809–823. [Google Scholar] [CrossRef]
| Month | Cantwell | Tok | CRB |
|---|---|---|---|
| January | 15 (8) | 27 (39) | 20 (13) |
| February | 11 (7) | 16 (15) | 62 (72) |
| March | 22 (16) | 11 (8) | 62 (86) |
| April | 45 (56) | 12 (11) | 82 (101) |
| May | 21 (14) | 12 (10) | 8 (5) |
| June | 96 (157) | 30 (27) | 168 (171) |
| July | 105 (146) | 1543 (1655) | 1925 (2281) |
| August | 4 (2) | 21 (70) | 67 (125) |
| September | 20 (21) | 49 (55) | 46 (83) |
| October | 17 (13) | 24 (25) | 32 (57) |
| November | 28 (65) | 19 (18) | 353 (604) |
| December | 12 (8) | 16 (27) | 18 (14) |
| Month | Tok | Cantwell | CRB | F | d.f. | p |
|---|---|---|---|---|---|---|
| January | 112 | 81 | 158 | 3.20 | 2, 48 | 0.050 * |
| February | 97 | 98 | 74 | 2.08 | 2, 45 | 0.138 |
| March | 90 | 85 | 88 | 0.09 | 2, 44 | 0.918 |
| April | 205 | 89 | 132 | 9.45 | 2, 49 | 0.000 * |
| May | 356 | 189 | 273 | 7.35 | 2, 41 | 0.002 * |
| June | 295 | 197 | 255 | 4.10 | 2, 39 | 0.025 * |
| July | 446 | 283 | 412 | 5.77 | 2, 38 | 0.007 * |
| August | 260 | 170 | 255 | 3.90 | 2, 36 | 0.030 * |
| September | 371 | 263 | 370 | 4.23 | 2, 34 | 0.023 * |
| October | 438 | 236 | 394 | 15.99 | 2, 50 | 0.000 * |
| November | 274 | 143 | 222 | 7.02 | 2, 50 | 0.002 * |
| December | 124 | 120 | 174 | 2.33 | 2, 50 | 0.108 |
| A. Year round | |||
| Model | AICC | Delta AICC | Model Weight |
| Group size + Ave. temp. + ABSADT | 2046.91 | 0.00 | 0.9867 |
| Group size + WR + Ave. temp. + ABSADT | 2055.67 | 8.76 | 0.0124 |
| Ave. temp. + ABSADT | 2062.19 | 15.28 | 0.0005 |
| Group size + Ave. temp. + ABSADT | 2062.36 | 15.45 | 0.0004 |
| B. Summer (June, July, August, and September) | |||
| Model | AICC | Delta AICC | Model Weight |
| Group size | 1828.76 | 0.00 | 0.9976 |
| Group size + WR | 1842.34 | 13.58 | 0.0011 |
| Group size. + ABSADT | 1843.26 | 14.50 | 0.0007 |
| Group size + Ave. temp. | 1843.96 | 15.20 | 0.0005 |
| C. Winter (December, January, February, and March) | |||
| Model | AICC | Delta AICC | Model Weight |
| Ave. temp. | 1984.61 | 0.00 | 0.9954 |
| Group size + Ave. temp. | 1996.17 | 11.56 | 0.0031 |
| Ave. temp.+ ABSADT | 1997.95 | 13.34 | 0.0013 |
| Group size | 2003.59 | 18.98 | 0.0001 |
| D. Migration (April, May, October, and November) | |||
| Model | AICC | Delta AICC | Model Weight |
| ABSADT | 2046.91 | 0.00 | 0.9867 |
| WR + ABSADT | 2055.67 | 8.76 | 0.0124 |
| Ave. temp.+ ABSADT | 2062.19 | 15.28 | 0.0005 |
| Group size + ABSADT | 2062.36 | 15.45 | 0.0004 |
| E. Late Winter (February, March, April, and May) | |||
| Model | AICC | Delta AICC | Model Weight |
| Ave. temp. + Snow depth | 1963.79 | 0.00 | 0.9874 |
| Ave. temp. + Snow depth + Snow%ave | 1972.87 | 9.08 | 0.0105 |
| Ave. temp. + Snow depth + ABSADT | 1976.96 | 13.17 | 0.0014 |
| Group size + Ave. temp. + Snow depth | 1978.29 | 14.50 | 0.0007 |
| F. Mid-Winter (February and March) | |||
| Model | AICC | Delta AICC | Model Weight |
| Group size | 921.59 | 0 | 0.3509 |
| Snow depth | 922.95 | 1.36 | 0.1778 |
| Ave. temp. | 923.11 | 1.52 | 0.1641 |
| Snow%ave | 923.26 | 1.67 | 0.1523 |
| ABSADT | 923.27 | 1.68 | 0.1515 |
| Group size + snow depth | 933.66 | 12.07 | 0.0008 |
| A. | |||||
| Model | AICC | Delta AICC | Model Weight | # Parameters | Deviance |
| All caribou the same | 117.28 | 0.00 | 0.86283 | 1 | 18.328 |
| Vary by wintering area | 120.95 | 3.68 | 0.13717 | 3 | 17.943 |
| B. | |||||
| Model | AICC | Delta AICC | Model Weight | # Parameters | Deviance |
| Vary by collar type | 289.92 | 0.00 | 0.90348 | 2 | 65.840 |
| All caribou the same | 294.39 | 4.47 | 0.09652 | 1 | 72.317 |
| C. | |||||
| Model | AICC | Delta AICC | Model Weight | # Parameters | Deviance |
| All caribou the same | 123.68 | 0.00 | 0.65937 | 1 | 36.725 |
| Vary by area and collar | 125.00 | 1.32 | 0.34063 | 6 | 27.796 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joly, K. Influence of Migratory Strategy, Group Size, and Environmental Conditions on the Movements of Caribou in Eastern Alaska. Animals 2025, 15, 1453. https://doi.org/10.3390/ani15101453
Joly K. Influence of Migratory Strategy, Group Size, and Environmental Conditions on the Movements of Caribou in Eastern Alaska. Animals. 2025; 15(10):1453. https://doi.org/10.3390/ani15101453
Chicago/Turabian StyleJoly, Kyle. 2025. "Influence of Migratory Strategy, Group Size, and Environmental Conditions on the Movements of Caribou in Eastern Alaska" Animals 15, no. 10: 1453. https://doi.org/10.3390/ani15101453
APA StyleJoly, K. (2025). Influence of Migratory Strategy, Group Size, and Environmental Conditions on the Movements of Caribou in Eastern Alaska. Animals, 15(10), 1453. https://doi.org/10.3390/ani15101453







