Development a Recombinant Protein (CrFSH) as a Reproductive Hormone for the Assisted Reproduction of Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Vectors
2.2. Design and Construction of the CrFSH Gene
2.3. The Lentiviral Vector Transfection
2.4. Monoclonal Cell Isolation and Identification
2.5. Western Blot Analysis
2.6. Purification of CrFSH
2.7. The Cell Proliferation Rate Was Detected by the CCK-8 Method
2.8. cAMP-Based Enzyme Immunoassay
2.9. RNA Isolation and qRT-PCR
2.10. Statistical Analyses
3. Results
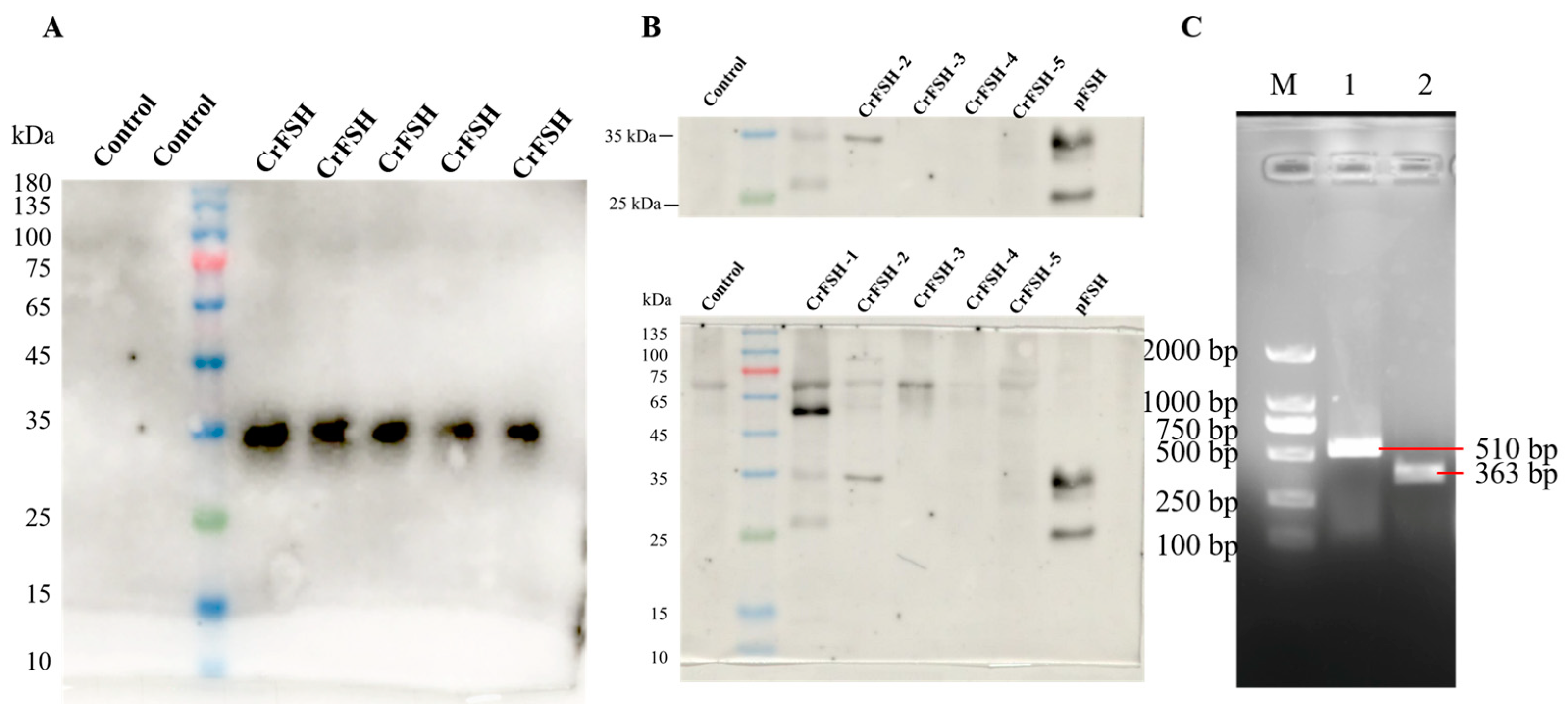
3.1. Construction and Identification of the rBFSH Expression Lentiviral Vector
3.2. Isolate the Genes of CrFSH-Carrying Monoclonal by the Limiting Dilution Method and Identify CrFSH
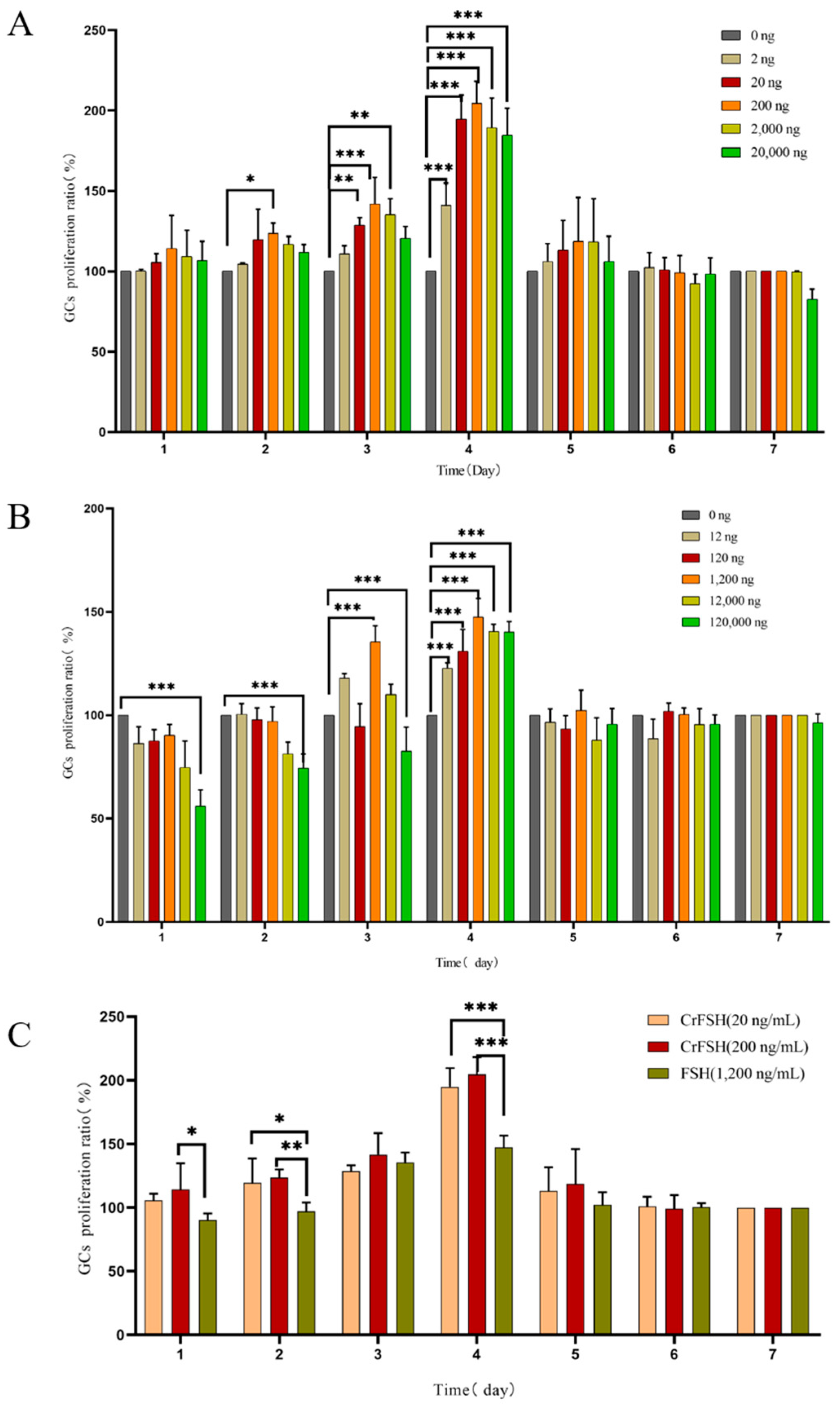
3.3. The GCs Proliferation Rate
3.4. cAMP Induction Assay
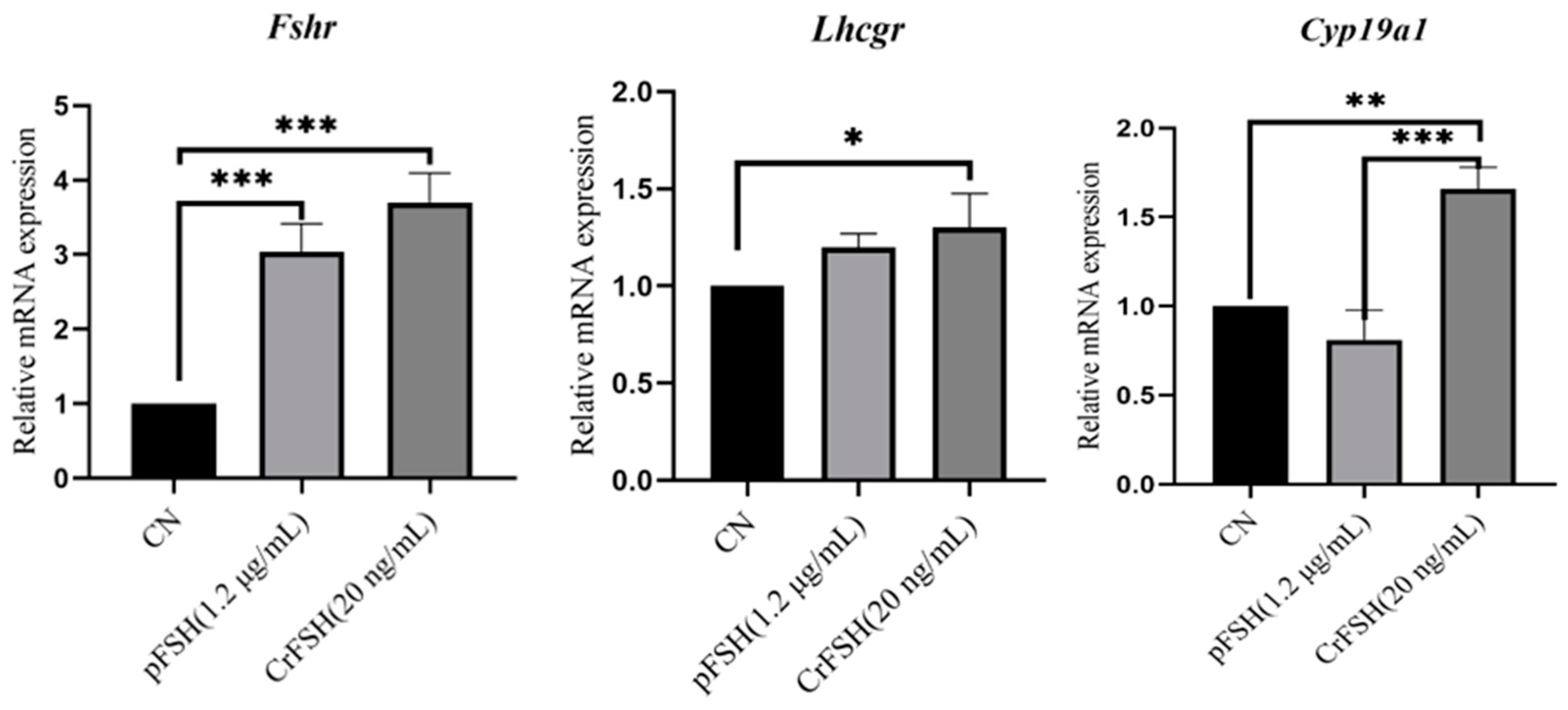
3.5. The Genes Expression in GCs Treated with pFSH and CrFSH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viana, J.H.M. 2022 Statistics of embryo production and transfer in domestic farm animals: The main trends for the world embryo industry still stand. Embryo Technol. Newsl. 2023, 41, 20–38. [Google Scholar]
- Pontes, J.H.; Silva, K.C.; Basso, A.C.; Rigo, A.G.; Ferreira, C.R.; Santos, G.M.; Sanches, B.V.; Porcionato, J.P.; Vieira, P.H.; Faifer, F.S.; et al. Large-scale in vitro embryo production and pregnancy rates from Bos taurus, Bos indicus, and indicus-taurus dairy cows using sexed sperm. Theriogenology 2010, 74, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Morotti, F.; Sanches, B.V.; Pontes, J.H.; Basso, A.C.; Siqueira, E.R.; Lisboa, L.A.; Seneda, M.M. Pregnancy rate and birth rate of calves from a large-scale IVF program using reverse-sorted semen in Bos indicus, Bos indicus-taurus, and Bos taurus cattle. Theriogenology 2014, 81, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Ferré, L.B.; Kjelland, M.E.; Strøbech, L.B.; Hyttel, P.; Mermillod, P.; Ross, P.J. Review: Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Anim. Int. J. Anim. Biosci. 2020, 14, 991–1004. [Google Scholar] [CrossRef]
