Dietary Soy Isoflavones Promote Feminization and Enhance Growth of Juvenile Japanese Eel (Anguilla japonica)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Experimental Eels and Conditions
2.2. Preparation of the Experimental Diets
2.3. Sample Collection
2.4. Chemical Composition of the Diets and Whole Body of Eels
2.5. RNA Extraction and Quantitative Real-Time PCR Analysis
2.6. Isoflavone Analysis of the Experimental Diets
2.7. Histological Analysis
2.8. Calculation and Statistical Analysis
3. Results
3.1. Growth Performance of Eels
3.2. Chemical Composition of the Whole Body of Eels
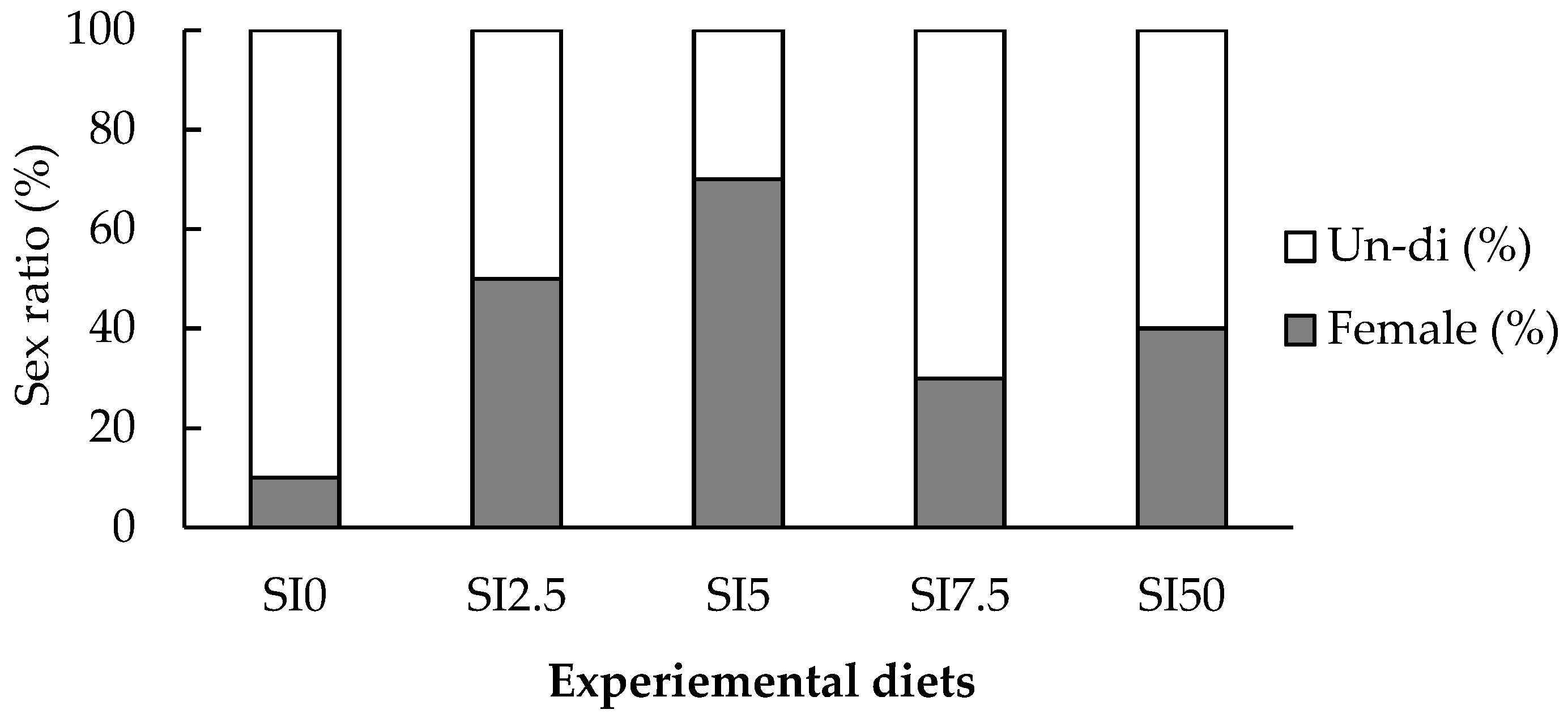
3.3. Sex Determination Ratio of Eels and Histological Analysis
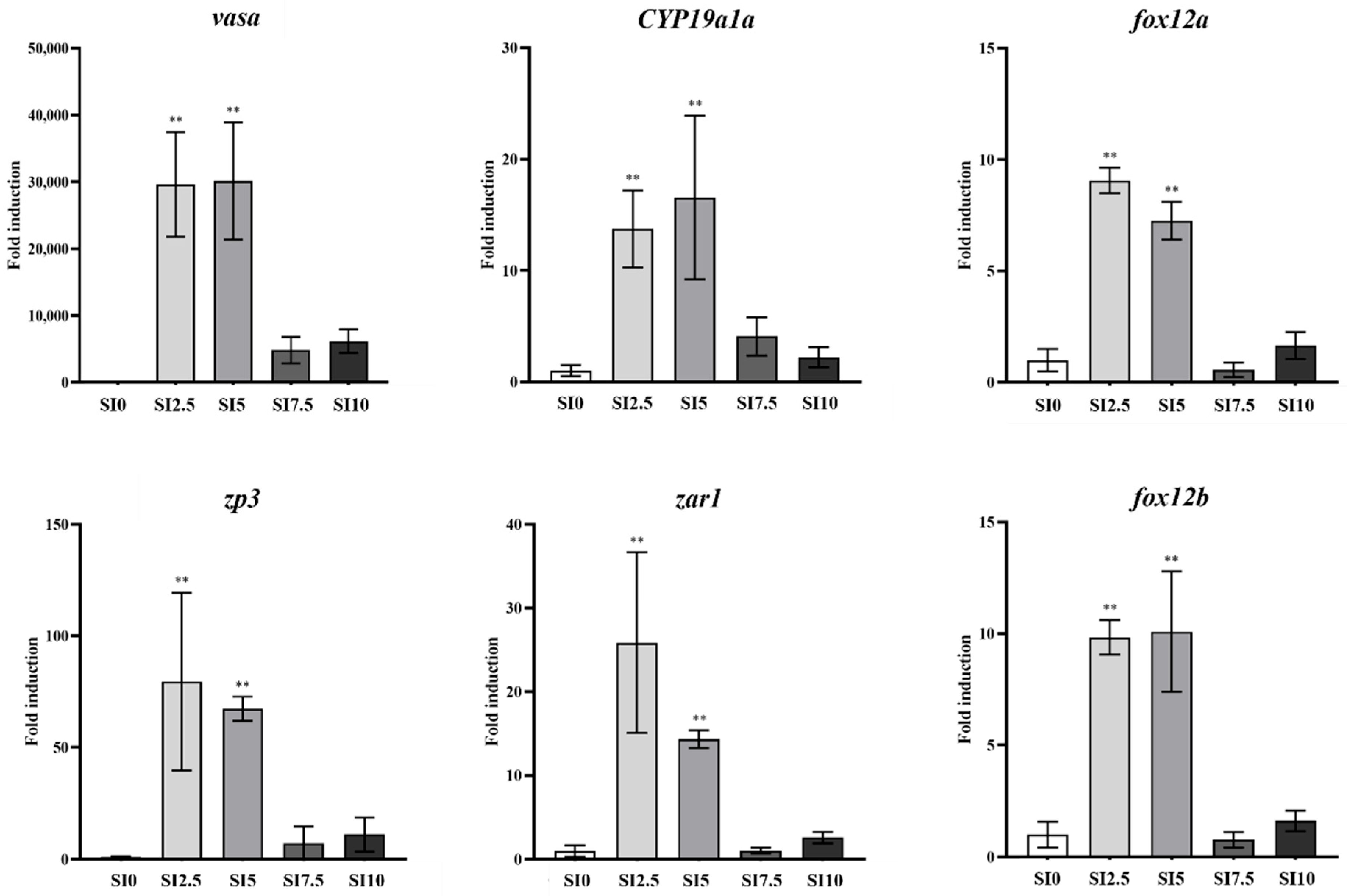
3.4. Expression of Sex-Specific Genes in the Gonads
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahn, J.C.; Chong, W.S.; Na, J.H.; Yun, H.B.; Shin, K.J.; Lee, K.W.; Park, J.T. An evaluation of major nutrients of four farmed freshwater eel species (Anguilla japonica, A. rostrata, A. bicolor pacifica and A. marmorata). Korean J. Fish. Aquat. Sci. 2015, 48, 44–50. [Google Scholar] [CrossRef]
- Hakoyama, H.; Faulks, L.; Rousseau, Y.; Kodama, S.; Okamoto, C.; Fujimori, H.; Sekino, M. Japanese Eel, Anguilla japonica. In Current Status of International Fishery Stocks in 2022; Fisheries Research Agency: Nagano, Japan, 2022; pp. 1–27. [Google Scholar]
- Hamidoghli, A.; Bae, J.; Won, S.; Lee, S.; Kim, D.J.; Bai, S.C. A review on Japanese eel (Anguilla japonica) aquaculture, with special emphasis on nutrition. Rev. Fish. Sci. Aquac. 2019, 27, 226–241. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, Y.; Dai, Y.; Gong, Y.; Yuan, Y. Development status and trends in the eel farming industry in Asia. N. Am. J. Aquac. 2022, 84, 3–17. [Google Scholar] [CrossRef]
- Korean Statistical Information Service (KOSIS). Available online: https://kosis.kr/statHtml/statHtml.do?sso=ok&returnurl=https%3A%2F%2Fkosis.kr%3A443%2FstatHtml%2FstatHtml.do%3Flist_id%3DK2_7%26obj_var_id%3D%26seqNo%3D%26tblId%3DDT_1EW0004%26vw_cd%3DMT_ZTITLE%26orgId%3D101%26path%3D%252FstatisticsList%252FstatisticsListIndex.do%26conn_path%3DMT_ZTITLE%26itm_id%3D%26lang_mode%3Dko%26scrId%3D%26 (accessed on 21 July 2025).
