A Randomised-Controlled Study Demonstrates That Diet Can Contribute to the Clinical Management of Feline Atopic Skin Syndrome (FASS)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Initial Clinical Scoring at Inclusion
2.3. Follow-Up Consultations and Drop-Outs
2.4. Diets
2.5. Outcomes Measures
2.6. Statistical Methods
3. Results
3.1. Study Population
3.2. Data Collection from the Cohort
3.3. Evolution of Scores
3.3.1. General Evolution
3.3.2. FeDESI
3.3.3. VAScat
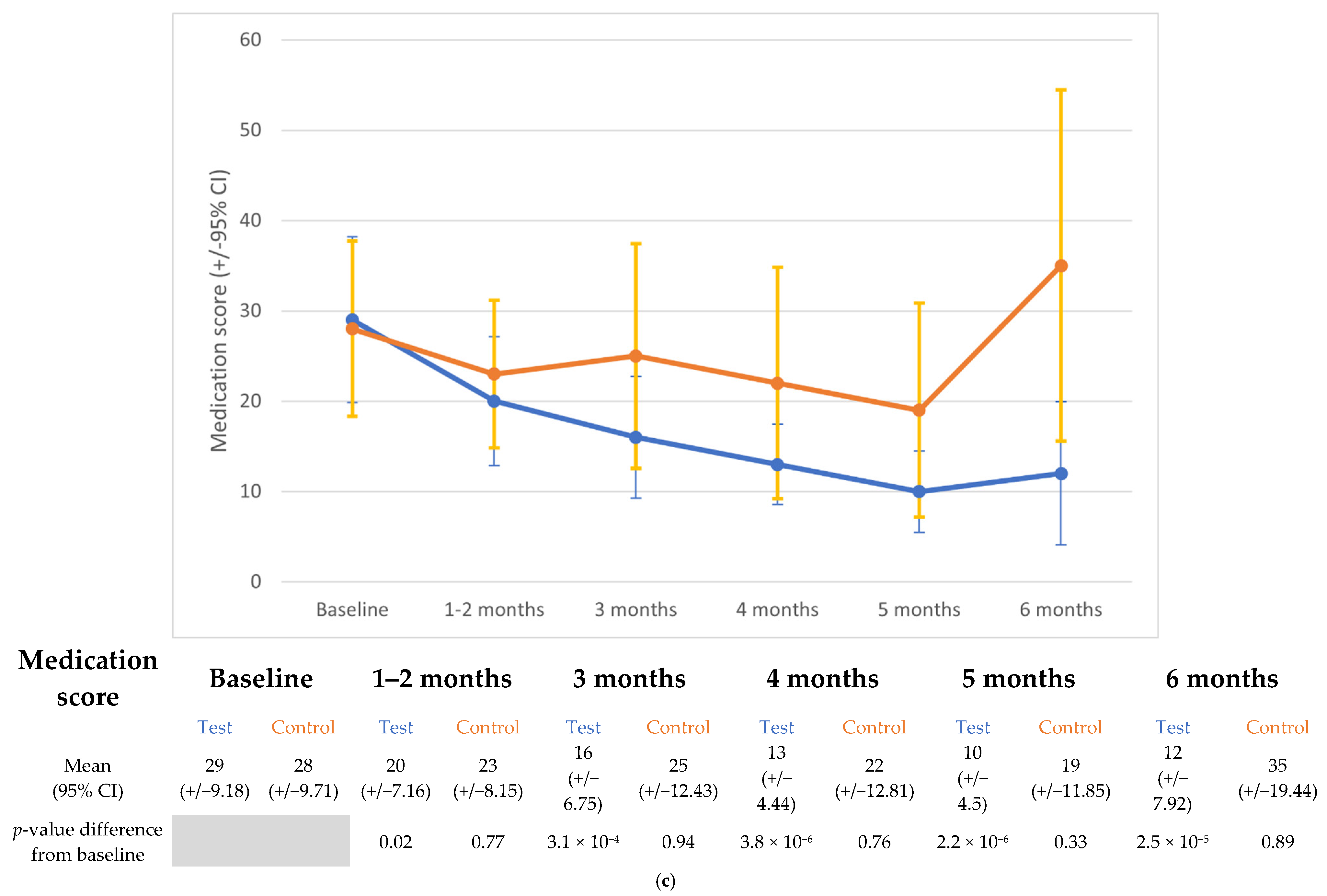
3.4. Medication Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halliwell, R.; Pucheu-Haston, C.M.; Olivry, T.; Prost, C.; Jackson, H.; Banovic, F.; Nuttall, T.; Santoro, D.; Bizikova, P.; Mueller, R.S. Feline allergic diseases: Introduction and proposed nomenclature. Vet. Dermatol. 2021, 32, 8-e2. [Google Scholar] [CrossRef]
- Hobi, S.; Linek, M.; Marignac, G.; Olivry, T.; Beco, L.; Nett, C.; Fontaine, J.; Roosje, P.; Bergvall, K.; Belova, S.; et al. Clinical characteristics and causes of pruritus in cats: A multicentre study on feline hypersensitivity-associated dermatoses. Vet. Dermatol. 2011, 22, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Favrot, C.; Steffan, J.; Seewald, W.; Hobi, S.; Linek, M.; Marignac, G.; Olivry, T.; Beco, L.; Nett, C.; Fontaine, J.; et al. Establishment of diagnostic criteria for feline non-flea-induced hypersensitivity dermatitis. Vet. Dermatol. 2011, 23, 45-e11. [Google Scholar] [CrossRef]
- Santoro, D.; Pucheu-Haston, C.M.; Prost, C.; Mueller, R.S.; Jackson, H. Clinical signs and diagnosis of feline atopic syndrome: Detailed guidelines for a correct diagnosis. Vet. Dermatol. 2021, 32, 26-e6. [Google Scholar] [CrossRef] [PubMed]
- Ravens, P.A.; Xu, B.J.; Vogelnest, L.J. Feline atopic dermatitis: A retrospective study of 45 cases (2001–2012). Vet. Dermatol. 2014, 25, 95–102. [Google Scholar] [CrossRef]
- Scott, D.W.; Miller, W.H., Jr. Feline atopic dermatitis: A retrospective study of 194 cases (1988–2003). Jpn. J. Vet. Dermatol. 2013, 19, 135–147. [Google Scholar] [CrossRef]
- Vapalahti, K.; Virtala, A.-M.; Joensuu, T.A.; Tiira, K.; Tähtinen, J.; Lohi, H. Health and behavioral survey of over 8000 Finnish cats. Front. Vet. Sci. 2016, 3, 70. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, R.; Banovic, F.; Mueller, R.S.; Olivry, T. Immunopathogenesis of the feline atopic syndrome. Vet. Dermatol. 2021, 32, 13-e4. [Google Scholar] [CrossRef]
- Bajwa, J. Atopic dermatitis in cats. Can. Vet. J. 2018, 59, 311–313. [Google Scholar]
- Nuttall, T.J.; Steen, R.V.; Cawood, M.I.; Houghton, C.A. Feline dermatitis extent and severity index: A pilot study. Vet Dermatol. 2004, 15 (Suppl. S1), 36. [Google Scholar] [CrossRef]
