The Function of Heat Shock Transcription Factors in Sex Differentiation in Cynoglossus semilaevis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fish and Tissue Collection
2.3. High-Temperature Breeding Experiment
2.4. Gene Family Identification and Evolutionary Analysis
2.5. Primer Design and Synthesis
2.6. Genomic DNA Extraction and Genetic Identification
2.7. Total RNA Extraction and cDNA Synthesis
2.8. Real-Time Fluorescent Quantitative PCR of the hsf Gene
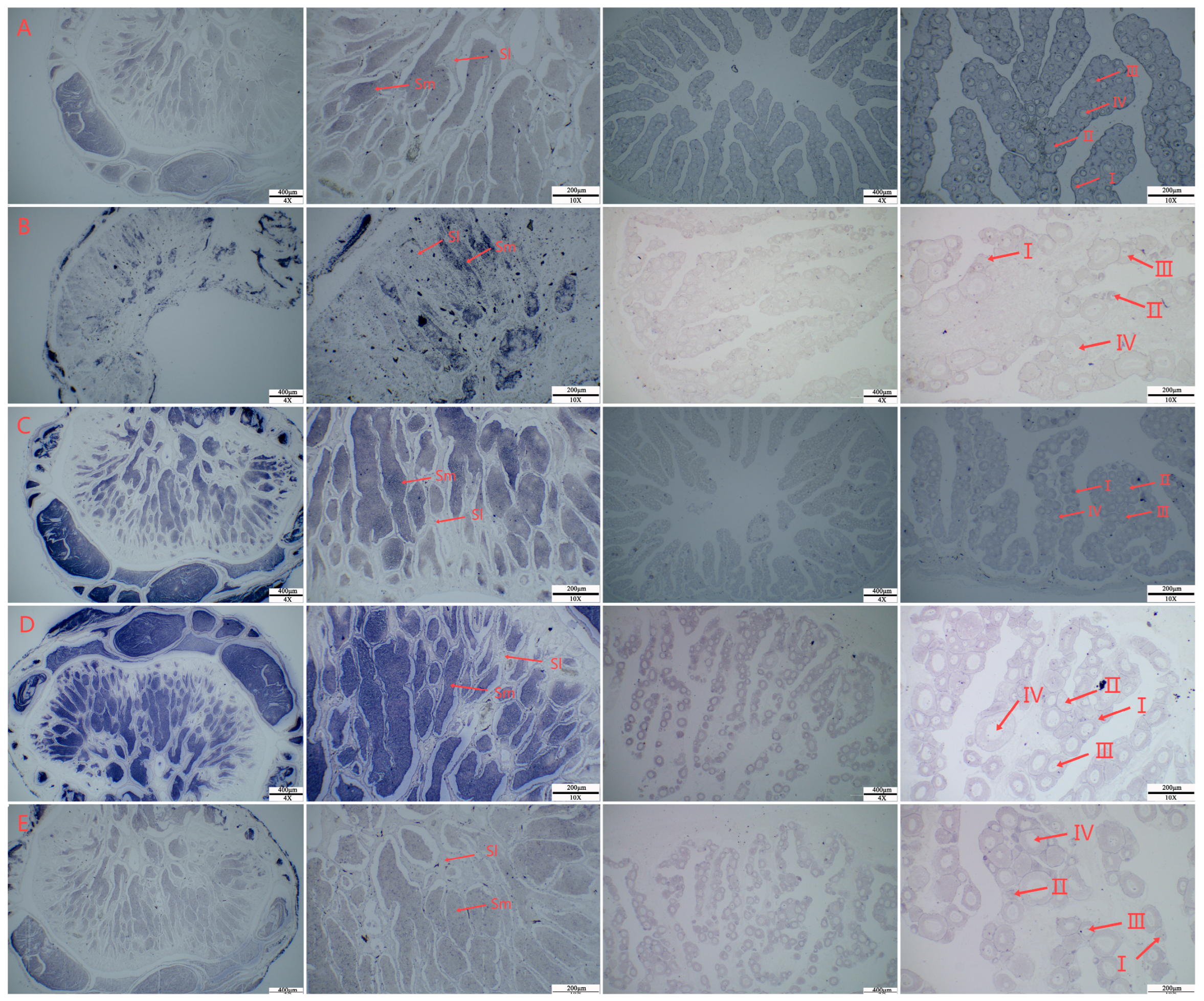
2.9. In Situ Hybridization of the hsf Gene
2.10. Interference of hsf Mediated by siRNA on Gonadal Cell Lines of C. semilaevis In Vivo and In Vitro
2.10.1. In Vitro siRNA-Mediated Knockdown
2.10.2. In Vivo siRNA-Mediated Knockdown
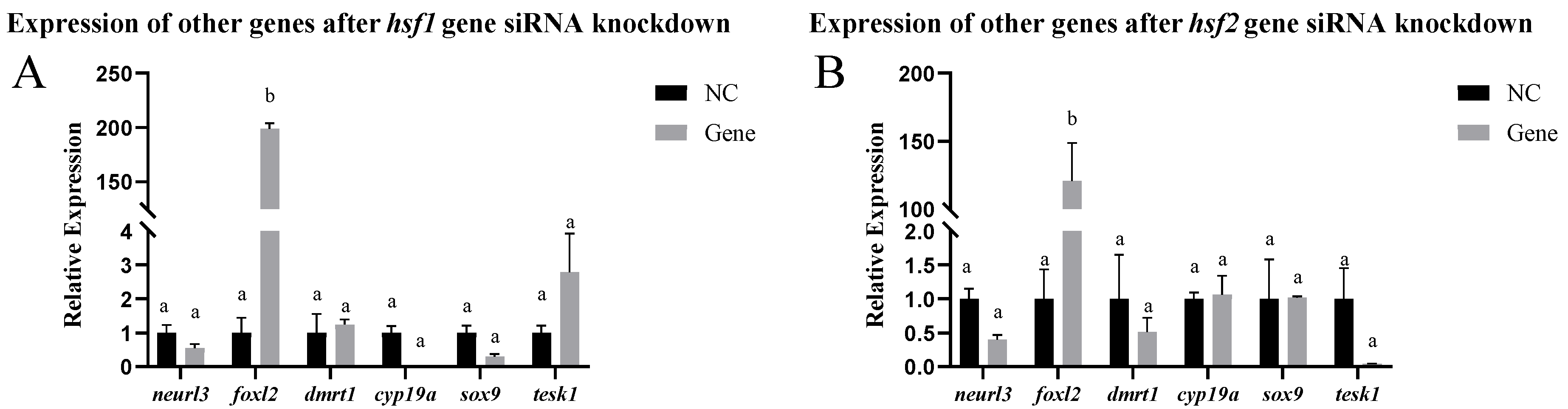
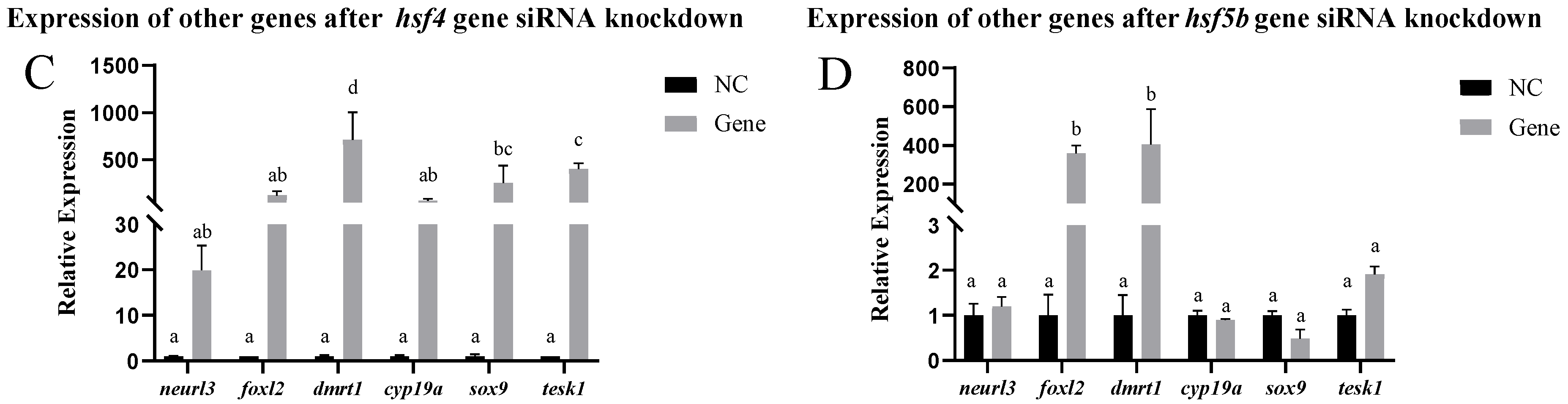
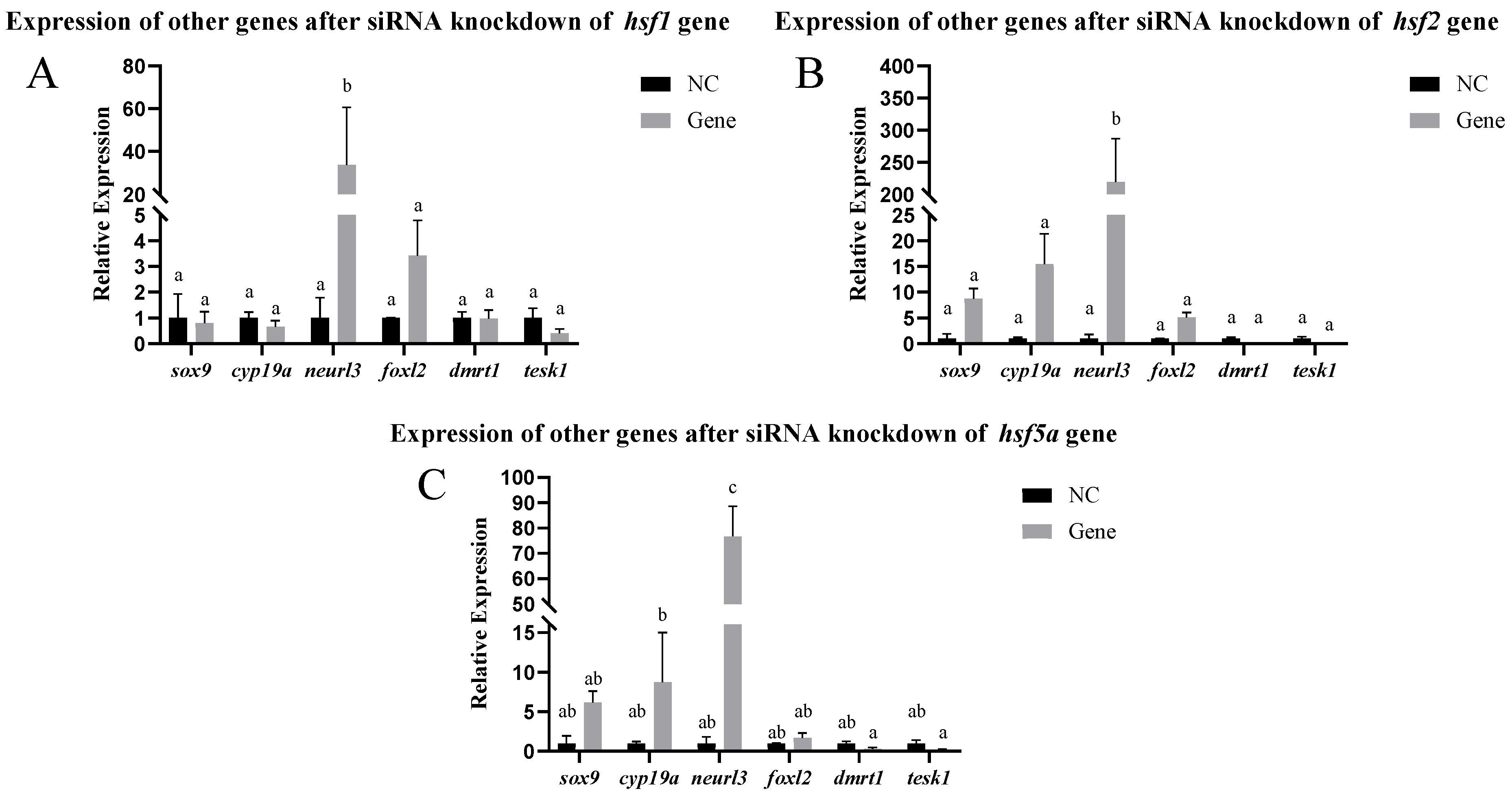
2.10.3. Expression of Other Genes After siRNA-Mediated Knockdown
3. Results
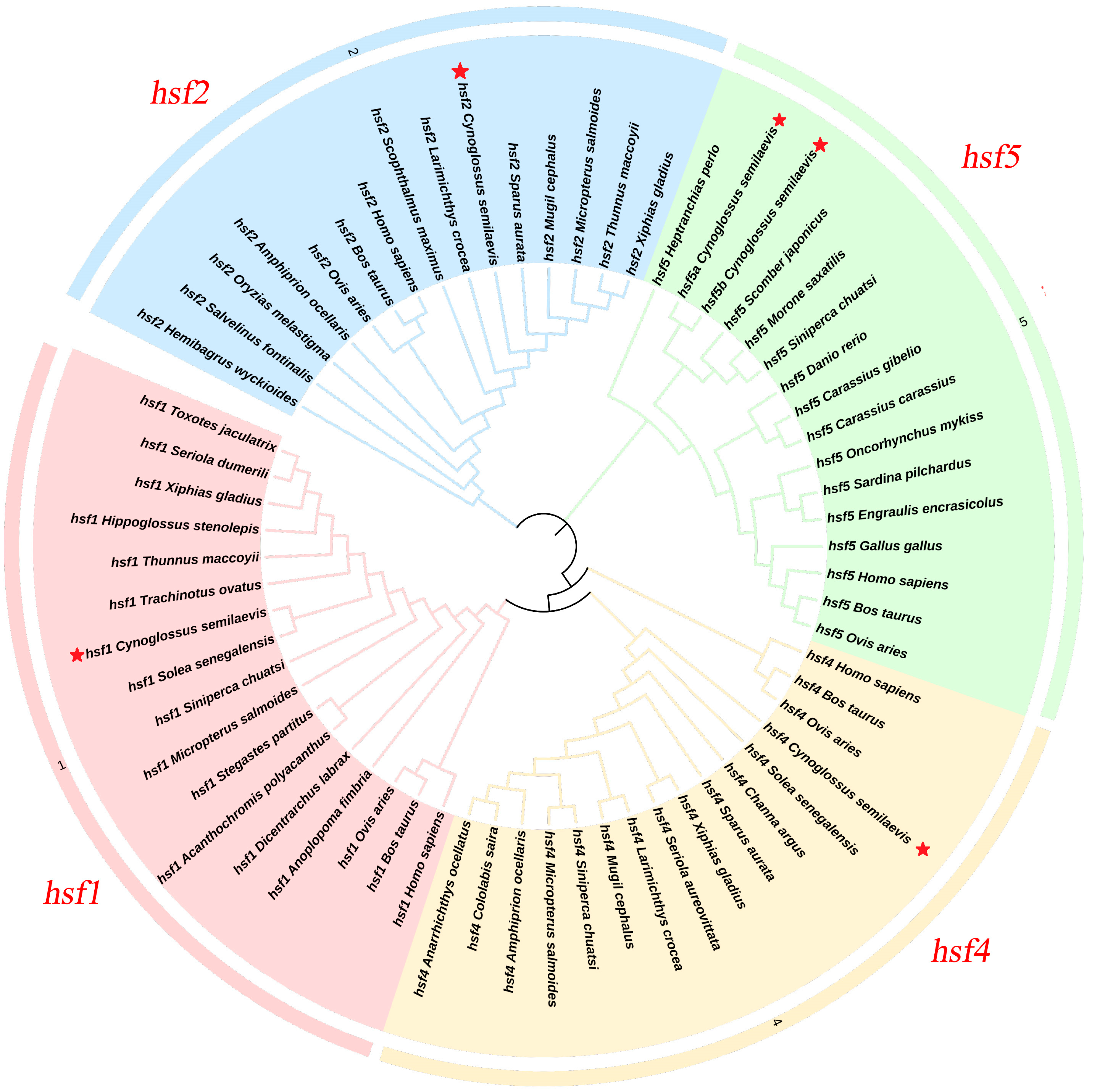
3.1. Cloning and Characterization of the hsf Gene
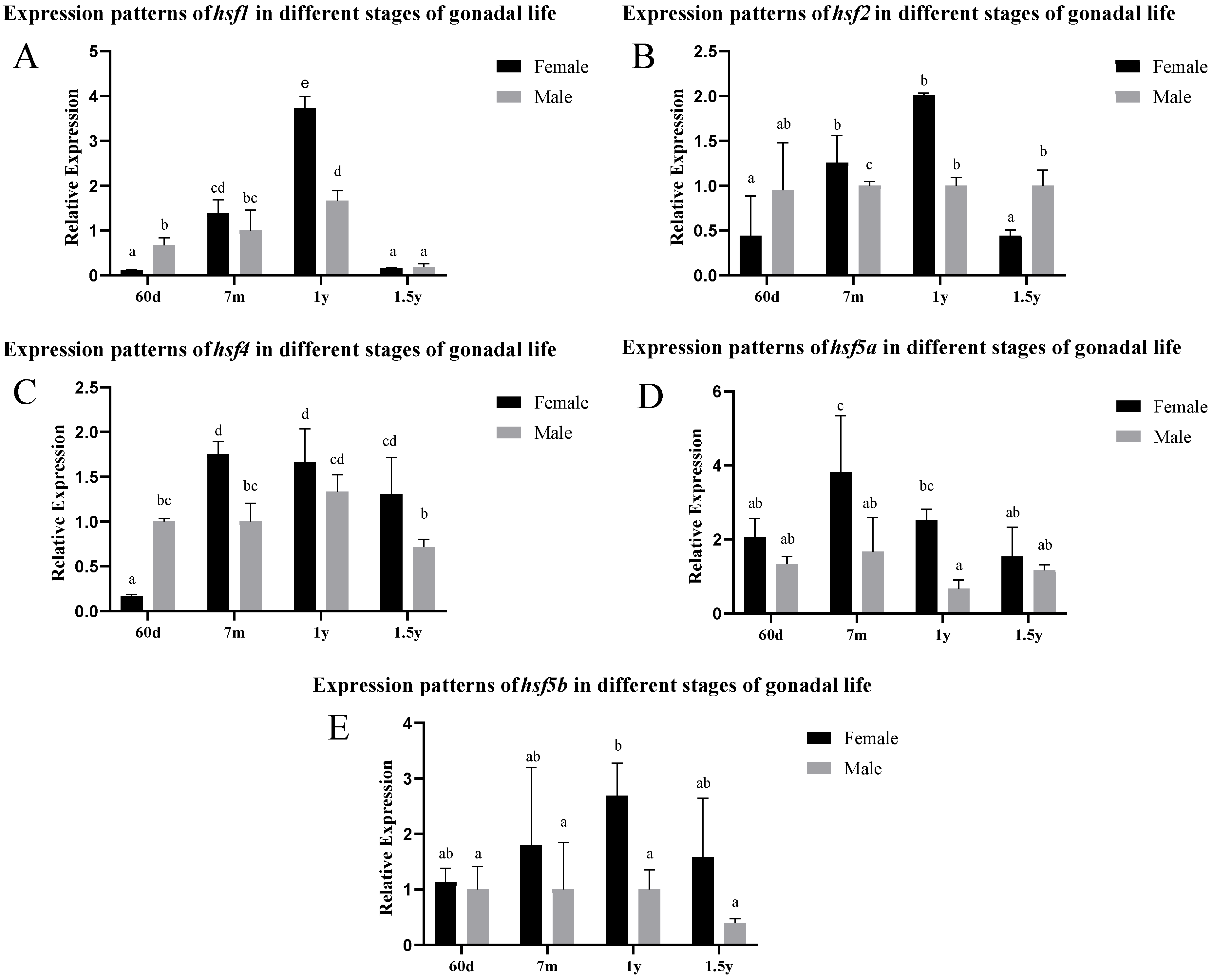
3.2. Differential Expression of the hsf Gene in Different Stages of Gonadal Development
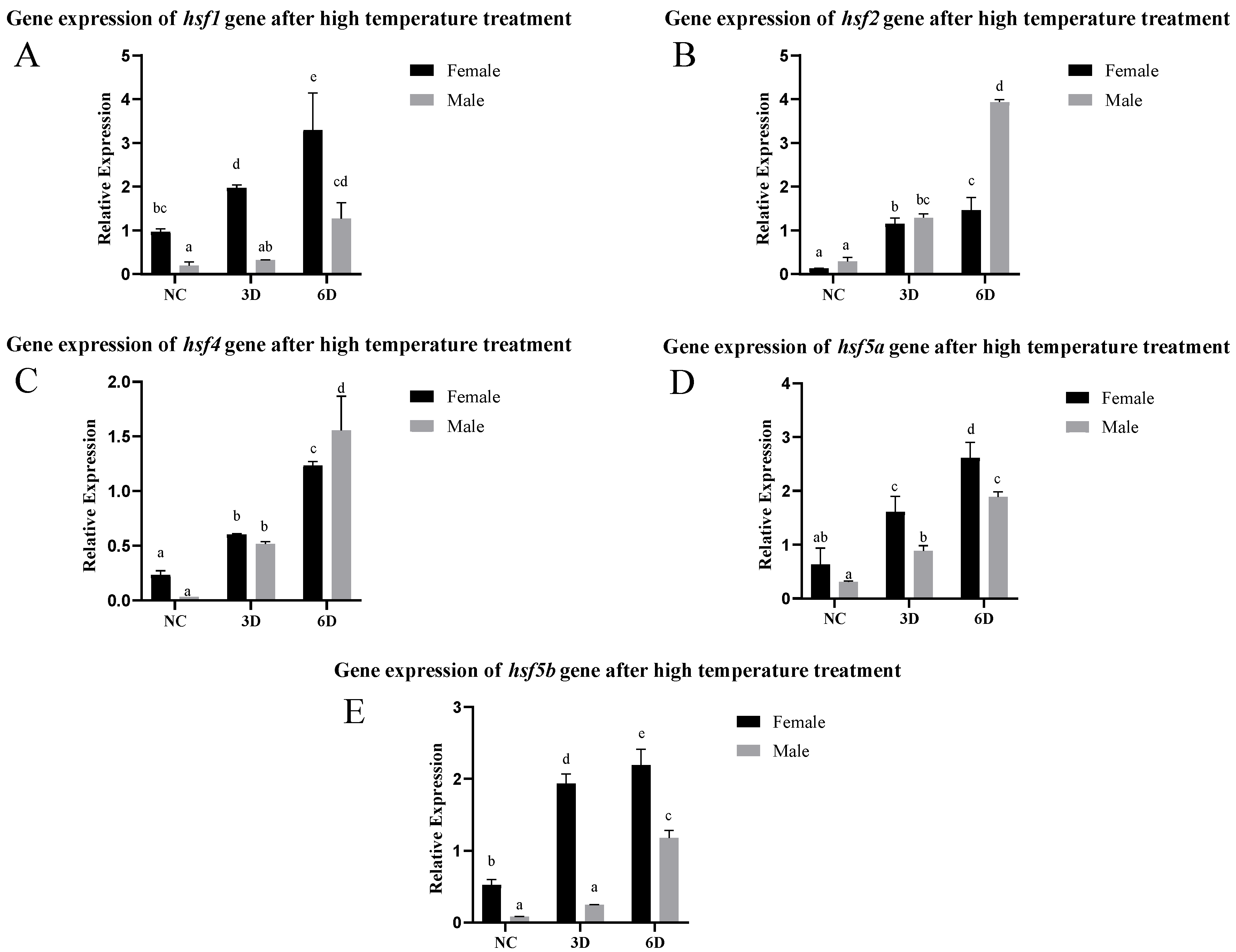
3.3. Expression Pattern Analysis of the hsf Gene in the Gonads After High-Temperature Treatment
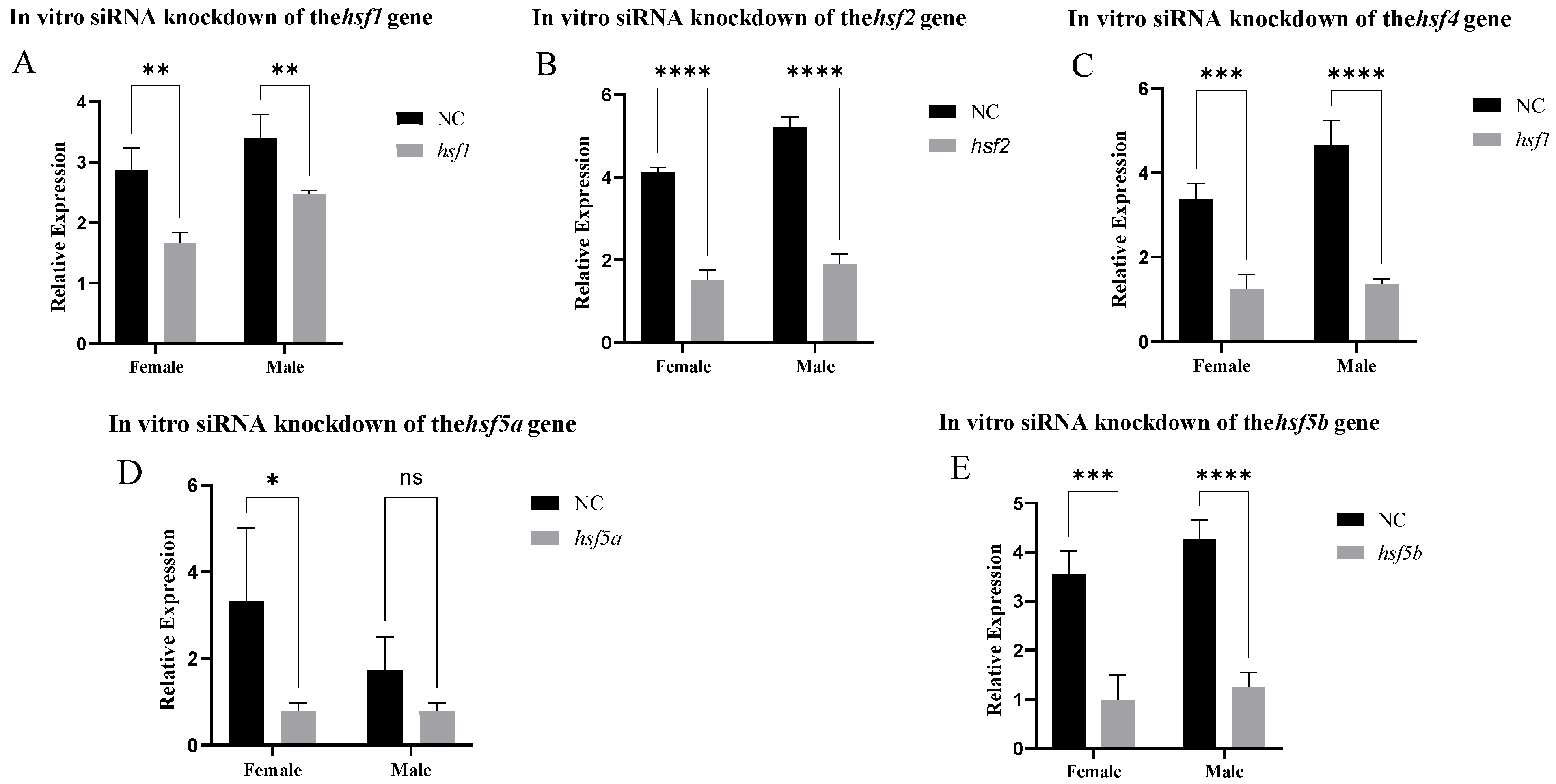
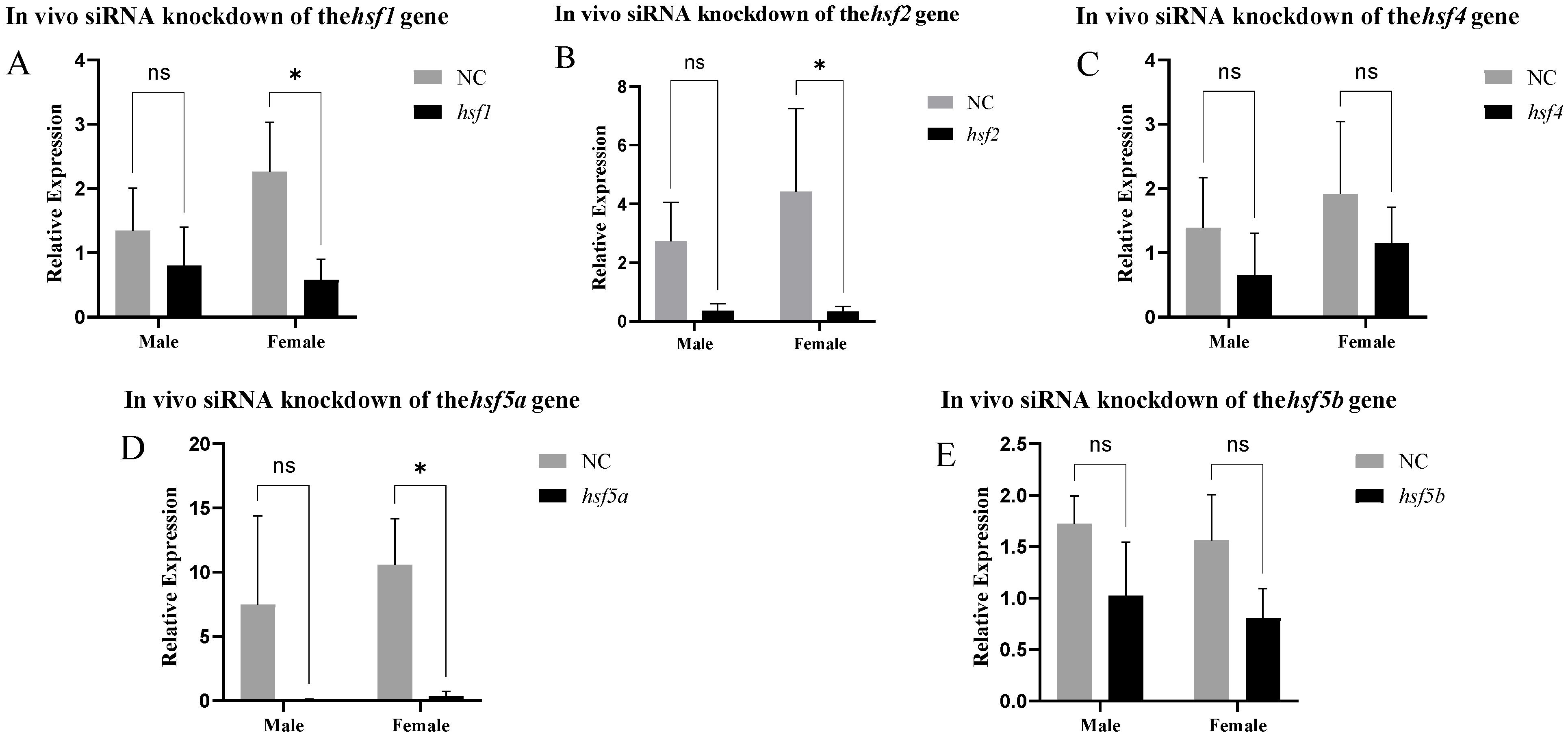
3.4. Analysis of Expression Patterns After siRNA-Mediated Knockout In Vitro and In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi-Yuan, L.; Lingkun, L. Histological study on the digestive gland of Cynoglossus semilaevis. Friends Farmers Get. Rich. 2015, 15, 45. (In Chinese) [Google Scholar]