- Higuchi, M.; Mekuchi, M.; Hano, T.; Imaizumi, H. Trans-omics analyses revealed differences in hormonal and nutritional status between wild and cultured female Japanese eel (Anguilla japonica). PLoS ONE 2019, 14, e0209063. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Iwata, Y.; Suzuki, T.; Horiuchi, M.; Surugaya, R.; Ijiri, S.; Uchiyama, A.; Takano, R.; Hara, S.; Yazawa, T. Induce feminization of Japanese eel (Anguilla japonica). Int. J. Mol. Sci. 2023, 24, 396. [Google Scholar] [CrossRef]
- Ohta, H.; Kagawa, H.; Tanaka, H.; Okuzawa, K.; Iinuma, N.; Hirose, K. Artificial induction of maturation and fertilization in the Japanese eel, Anguilla japonica. Fish Physiol. Biochem. 1997, 17, 163–169. [Google Scholar] [CrossRef]
- Chai, Y.; Tosaka, R.; Abe, T.; Sago, K.; Sago, Y.; Hatanaka, E.; Ijiri, S.; Adachi, S. The relationship between the developmental stage of oocytes in various seasons and the quality of the egg obtained by artificial maturation in the feminized Japanese eel Anguilla japonica. Aquac. Sci. 2010, 58, 269–278. [Google Scholar]
- Chiba, H.; Iwatsuki, K.; Hayami, K.; Yamauchi, K. Effects of dietary Estradiol-17β on feminization, growth and body composition in the Japanese eel (Anguilla japonica). Comp. Biochem. Physiol. 1993, 106, 367–371. [Google Scholar]
- Satoh, H.; Nimura, Y.; Hibiya, T. Sex control of the Japanese eel by an estrogen (DES-Na) in feed. Nippon Suisan Gakkaishi 1992, 58, 1211–1218. [Google Scholar] [CrossRef]
- Yokouchi, K.; Kaneko, Y.; Kaifu, K.; Aoyama, J.; Uchida, K.; Tsukamoto, K. Demographic survey of the yellow-phase Japanese eel in Japan. Fish. Sci. 2014, 80, 543–554. [Google Scholar] [CrossRef]
- Hwang, J.A.; Park, J.; Kim, J.E.; Lee, J.H.; Kim, H.S. Estradiol-17β levels as a tool for sex determination in Farmed Anguilla japonica. Biochem. Biophys. Res. Commun. 2022, 634, 108–113. [Google Scholar] [CrossRef]
- Tzchori, I.; Degani, G.; Elisha, R.; Eliyahu, R.; Hurvitz, A.; Vaya, J.; Moav, B. The influence of phytoestrogens and oestradiol-17 β on growth and sex determination in the European eel (Anguilla anguilla). Aquac. Res. 2004, 35, 1213–1219. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, B.I.; Kim, K.K.; Kim, E.O.; Son, M.H.; Seong, K.B. Effects of Estradiol-17β on the feminization of Japanase eel, Anguilla japonica. J. Life Sci. 2013, 23, 998–1003. [Google Scholar] [CrossRef]