- Schmidt, V.; Buckley, L.M.; McEwan, N.A.; Rème, C.A.; Nuttall, T.J. Efficacy of a 0.0584% hydrocortisone aceponate spray in presumed feline allergic dermatitis: An open label pilot study. Vet. Dermatol. 2011, 23, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; Sartori, R.; Schievano, C.; Borio, S. Development and validation of an owner-assessed Visual Analog Scale for feline pruritus severity scoring (VAScat). Vet. Dermatol. 2022, 33, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Tarpataki, N.; Leidi, F.; Rostaher, A.; Favrot, C. An open study on the efficacy of a recombinant Der f 2 (Dermatophagoides farinae) immunotherapy in atopic dogs in Hungary and Switzerland. Vet. Dermatol. 2018, 29, 337-e118. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Atopic Dermatitis. J. Nippon. Med. Sch. 2021, 88, 171–177. [Google Scholar] [CrossRef]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef]
- Watson, A.; Rostaher, A.; Fischer, N.M.; Favrot, C. A novel therapeutic diet can significantly reduce the medication score and pruritus of dogs with atopic dermatitis during a nine-month Controlled study. Vet. Dermatol. 2022, 33, 55-e18. [Google Scholar] [CrossRef]
- de Santiago, M.S.; Arribas, J.L.G.; Llamas, Y.M.; Becvarova, I.; Meyer, H. Randomized, double-blind, placebo-Controlled clinical trial measuring the effect of a dietetic food on dermatologic scoring and pruritus in dogs with atopic dermatitis. BMC Vet. Res. 2021, 17, 354. [Google Scholar] [CrossRef]
- Harvey, R.G. Management of feline miliary dermatitis by supplementing the diet with essential fatty acids. Vet. Rec. 1991, 128, 326–329. [Google Scholar] [CrossRef]
- Harvey, R.G. Effect of varying proportions of evening primrose oil and fish oil on cats with crusting dermatosis (‘miliary dermatitis’). Vet. Rec. 1993, 133, 208–211, Erratum in: Vet. Rec. 1993, 133, 338. [Google Scholar] [CrossRef]
- Noli, C.; Della Valle, M.F.; Miolo, A.; Medori, C.; Schievano, C.; Skinalia Clinical Research Group. Effect of dietary supplementation with ultramicronized palmitoylethanolamide in maintaining remission in cats with non-flea hypersensitivity dermatitis: A double-blind, multicentre, randomized, placebo-Controlled study. Vet. Dermatol. 2019, 30, 387-e117. [Google Scholar] [CrossRef]
- Lesponne, I.; Boutigny, L.; Rochon, J.; Laxalde, J.; Langon, X. Nutritionally based improvement of cats’ skin & coat health in Non-Flea, Non-Food-Induced Hypersensitive Dermatitis. In Proceedings of the BSAVA Congress Proceedings, Birmingham, UK, 2–5 April 2020; p. 448. [Google Scholar]
- NRC (National Research Council). Nutrient Requirements of Dogs and Cats; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. R Core Team. 2021. Available online: https://www.R-project.org/ (accessed on 15 February 2024).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 15 February 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.8.2. 2022. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 15 February 2024).