- Shengli, L.; Kun, Y.; Changquan, L.; Yachun, W.; Zhonghua, W.; Jianxi, L.; Jiaqi, W.; Heping, Z.; Zhijun, C.; Weixuan, T. Report on the Development of Dairy Industry and Technology in 2024. Chin. J. Anim. Sci. 2025, 61, 13–22. [Google Scholar]
- Evans, R.D.; Wallace, M.; Garrick, D.J.; Dillon, P.; Berry, D.P.; Olori, V. Effects of calving age, breed fraction and month of calving on calving interval and survival across parities in Irish spring-calving dairy cows. Livest. Sci. 2006, 100, 216–230. [Google Scholar] [CrossRef]
- Gallo, L.; Berton, M.; Piazza, M.; Sturaro, E.; Schiavon, S.; Bittante, G. Environmental impact of Holstein Friesian and 3-breed crossbred dairy cows using a life cycle assessment approach applied to individual animals. J. Dairy Sci. 2024, 107, 4670–4684. [Google Scholar] [CrossRef]
- Hasler, J.F. Looking back at five decades of embryo technology in practice. Reprod. Fertil. Dev. 2023, 36, 1–15. [Google Scholar] [CrossRef]
- Viana, J.H.M. Development of the world farm animal embryo industry over the past 30 years. Theriogenology 2024, 230, 151–156. [Google Scholar] [CrossRef]
- Blondin, P.; Bousquet, D.; Twagiramungu, H.; Barnes, F.; Sirard, M.A. Manipulation of follicular development to produce developmentally competent bovine oocytes. Biol. Reprod. 2002, 66, 38–43. [Google Scholar] [CrossRef]
- Schuler, G. Equine chorionic gonadotrophin: Biology and veterinary use. Tierarztl. Praxis. Ausg. G Grosstiere/Nutztiere 2020, 48, 344–354. [Google Scholar] [CrossRef]
- Available online: https://www.animal-welfare-foundation.org (accessed on 20 October 2021).
- Sakaguchi, K. Optimization of ovum pick-up-in vitro fertilization and in vitro growth of immature oocytes in ruminants. J. Reprod. Dev. 2025, 71, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.; del Niño Jesus, A.; Josa, A.; Espinosa, E.; Gil, I. Use of follicle-stimulating hormone (FSH) to increase the in vitro fertilization (IVF) efficiency of mice. J. Assist. Reprod. Genet. 1995, 12, 738–743. [Google Scholar] [CrossRef]