- Furuita, H.; Ohta, H.; Unuma, T.; Tanaka, H.; Kagawa, H.; Suzuki, N.; Yamamoto, T. Biochemical composition of eggs in relation to egg quality in the Japanese eel, Anguilla japonica. Fish Physiol. Biochem. 2003, 29, 37–46. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M.; Abdel-Aziz, E.S.H.; Abdel-Ghani, H.M. Effects of phytoestrogens on sex reversal of Nile tilapia (Oreochromis niloticus) larvae fed diets treated with 17α-Methyltestosterone. Aquaculture 2012, 360–361, 58–63. [Google Scholar] [CrossRef]
- Pelissero, C.; Le Menn, F.; Kaushick, S. Estrogenic effect of dietary soya bean meal on vitellogenesis in cultured Siberian sturgeon Acipenser baeri. Gen. Comp. Endocrinol. 1991, 83, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Farooq, S.; Bhat, N.; Dar, S.A.; Malik, M.A. Phytoestrogens in aquaculture: Friend or foe to fish growth and reproductive health? Blue Biotechnol. 2025, 2, 15. [Google Scholar] [CrossRef]
- Pastore, M.R.; Negrato, E.; Poltronieri, C.; Barion, G.; Messina, M.; Tulli, F.; Ballarin, C.; Maccatrozzo, L.; Radaelli, G.; Bertotto, D. Effects of dietary soy isoflavones on estrogenic activity, cortisol level, health and growth in rainbow trout, Oncorhynchus mykiss. Aquac. Res. 2018, 49, 1469–1479. [Google Scholar] [CrossRef]
- He, L.; Wang, H.; Li, E.; Huang, Q.; Wang, X.; Qiao, F.; Qin, C.; Qin, J.; Chen, L. Effects of soy isoflavones on growth performance, antioxidant capacity, non-specific immunity and lipid metabolism of juvenile Chinese mitten crab, Eriocheir sinensis. Aquaculture 2024, 581, 740470. [Google Scholar] [CrossRef]
- DiMaggio, M.A.; Kenter, L.W.; Breton, T.S.; Berlinsky, D.L. Effects of dietary genistein administration on growth, survival and sex determination in southern flounder, Paralichthys lethostigma. Aquac. Res. 2016, 47, 82–90. [Google Scholar] [CrossRef]