- Mueller, R.S.; Nuttall, T.; Prost, C.; Schulz, B.; Bizikova, P. Treatment of the feline atopic syndrome—A systematic review. Vet. Dermatol. 2021, 32, 43-e8. [Google Scholar] [CrossRef]
- Silva, P.T.R.F.; Coura, F.M.; Costa-Val, A.P. Caregiver Burden in Small Animal Clinics: A Comparative Analysis of Dermatological and Oncological Cases. Animals 2024, 14, 276. [Google Scholar] [CrossRef]
- Mueller, R.S. A systematic review of allergen immunotherapy, a successful therapy for canine atopic dermatitis and feline atopic skin syndrome. J. Am. Vet. Med. Assoc. 2023, 261 (Suppl. S1), S30–S35. [Google Scholar] [CrossRef] [PubMed]
- Taglinger, K.; Day, M.J.; Foster, A.P. Characterization of inflammatory cell infiltration in feline allergic skin disease. J. Comp. Pathol. 2007, 137, 211–223. [Google Scholar] [CrossRef]
- Roosje, P.J.; Whitaker-Menezes, D.; Goldschmidt, M.H.; Moore, P.F.; Willemse, T.; Murphy, G.F. Feline atopic dermatitis. A model for Langerhans cell participation in disease pathogenesis. Am. J. Pathol. 1997, 151, 927–932. [Google Scholar] [PubMed]
- Roosje, P.J.; Dean, G.A.; Willemse, T.; Rutten, V.P.; Thepen, T. Interleukin 4-producing CD4+ T cells in the skin of cats with allergic dermatitis. Vet. Pathol. 2002, 39, 228–233. [Google Scholar] [CrossRef]
- Meason-Smith, C.; Diesel, A.; Patterson, A.P.; Older, C.E.; Johnson, T.J.; Mansell, J.M.; Suchodolski, J.S.; Rodrigues Hoffmann, A. Characterization of the cutaneous mycobiota in healthy and allergic cats using next generation sequencing. Vet. Dermatol. 2017, 28, 71-e17. [Google Scholar] [CrossRef] [PubMed]
- Older, C.E.; Hoffmann, A.R.; Diesel, A.B. The feline skin microbiome: Interrelationship between health and disease. J. Feline Med. Surg. 2023, 25, 1098612X231180231. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Product-evaluation registry of Meriva®, a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010, 52 (Suppl. S1), 55–62. [Google Scholar]
- Saevik, B.K.; Bergvall, K.; Holm, B.R.; Saijonmaa-Koulumies, L.E.; Hedhammar, A.; Larsen, S.; Kristensen, F. A randomized, Controlled study to evaluate the steroid sparing effect of essential fatty acid supplementation in the treatment of canine atopic dermatitis. Vet. Dermatol. 2004, 15, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Plevnik Kapun, A.; Salobir, J.; Levart, A.; Tavčar Kalcher, G.; Nemec Svete, A.; Kotnik, T. Vitamin E supplementation in canine atopic dermatitis: Improvement of clinical signs and effects on oxidative stress markers. Vet. Rec. 2014, 175, 560. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Tremblay, A.; Morin, S.; Martin, C.; Julien, P.; Fradette, J.; Flamand, N.; Pouliot, R. α-Linolenic acid and linoleic acid modulate the lipidome and the skin barrier of a tissue-engineered skin model. Acta Biomater. 