- Liu, L.; Haige, D. Biological characteristics of Cynoglossus semilaevis and its development prospect of aquaculture. Chin. Aquat. Prod. 2005, 3, 63–64. (In Chinese) [Google Scholar]
- Wang, P.; Zheng, M.; Liu, J.; Liu, Y.; Lu, J.; Sun, X. Sexually Dimorphic Gene Expression Associated with Growth and Reproduction of Tongue Sole (Cynoglossus semilaevis) Revealed by Brain Transcriptome Analysis. Int. J. Mol. Sci. 2016, 17, 1402. [Google Scholar] [CrossRef]
- Roberts, R.J.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, K.; Feng, B.; Zhang, Z.; Wang, R.; Tang, L.; Li, W.; Li, Q.; Piferrer, F.; Shao, C. Transcriptome of gonads from high temperature induced sex reversal during sex determination and differentiation in chinese tongue sole, Cynoglossus semilaevis. Front. Genet. 2019, 10, 1128. [Google Scholar] [CrossRef]
- Hietakangas, V.; Sistonen, L. Regulation of the heat shock response by the heat shock transcription factors. Chaperons Top. Curr. Genet. 2005, 15, 1118–1131. [Google Scholar]
- Pelham, H.R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell 1982, 30, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Feng, H.; Wang, S.; Punekar, A.S.; Ladenstein, R.; Wang, D.C.; Zhang, Q.; Ding, J.; Liu, W. Structures of heat shock factor trimers bound to DNA. iScience 2021, 24, 102951. [Google Scholar] [CrossRef]
- Telonis-Scott, M.; Ali, Z.; Hangartner, S.; Sgrò, C.M. Temporal specific coevolution of Hsp70 and co-chaperone stv expression in Drosophila melanogaster under selection for heat tolerance. J. Therm. Biol. 2021, 102, 103110. [Google Scholar] [CrossRef]
- Pirkkala, L.; Nykänen, P.; Sistonen, L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001, 15, 1118–1131. [Google Scholar] [CrossRef]