- Kiparissis, Y.; Balch, G.C.; Metcalfe, T.L.; Metcalfe, C.D. Effects of the isoflavones genistein and equol on the gonadal development of Japanese medaka (Oryzias latipes). Environ. Health Perspect. 2003, 111, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Fajkowska, M.; Adamek-Urbańska, D.; Ostaszewska, T.; Szczepkowski, M.; Rzepkowska, M. Effect of genistein, daidzein and coumestrol on sex-related genes expression in Russian sturgeon (Acipenser gueldenstaedtii). Aquaculture 2021, 530, 735872. [Google Scholar] [CrossRef]
- Turan, F.; Yigitarslan, K.D. Effect of immersion treatment of soybean isoflavones extract on sex reversal in the rainbow trout (Oncorhynchus mykiss, Walbaum, 1792). Biharean Biol. 2019, 13, 32–35. [Google Scholar]
- Hwang, J.A.; Park, J.S.; Jeong, H.S.; Hwang, S.D. Influence of 17α-methyltestosterone on morphological deformities and pigmentation development in juvenile Japanese eels, Anguilla japonica. Animals 2024, 14, 2684. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; AOAC: Arlington, VA, USA, 1990; p. 26. [Google Scholar]
- Jeong, H.S.; Kim, J.; Olowe, O.S.; Cho, S.H. Dietary optimum inclusion level of jack mackerel meal for olive flounder (Paralichthys olivaceus, Temminck & Schlegel, 1846). Aquaculture 2022, 559, 738432. [Google Scholar] [CrossRef]
- Jeng, S.R.; Wu, G.C.; Yueh, W.S.; Kuo, S.F.; Dufour, S.; Chang, C.F. Gonadal development and expression of sex-specific genes during sex differentiation in the Japanese eel. Gen. Comp. Endocrinol. 2018, 257, 74–85. [Google Scholar] [CrossRef]
- Horiuchi, M.; Hagihara, S.; Kume, M.; Chushi, D.; Hasegawa, Y.; Itakura, H.; Yamashita, Y.; Adachi, S.; Ijiri, S. Morphological and molecular gonadal sex differentiation in the wild Japanese eel Anguilla japonica. Cells 2022, 11, 1554. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, N.S.; Kim, S.K.; Lee, B.I.; Seong, K.B.; Kim, K.K. Effects of water temperature and estradiol-17b on the sex ratio and growth of the japanese eel Anguilla japonica. J. Life Sci. 2013, 23, 1454–1459. [Google Scholar] [CrossRef]
- Mai, K.; Zhang, Y.; Chen, W.; Xu, W.; Ai, Q.; Zhang, W. Effects of dietary soy isoflavones on feed intake, growth performance and digestibility in juvenile Japanese flounder (Paralichthys olivaceus). J. Ocean. Univ. China 2012, 11, 511–516. [Google Scholar] [CrossRef]
- Gu, M.; Gu, J.N.; Penn, M.; Bakke, A.M.; Lein, I.; Krogdahl, Å. Effects of diet supplementation of soya-saponins, isoflavones and phytosterols on Atlantic salmon (Salmo salar, L.) fry fed from start-feeding. Aquac. Nutr. 2015, 21, 604–613. [Google Scholar] [CrossRef]