- Sugano, M.; Watanabe, S. Use of Highly Purified Porcine FSH Preparation for Superovulation in Japanese Black Cattle. J. Vet. Med. Sci. 1997, 59, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Boime, I.; Ben-Menahem, D. Glycoprotein hormone structure-function and analog design. Recent Prog. Horm. Res. 1999, 54, 271–288; discussion 288–279. [Google Scholar]
- Ceaglio, N.; Gugliotta, A.; Tardivo, M.B.; Cravero, D.; Etcheverrigaray, M.; Kratje, R.; Oggero, M. Improvement of in vitro stability and pharmacokinetics of hIFN-α by fusing the carboxyl-terminal peptide of hCG β-subunit. J. Biotechnol. 2016, 221, 13–24. [Google Scholar] [CrossRef]
- Hershkovitz, O.; Bar-Ilan, A.; Guy, R.; Felikman, Y.; Moschcovich, L.; Hwa, V.; Rosenfeld, R.G.; Fima, E.; Hart, G. In Vitro and in Vivo Characterization of MOD-4023, a Long-Acting Carboxy-Terminal Peptide (CTP)-Modified Human Growth Hormone. Mol. Pharm. 2016, 13, 631–639. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Chen, Y.-Q.; Tan, J.-C.; Zhou, J.-A.; Chen, W.-N.; Jiang, T.; Zha, J.-Y.; Zeng, X.-K.; Li, B.-W.; Wei, L.-Q.; et al. Global fungal-host interactome mapping identifies host targets of candidalysin. Nat. Commun. 2024, 15, 1757. [Google Scholar] [CrossRef]
- Agwuegbo, U.T.; Colley, E.; Albert, A.P.; Butnev, V.Y.; Bousfield, G.R.; Jonas, K.C. Differential FSH Glycosylation Modulates FSHR Oligomerization and Subsequent cAMP Signaling. Front. Endocrinol. 2021, 12, 765727. [Google Scholar] [CrossRef]
- Khan, D.R.; Guillemette, C.; Sirard, M.A.; Richard, F.J. Transcriptomic analysis of cyclic AMP response in bovine cumulus cells. Physiol. Genom. 2015, 47, 432–442. [Google Scholar] [CrossRef]
- Pierce, J.G.; Parsons, T.F. Glycoprotein hormones: Structure and function. Annu. Rev. Biochem. 1981, 50, 465–495. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.C.; Kalmijn, J.; Hsueh, A.J. Growth hormone enhances follicle-stimulating hormone-induced differentiation of cultured rat granulosa cells. Endocrinology 1986, 118, 1401–1409. [Google Scholar] [CrossRef]