- Minsky, N.; Roeder, R.G. Direct link between metabolic regulation and the heat-shock response through the transcriptional regulator PGC-1α. Proc. Natl. Acad. Sci. USA 2015, 112, E5669–E5678. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.; Sarge, K.; Abravaya, K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J. Biol. Chem. 1992, 267, 21987–21990. [Google Scholar] [CrossRef]
- Mathew, A.; Mathur, S.K.; Jolly, C.; Fox, S.G.; Kim, S.; Morimoto, R.I. Stress-specific activation and repression of heat shock factors 1 and 2. Mol. Cell Biol. 2001, 21, 7163–7171. [Google Scholar] [CrossRef]
- Yamamoto, N.; Takemori, Y.; Sakurai, M.; Sugiyama, K.; Sakurai, H. Differential recognition of heat shock elements by members of the heat shock transcription factor family. FEBS J. 2009, 276, 1962–1974. [Google Scholar] [CrossRef] [PubMed]
- Yoshima, T.; Yura, T.; Yanagi, H. Novel testis-specific protein that interacts with heat shock factor 2. Gene 1998, 214, 139–146. [Google Scholar] [CrossRef]
- Prasad, K.V.; Taiyab, A.; Jyothi, D.; Srinivas, U.K.; Sreedhar, A.S. Heat shock transcription factors regulate heat induced cell death in a rat histiocytoma. J. Biosci. 2007, 32, 585–593. [Google Scholar] [CrossRef]
- Kawazoe, Y.; Nakai, A.; Tanabe, M.; Nagata, K. Proteasome inhibition leads to the activation of all members of the heat-shock-factor family. Eur. J. Biochem. 1998, 255, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Mathur, S.K.; Morimoto, R.I. Heat Shock Response and protein degradation: Regulation of HSF2 by the ubiquitin-proteasome pathway. Mol. Cell Biol. 1998, 18, 5091–5098. [Google Scholar] [CrossRef]