- Cao, S.; Xiong, D.; Luo, W.; Tang, J.; Qu, F.; Zhou, Y.; He, Z.; Xie, S.; Liu, Z. Effects of dietary soy isoflavones on growth, antioxidant status, immune response and resistance of juvenile grass carp (Ctenopharyngodon idella) to Aeromonas hydrophila challenge. Aquac. Res. 2020, 51, 2472–2482. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Ge, X.; Niu, J.; Wang, J.; Wang, Y.; Chen, L.; Huang, Z.; Yu, W.; Tan, X. The Effects of dietary soybean isoflavones on growth, innate immune responses, hepatic antioxidant abilities and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 2015, 43, 158–166. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Xue, J.; Chu, W.; Hu, Y. Effects of dietary soy isoflavone and soy saponin on growth performance, intestinal structure, intestinal immunity and gut microbiota community on rice field eel (Monopterus albus). Aquaculture 2021, 537, 736506. [Google Scholar] [CrossRef]
- Jeong, H.S.; Cho, S.H.; Lee, K.W. Dietary substitution effect of Undaria pinnatifida with onion extract byproduct on growth, chemical composition and air exposure stress of juvenile abalone (Haliotis discus, Reeve 1846). Aquaculture 2020, 529, 735718. [Google Scholar] [CrossRef]
- Dai, Q.; Cho, S.H. Dietary inclusion effect of citrus peel by-product as an additive on the growth performance, body composition, and various stress resistance of juvenile abalone (Haliotis discus) compared to ethoxyquin. Aquac. Rep. 2022, 24, 101187. [Google Scholar] [CrossRef]
- Heinsbroek, L.T.N.; Van Hooff, P.L.A.; Swinkels, W.; Tanck, M.W.T.; Schrama, J.W.; Verreth, J.A.J. Effects of feed composition on life history developments in feed intake, metabolism, growth and body composition of European eel, Anguilla anguilla. Aquaculture 2007, 267, 175–187. [Google Scholar] [CrossRef]
- Ramseyer, L.J. Predicting whole-fish nitrogen content from fish wet weight using regression analysis. N. Am. J. Aquac. 2002, 64, 195–204. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Panagiotidou, M.; Papaioannou, N.; Pavlidis, M.; Nengas, I.; Mylonas, C. Effect of dietary lipid levels on growth, feed utilization, body composition and serum metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture 2010, 307, 65–70. [Google Scholar] [CrossRef]