- Bouloux, P.M.; Handelsman, D.J.; Jockenhövel, F.; Nieschlag, E.; Rabinovici, J.; Frasa, W.L.; de Bie, J.J.; Voortman, G.; Itskovitz-Eldor, J. First human exposure to FSH-CTP in hypogonadotrophic hypogonadal males. Hum. Reprod. 2001, 16, 1592–1597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duijkers, I.J.; Klipping, C.; Boerrigter, P.J.; Machielsen, C.S.; De Bie, J.J.; Voortman, G. Single dose pharmacokinetics and effects on follicular growth and serum hormones of a long-acting recombinant FSH preparation (FSH-CTP) in healthy pituitary-suppressed females. Hum. Reprod. 2002, 17, 1987–1993. [Google Scholar] [CrossRef]
- Klein, J.; Lobel, L.; Pollak, S.; Lustbader, B.; Ogden, R.T.; Sauer, M.V.; Lustbader, J.W. Development and characterization of a long-acting recombinant hFSH agonist. Hum. Reprod. 2003, 18, 50–56. [Google Scholar] [CrossRef]
- Klein, J.; Lobel, L.; Pollak, S.; Ferin, M.; Xiao, E.; Sauer, M.; Lustbader, J.W. Pharmacokinetics and pharmacodynamics of single-chain recombinant human follicle-stimulating hormone containing the human chorionic gonadotropin carboxyterminal peptide in the rhesus monkey. Fertil. Steril. 2002, 77, 1248–1255. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Hsueh, A.J.; Lapolt, P.; Tsafriri, A.; Keene, J.L.; Boime, I. The biological role of the carboxyl-terminal extension of human chorionic gonadotropin [corrected] beta-subunit. Endocrinology 1990, 126, 376–383. [Google Scholar] [CrossRef]
- Karl, K.R.; Jimenez-Krassel, F.; Gibbings, E.; Ireland, J.L.H.; Clark, Z.L.; Tempelman, R.J.; Latham, K.E.; Ireland, J.J. Negative impact of high doses of follicle-stimulating hormone during superovulation on the ovulatory follicle function in small ovarian reserve dairy heifers. Biol. Reprod. 2021, 104, 695–705. [Google Scholar] [CrossRef]
- Khan, D.R.; Guillemette, C.; Sirard, M.A.; Richard, F.J. Characterization of FSH signalling networks in bovine cumulus cells: A perspective on oocyte competence acquisition. Mol. Hum. Reprod. 2015, 21, 688–701. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hwang, J.L.; Seow, K.M.; Huang, L.W.; Hsieh, B.C.; Chen, H.J.; Tzeng, C.R.; Bai, C.H. Effect of incubation with different concentrations and durations of FSH for in-vitro maturation of murine oocytes. Reprod. Biomed. Online 2011, 23, 111–117. [Google Scholar] [CrossRef]