- Tanabe, M.; Kawazoe, Y.; Takeda, S.; Morimoto, R.I.; Nagata, K.; Nakai, A. Disruption of the HSF3 gene results in the severe reduction of heat shock gene expression and loss of thermotolerance. EMBO J. 1998, 17, 1750–1758. [Google Scholar] [CrossRef]
- Elsing, A.N.; Aspelin, C.; Björk, J.K.; Bergman, H.A.; Himanen, S.V.; Kallio, M.J.; Roos-Mattjus, P.; Sistonen, L. Expression of HSF2 decreases in mitosis to enable stress-inducible transcription and cell survival. J. Cell Biol. 2014, 206, 735–749. [Google Scholar] [CrossRef]
- Somasundaram, T.; Bhat, S.P. Developmentally dictated expression of heat shock factors: Exclusive expression of HSF4 in the postnatal lens and its specific interaction with alphaB-crystallin heat shock promoter. J. Biol. Chem. 2004, 279, 44497–44503. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Tanabe, M.; Kawazoe, Y.; Inazawa, J.; Morimoto, R.I. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol. Cell Biol. 1997, 17, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Wieslawa, W.; Natalia, V. The Role of Heat Shock Factors in Mammalian Spermatogenesis. In Advances in Anatomy, Embryology and Cell Biology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 222, pp. 45–65. [Google Scholar]
- Liu, Y.; Chen, S.; Gao, F.; Meng, L.; Hu, Q.; Song, W.; Shao, C.; Lv, W. SCAR transformation of sex-specific microsatellite markers in Cynoglossus semilaevis and its application. J. Agric. Biotechnol. 2014, 22, 787–792. (In Chinese) [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Andrási, N.; PettkóSzandtner, A.; Szabados, L. Diversity of Plant Heat Shock Factors: Regulation, Interactions and Functions. J. Exp. Bot. 2020, 72, 1558–1575. [Google Scholar] [CrossRef]