- Tesch, F.W. The Eel, 5th ed.; Blackwell Publishing Science: Oxford, UK, 2003; p. 408. [Google Scholar]
- Uchida, D.; Yamashita, M.; Kitano, T.; Iguchi, T. An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 137, 11–20. [Google Scholar] [CrossRef]
- Guerrero-Estévez, S.; Moreno-Mendoza, N. Gonadal morphogenesis and sex differentiation in the viviparous fish Chapalichthys encaustus (Teleostei, Cyprinodontiformes, Goodeidae). J. Fish Biol. 2012, 80, 572–594. [Google Scholar] [CrossRef]
- Karube, M.; Fernandino, J.I.; Strobl, M.P.; Strussmann, C.A.; Yoshizaki, G.; Somoza, G.M.; Patino, R. Characterization and expression profile of the ovarian cytochrome p-450 aromatase (cyp19A1) gene during thermolabile sex determination in pejerrey, Odontesthes bonariensis. J. Exp. Zool. A Ecol. Genet. Physiol. 2007, 308, 625–636. [Google Scholar] [CrossRef]
- Robledo, D.; Ribas, L.; Cal, R.; Sánchez, L.; Piferrer, F.; Martínez, P.; Viñas, A. Gene expression analysis at the onset of sex differentiation in turbot (Scophthalmus maximus). BMC Genom. 2015, 16, 973. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Hara, S.; Horiuchi, M.; Ijiri, S.; Kitano, T. Gonadal expression profiles of sex-specific genes during early sexual differentiation in Japanese eel Anguilla japonica. Fish. Sci. 2021, 87, 203–209. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yamaguchi, S.; Hirai, T.; Kitano, T. Follicle-stimulating hormone signaling and Foxl2 are involved in transcriptional regulation of aromatase gene during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochem. Biophys. Res. Commun. 2007, 359, 935–940. [Google Scholar] [CrossRef]
- Geffroy, B.; Guilbaud, F.; Amilhat, E.; Beaulaton, L.; Vignon, M.; Huchet, E.; Rives, J.; Bobe, J.; Fostier, A.; Guiguen, Y. Sexually dimorphic gene expressions in eels: Useful markers for early sex assessment in a conservation context. Sci. Rep. 2016, 6, 34041. [Google Scholar] [CrossRef]
- Geffroy, B.; Bardonnet, A. Sex differentiation and sex determination in eel: Consequences for management. Fish Fish. 2016, 17, 375–398. [Google Scholar] [CrossRef]