- Caixeta, E.S.; Machado, M.F.; Ripamonte, P.; Price, C.; Buratini, J. Effects of FSH on the expression of receptors for oocyte-secreted factors and members of the EGF-like family during in vitro maturation in cattle. Reprod. Fertil. Dev. 2013, 25, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Clark, Z.L.; Thakur, M.; Leach, R.E.; Ireland, J.J. FSH dose is negatively correlated with number of oocytes retrieved: Analysis of a data set with ~650,000 ART cycles that previously identified an inverse relationship between FSH dose and live birth rate. J. Assist. Reprod. Genet. 2021, 38, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, F.; Manandhar, S.; Conti, M. FSH Regulates mRNA Translation in Mouse Oocytes and Promotes Developmental Competence. Endocrinology 2016, 157, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Barati, F.; Niasari-Naslaji, A.; Bolourchi, M.; Sarhaddi, F.; Razavi, K.; Naghzali, E.; Thatcher, W.W. Superovulatory response of Sistani cattle to three different doses of FSH during winter and summer. Theriogenology 2006, 66, 1149–1155. [Google Scholar] [CrossRef]
- Clark, Z.L.; Ruebel, M.L.; Schall, P.Z.; Karl, K.R.; Ireland, J.J.; Latham, K.E. Follicular Hyperstimulation Dysgenesis: New Explanation for Adverse Effects of Excessive FSH in Ovarian Stimulation. Endocrinology 2022, 163, bqac100. [Google Scholar] [CrossRef]
- Durai, L.; Vijayalakshmi, R.; Karunagaran, D. A novel reporter system for cyclic AMP mediated gene expression in mammalian cells based on synthetic transgene expression system. Eur. J. Pharmacol. 2019, 855, 56–64. [Google Scholar] [CrossRef]
- Minegishi, T.; Tano, M.; Igarashi, M.; Rokukawa, S.; Abe, Y.; Ibuki, Y.; Miyamoto, K. Expression of follicle-stimulating hormone receptor in human ovary. Eur. J. Clin. Investig. 1997, 27, 469–474. [Google Scholar] [CrossRef]
- Francis, S.H.; Corbin, J.D. Cyclic nucleotide-dependent protein kinases: Intracellular receptors for cAMP and cGMP action. Crit. Rev. Clin. Lab. Sci. 1999, 36, 275–328. [Google Scholar] [CrossRef]