- Liu, B.B.; Hu, J.J.; Zhang, J. Evolutionary Divergence of Duplicated Hsf Genes in Populus. Cells 2019, 8, 438. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Gao, Y.; Zhou, Y.; Zhang, E.; Hu, Y.; Yuan, Y.; Liang, G.; Xu, C. Adaptive evolution and divergent expression of heat stress transcription factors in grasses. BMC Evol. Biol. 2014, 14, 147. [Google Scholar] [CrossRef]
- Kovács, D.; Kovács, M.; Ahmed, S.; Barna, J. Functional diversification of heat shock factors. Biol. Futur. 2022, 73, 427–439. [Google Scholar] [CrossRef]
- Akerfelt, M.; Morimoto, R.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef]
- Takii, R.; Fujimoto, M.; Pandey, A.; Jaiswal, K.; Shearwin-Whyatt, L.; Grutzner, F.; Nakai, A. HSF1 is required for cellular adaptation to daily temperature fluctuations. Sci. Rep. 2024, 14, 21361. [Google Scholar] [CrossRef]
- Dutta, N.; Garcia, G.; Higuchi, S.R. Hijacking Cellular Stress Responses to Promote Lifespan. Front. Aging 2022, 24, 860404. [Google Scholar] [CrossRef] [PubMed]
- Phornphan, S.; Wisarut, J.; Warumporn, Y.; Anchalee, T. Heat shock factor 1 regulates heat shock proteins and immune-related genes in Penaeus monodon under thermal stress. Dev. Comp. Immunol. 2018, 88, 19–27. [Google Scholar]
- Råbergh, C.M.; Airaksinen, S.; Soitamo, A.; Björklund, H.V.; Johansson, T.; Nikinmaa, M.; Sistonen, L. Tissue-specific expression of zebrafish (Danio rerio) heat shock factor 1 mRNAs in response to heat stress. J. Exp. Biol. 2000, 203, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Tan, L.L.; Ma, W.; Zhao, X.N.; Shao, C.W.; Wang, Q. The Role of the Heat Shock Cognate Protein 70 Genes in Sex Determination and Differentiation of Chinese Tongue Sole (Cynoglossus semilaevis). Int. J. Mol. Sci. 2023, 24, 3761. [Google Scholar] [CrossRef] [PubMed]