- Amin, E.M. Gonad differentiation and early gonadal development of the European eel Anguilla anguilla L. in Egyptian waters. Arab. Gulf J. Sci. Res. 1997, 15, 175–186. [Google Scholar]
- Krueger, W.H.; Oliveira, K. Evidence for environmental sex determination in the American eel, Anguilla rostrata. Environ. Biol. Fishes 1999, 55, 381–389. [Google Scholar] [CrossRef]

| SI0 | SI2.5 | SI5 | SI7.5 | SI10 | |
|---|---|---|---|---|---|
| Ingredients (%) | |||||
| Jack mackerel meal | 75 | 75 | 75 | 75 | 75 |
| α-Starch | 23 | 20.5 | 18 | 15.5 | 13 |
| Soy isoflavone | 0 | 2.5 | 5 | 7.5 | 10 |
| Vitamin C | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Vitamin E | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Vitamin Mix 1 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Mineral Mix 2 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| MCP 3 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Chemical composition (%) | |||||
| Dry matter | 90.8 | 91.0 | 91.4 | 91.8 | 92.2 |
| Crude protein | 55.5 | 55.5 | 55.6 | 55.7 | 55.7 |
| Crude lipid | 6.9 | 7.0 | 7.1 | 7.3 | 7.4 |
| Ash | 10.3 | 10.4 | 10.4 | 10.5 | 10.5 |
| Isoflavone contents (%) | |||||
| Daidzin | 0.182 | 0.306 | 0.470 | 0.638 | |
| Daidzein | 0.807 | 1.582 | 1.811 | 2.123 | |
| Genistin | 0.041 | 0.070 | 0.104 | 0.140 | |
| Genistein | 0.001 | 0.001 | 0.002 | 0.002 | |
| Glycitein | 0.001 | 0.001 | 0.002 | 0.002 |
| Genes | Forward (5′→3′) | Reverse (5′→3′) | Reference |
|---|---|---|---|
| vasa | CGTGATTCAGGTGACCCAGTT | GCCCGTGGTGTTCAGGAA | [30] |
| cyp19a1a | CAGAGAAGTTGGATGATGCTGACT | GCTCCCCGTGGTTCTGAGC | [31] |
| foxl2a | CCACCCACTCCTATGCCCTAT | GCCGACAGTCCTTTGACGTT | [31] |
| zp3 | GAGTTGGTGGTGGTCAAAG | CATACTGTCCACCATACAGCC | [31] |
| zar1 | CATCTCTGGAACCAATAAGGTG | CCACCCTGTACGGATTGAAC | [31] |
| foxl2b | CATTCTGACGCTCACCACCTT | CTTGTTGCGTCTGGAGAGGAA | [31] |
| β-actin | AATCCACGAGACCACCTTCAACT | TGATCTCTTTCTGCATTCTGTCG | [31] |
| Parameters | SI0 | SI2.5 | SI5 | SI7.5 | SI10 | p-Value |
|---|---|---|---|---|---|---|
| Initial weight (g/fish) | 1.34 ± 0.10 | 1.33 ± 0.03 | 1.25 ± 0.03 | 1.23 ± 0.06 | 1.24 ± 0.03 | 0.524 |
| Final weight (g/fish) | 5.82 ± 0.41 b | 12.60 ± 3.14 a | 9.52 ± 0.79 ab | 7.59 ± 1.33 ab | 7.86 ± 0.43 ab | 0.043 |