- Corbin, J.D.; Francis, S.H. Cyclic GMP phosphodiesterase-5: Target of sildenafil. J. Biol. Chem. 1999, 274, 13729–13732. [Google Scholar] [CrossRef]
- Mann, O.N.; Kong, C.S.; Lucas, E.S.; Brosens, J.J.; Hanyaloglu, A.C.; Brighton, P.J. Expression and function of the luteinizing hormone choriogonadotropin receptor in human endometrial stromal cells. Sci. Rep. 2022, 12, 8624. [Google Scholar] [CrossRef]
- Bhartiya, D.; Patel, H. An overview of FSH-FSHR biology and explaining the existing conundrums. J. Ovarian Res. 2021, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Parakh, T.N.; Hernandez, J.A.; Grammer, J.C.; Weck, J.; Hunzicker-Dunn, M.; Zeleznik, A.J.; Nilson, J.H. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires β-catenin. Proc. Natl. Acad. Sci. USA 2006, 103, 12435–12440. [Google Scholar] [CrossRef] [PubMed]
- Daya, S. Updated meta-analysis of recombinant follicle-stimulating hormone (FSH) versus urinary FSH for ovarian stimulation in assisted reproduction. Fertil. Steril. 2002, 77, 711–714. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Zhang, H.; Liu, T.; Cui, Z.; Gao, K.; Lin, P.; Jin, Y. Development a Recombinant Protein (CrFSH) as a Reproductive Hormone for the Assisted Reproduction of Dairy Cows. Animals 2025, 15, 1430. https://doi.org/10.3390/ani15101430
Qin X, Zhang H, Liu T, Cui Z, Gao K, Lin P, Jin Y. Development a Recombinant Protein (CrFSH) as a Reproductive Hormone for the Assisted Reproduction of Dairy Cows. Animals. 2025; 15(10):1430. https://doi.org/10.3390/ani15101430
Chicago/Turabian StyleQin, Xinxi, Haisen Zhang, Tian Liu, Zhenliang Cui, Kangkang Gao, Pengfei Lin, and Yaping Jin. 2025. "Development a Recombinant Protein (CrFSH) as a Reproductive Hormone for the Assisted Reproduction of Dairy Cows" Animals 15, no. 10: 1430. https://doi.org/10.3390/ani15101430
APA StyleQin, X., Zhang, H., Liu, T., Cui, Z., Gao, K., Lin, P., & Jin, Y. (2025). Development a Recombinant Protein (CrFSH) as a Reproductive Hormone for the Assisted Reproduction of Dairy Cows. Animals, 15(10), 1430. https://doi.org/10.3390/ani15101430