- Rocio, G.-P.; Eileen, B.; Dennis, T. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar]
- Saju, J.M.; Hossain, M.S.; Liew, W.C.; Ajay, P.; Natascha, M.T.; Lydia, S.T.; Amit, A.; Per-Erik, O.; László, O. Heat Shock Factor 5 Is Essential for Spermatogenesis in Zebrafish. Cell Rep. 2018, 25, 3252–3261. [Google Scholar] [CrossRef]
- Wang, G.; Ying, Z.; Jin, X.; Tu, N.; Zhang, Y.; Michele, P.; Demetrius, M.; Nahid, F.M. Essential requirement for both hsf1 and hsf2 transcriptional activity in spermatogenesis and male fertility. Genesis 2004, 38, 66–80. [Google Scholar] [CrossRef]
- Hemati, A.; Modarressi, M.H.; Kolivand, S.; Azarnia, M. Heat shock factor 5 is essential for spermatogenesis in mice: Detected by a new monoclonal antibody. Iran. J. Basic Med. Sci. 2020, 23, 293–297. [Google Scholar]
- Carlsson, P.; Mahlapuu, M. Forkhead transcription factors: Key players in development and metabolism. Dev. Biol. 2002, 250, 1–23. [Google Scholar] [CrossRef]
- Xu, W.; Li, H.; Dong, Z.; Cui, Z.; Zhang, N.; Meng, L.; Zhu, Y.; Liu, Y.; Li, Y.; Guo, H.; et al. Ubiquitin ligase gene neurl3 plays a role in spermatogenesis of half-smooth tongue sole (Cynoglossus semilaevis) by regulating testis protein ubiquitination. Gene 2016, 592, 215–220. [Google Scholar] [CrossRef]

| Gene | ID | Chromosome Position | Gene Full Length (bp) | Accession Number | CDS (bp) | Protein Length (aa) | |
|---|---|---|---|---|---|---|---|
| mRNA | Protein | ||||||
| hsf1 | 103393760 | 18 | 4558 | XM_008330832.3 | XP_008329054.1 | 1551 | 516 |
| XM_008330833.3 | XP_008329055.1 | 1500 | 499 | ||||