| Total length (cm) | 20.0 ± 0.23 b | 25.3 ± 1.02 a | 25.5 ± 0.66 a | 22.9 ± 1.08 ab | 22.6 ± 0.44 ab | 0.003 |
| Survival (%) | 88.5 ± 1.75 | 90.8 ± 4.99 | 95.9 ± 1.60 | 85.3 ± 5.68 | 85.4 ± 1.18 | 0.386 |
| WG (g/fish) | 4.48 ± 0.48 b | 11.27 ± 3.1 a | 8.27 ± 0.77 ab | 6.36 ± 1.28 ab | 6.62 ± 0.42 ab | 0.045 |
| SGR (%/day) | 0.70 ± 0.06 b | 1.04 ± 0.12 a | 0.96 ± 0.04 ab | 0.85 ± 0.06 ab | 0.88 ± 0.02 ab | 0.040 |
| CF (g/cm3) | 0.12 ± 0.024 | 0.10 ± 0.002 | 0.10 ± 0.002 | 0.10 ± 0.002 | 0.10 ± 0.003 | 0.564 |
| VSI (%) | 4.27 ± 0.23 | 3.77 ± 0.24 | 3.95 ± 0.22 | 3.44 ± 0.21 | 3.43 ± 0.09 | 0.076 |
| HSI (%) | 1.39 ± 0.03 b | 1.37 ± 0.16 ab | 1.45 ± 0.08 a | 1.11 ± 0.11 ab | 0.96 ± 0.05 ab | 0.020 |
| Parameters | SI0 | SI2.5 | SI5 | SI7.5 | SI10 | p-Value |
|---|---|---|---|---|---|---|
| Moisture | 69.1 ± 0.23 | 68.6 ± 0.29 | 69.4 ± 0.21 | 69.9 ± 0.48 | 69.3 ± 0.20 | 0.125 |
| Crude protein | 16.4 ± 0.09 d | 18.1 ± 0.18 a | 17.6 ± 0.20 ab | 17.1 ± 0.14 bc | 16.7 ± 0.13 cd | <0.001 |
| Crude lipid | 11.7 ± 0.15 a | 10.9 ± 0.08 bc | 10.4 ± 0.21 c | 11.2 ± 0.05 ab | 11.3 ± 0.18 ab | 0.001 |
| Ash | 2.2 ± 0.04 | 2.3 ± 0.04 | 2.1 ± 0.05 | 2.0 ± 0.10 | 2.1 ± 0.0 | 0.127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.S.; Hwang, S.D.; Won, K.M.; Hwang, J.-a. Dietary Soy Isoflavones Promote Feminization and Enhance Growth of Juvenile Japanese Eel (Anguilla japonica). Animals 2025, 15, 2513. https://doi.org/10.3390/ani15172513
Jeong HS, Hwang SD, Won KM, Hwang J-a. Dietary Soy Isoflavones Promote Feminization and Enhance Growth of Juvenile Japanese Eel (Anguilla japonica). Animals. 2025; 15(17):2513. https://doi.org/10.3390/ani15172513
Chicago/Turabian StyleJeong, Hae Seung, Seong Don Hwang, Kyoung Mi Won, and Ju-ae Hwang. 2025. "Dietary Soy Isoflavones Promote Feminization and Enhance Growth of Juvenile Japanese Eel (Anguilla japonica)" Animals 15, no. 17: 2513. https://doi.org/10.3390/ani15172513
APA StyleJeong, H. S., Hwang, S. D., Won, K. M., & Hwang, J.-a. (2025). Dietary Soy Isoflavones Promote Feminization and Enhance Growth of Juvenile Japanese Eel (Anguilla japonica). Animals, 15(17), 2513. https://doi.org/10.3390/ani15172513