| hsf2 | 103380605 | 7 | 15,272 | XM_008312623.3 | XP_008310845.1 | 1629 | 542 |
| hsf4 | 103379546 | 6 | 10,718 | XM_008311129.3 | XP_008309351.1 | 1290 | 429 |
| XM_017032938.2 | XP_016888427.1 | 1290 | 429 | ||||
| hsf5a (LOC103395174) | 103395174 | 19 | 4420 | XM_008332788.3 | XP_008331010.1 | 1050 | 349 |
| XM_008332789.3 | XP_008331011.1 | 1050 | 349 | ||||
| XM_017041700.2 | XP_016897189.1 | 1050 | 349 | ||||
| hsf5b (LOC103395175) | 103395175 | 19 | 4567 | XM_025065908.1 | XP_024921676.1 | 1119 | 372 |
| XM_017041699.2 | XP_016897188.1 | 504 | 167 | ||||
| XM_008332790.3 | XP_008331012.1 | 1146 | 381 | ||||
| Primer | Sequence (5′~3′) |
|---|---|
| qhsf1-F | ATCGACTCCAGGATCAACGC |
| qhsf1-R | ATATTTGGGCACGGAGTGGG |
| qhsf2-F | GACTGAGAACGGAGCGATTT |
| qhsf2-R | GTTTGCCGGTTTGCATCATT |
| qhsf4-F | CTCCAGTCCTGCTTCAAAGT |
| qhsf4-R | GCCTTTGTCTCTGGTGTCA |
| qhsf5a-F | AACTCAGCGCTGGTCTCAAA |
| qhsf5a-R | GTCGCAGCATGAGCTGTTTT |
| qhsf5b-F | TTGATGCCACCTCCAACCAA |
| qhsf5b-R | CTTGAATCAAAGCGAGCGGG |
| β-actin-F | TTCCAGCCTTCCTTCCTT |
| β-actin-R | TACCTCCAGACAGCACAG |
| ISH-hsf1-sp6 | AAGGGGAAACAGGAAACCA |
| ISH-hsf1-T7 | GGACCAGAGACACCAGGAA |
| ISH-hsf2-sp6 | AGGGATGGTCCTGTGGAGTT |
| ISH-hsf2-T7 | TGGGCATCTTTCCATCGTCC |
| ISH-hsf4-sp6 | AGAGGGGGAGTAGGAAAGG |
| ISH-hsf4-T7 | ATGTATGGTCGGAGGTTGG |
| ISH-hsf5a-sp6 | TTTCTGCAGCTTTGTGCGTC |
| ISH-hsf5a-T7 | CCAGACAGTTGGGAGTCGTC |
| ISH-hsf5b-sp6 | ATCTTGTCTCCTTCCCCCAG |
| ISH-hsf5b-T7 | GTTTTTGCATTCGCCACTTC |
| Sox-9-qF | AAGAACCACACAGATCAAGACAGA |
| Sox-9-qR | TAGTCATACTGTGCTCTGGTGATG |
| cyp19a-qF | GGTGAGGATGTGACCCAGTGT |
| cyp19a-qR | ACGGGCTGAAATCGCAAG |
| neurl3-qF | CTGGTGTTTAGCAGCCGTCCT |
| neurl3-qR | CCAGAACTCCAGCACTGACCC |
| foxl2-qF | GAGAGGAAGGGCAACTACTGGA |
| foxl2-qR | TGGTTGGAAGTGCGTGGG |
| dmrt1-qF | GGAGGAAGAACTTGGGATTTG |
| dmrt1-qR | AGGTAGGAGGTTGCTGGG |
| tesk1-qF | GCAGAAACTCTCTCACCCCAACA |
| tesk1-qR | CCAGACCAAAGTCCGTCACCA |
| Primer | Sequence (5′~3′) |
|---|---|
| si-hsf1-F | AAGAAGUUCUGCCAAAGUATT |
| si-hsf1-R | UACUUUGGCAGAACUUCUUTT |
| si-hsf2-F | AUGGAAAGAUGCCCAAAUGTT |
| si-hsf2-R | CAUUUGGGCAUCUUUCCAUTT |
| si-hsf4-F | AAGAAGUUCUUCCAAAGUATT |
| si-hsf4-R | UACUUUGGAAGAACUUCUUTT |
| si-hsf5a-F | GGCACCUUAUCGAGUAACATT |
| si-hsf5a-R | UGUUACUCGAUAAGGUGCCTT |
| si-hsf5b-F | UGCUGAUAACAAGGCUAAATT |
| si-hsf5b-R | UUUAGCCUUGUUAUCAGCATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Sun, X.; Yan, H.; Wang, L.; Li, X.; Wang, N.; Wei, M.; Xu, W. The Function of Heat Shock Transcription Factors in Sex Differentiation in Cynoglossus semilaevis. Animals 2025, 15, 1443. https://doi.org/10.3390/ani15101443
Li Z, Sun X, Yan H, Wang L, Li X, Wang N, Wei M, Xu W. The Function of Heat Shock Transcription Factors in Sex Differentiation in Cynoglossus semilaevis. Animals. 2025; 15(10):1443. https://doi.org/10.3390/ani15101443
Chicago/Turabian StyleLi, Zhijie, Xuexue Sun, Haipeng Yan, Lijun Wang, Xihong Li, Na Wang, Min Wei, and Wenteng Xu. 2025. "The Function of Heat Shock Transcription Factors in Sex Differentiation in Cynoglossus semilaevis" Animals 15, no. 10: 1443. https://doi.org/10.3390/ani15101443
APA StyleLi, Z., Sun, X., Yan, H., Wang, L., Li, X., Wang, N., Wei, M., & Xu, W. (2025). The Function of Heat Shock Transcription Factors in Sex Differentiation in Cynoglossus semilaevis. Animals, 15(10), 1443. https://doi.org/10.3390/ani15101443