2022, 140, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Janeke, G.; Siefken, W.; Carstensen, S.; Springmann, G.; Bleck, O.; Steinhart, H.; Höger, P.; Wittern, K.P.; Wenck, H.; Stäb, F.; et al. Role of taurine accumulation in keratinocyte hydration. J. Investig. Dermatol. 2003, 121, 354–361. [Google Scholar]

| Medication/Treatment | Dose/Schedule | Daily Score |
|---|---|---|
| No concurrent medication | 0 | |
| Prednisolone/Dexamethasone | ≥1 mg/kg/d | 40 |
| 0.5–1 mg/kg/d | 30 | |
| 0.2–0.5 mg/kg/d | 20 | |
| ≤0.2 mg/kg/d | 10 | |
| Atopica (7 mg/kg bwt) | daily | 30 |
| every other day | 20 | |
| every third day | 10 | |
| every fourth day | 5 | |
| Apoquel (1 mg/kg) | twice daily | 40 |
| daily | 30 | |
| every other day | 20 | |
| every third day | 10 | |
| Antihistamine | 10 | |
| Antibiotics (>21 days) * | 20 | |
| Antibiotics (<21 days) * | 10 | |
| Topical steroids | 5 | |
| Topical hydrocortisone | 5 | |
| Shampoo | 5 | |
| Ear wash | 5 | |
| Ear drops | 5 |
| Diet Component | Units | Test | Control |
|---|---|---|---|
| Dry Matter | % | 94.8 | 94.7 |
| Moisture | % | 5.2 | 5.3 |
| Protein | % | 33.5 | 33.2 |
| Fat | % | 22 | 22.5 |
| Ash | % | 6.7 | 6.6 |
| Total Fibre | % | 9.7 | 10.1 |
| Linoleic Acid | % | 4.4 | 2.9 |
| EPA/DHA | % | 0.55 | 0.034 |
| Vitamin E | mg/kg | 1120 | 536 |
| Vitamin C | mg/kg | 396 | 338 |
| Taurine | mg/kg | 4500 | 2600 |
| Lutein | mg/kg | 13 | 9 |
| Curcuma | mg/kg | 1425 | 0 |
| Clinical Presentation | Test (n) | Control (n) |
|---|---|---|
| EGC | 3 | 2 |
| HNP | 6 | 4 |
| SIAH | 2 | 0 |
| MD | 0 | 0 |
| EGC/SIAH | 2 | 3 |
| HNP/SIAH | 2 | 0 |
| SIAH/MD | 0 | 1 |
| Outcome | Emmean (95% CI); p-Values and Effect Sizes (95%) for Differences from Baseline | ||||||
|---|---|---|---|---|---|---|---|
| Test | Control | ||||||
| Baseline | 3 mo | 6 mo | Baseline | 3 mo | 6 mo | ||
| FeDESI | Emmean (95%CI) | 17.35 (9.95–24.76) | 7.92 (0.32–15.52) | 4.94 (−4.04–13.91) | 28.12 (18.32–37.92) | 23.88 (14.07–33.69) | 14.89 (2.41–27.34) |
| p-value | 0.037 | 0.02 | 0.64 | 0.09 | |||
| Effect size (95%CI) | 1.01 (0.19–1.83) | 1.33 (0.34–2.32) | 0.46 (0.57–1.48) | 1.42 (0.06–2.78) | |||
| VAScat | Emmean (95%CI) | 7.2 (6.21–8.19) | 4.35 (3.33–5.37) | 3.81 (2.61–5.01) | 5.89 (4.61–7.16) | 5.56 (4.28–6.82) | 5.35 (3.81–6.9) |
| p-value | 1.5 × 10−4 | 5.5 × 10−5 | 0.9 | 0.82 | |||
| Effect size 95%CI) | 1.71 (0.88–2.53) | 2.03 (1.1–2.97) | 0.2 (−0.76–1.16) | 0.32 (−0.77–1.41) | |||
| Outcome | Emmean (95%CI); p-Values and Effect Sizes (95%) for Differences from Baseline | ||||||
|---|---|---|---|---|---|---|---|
| Test Group | |||||||
| Baseline | 2mo | 3mo | 4mo | 5mo | 6mo | ||
| Medication score | Emmean (95%CI) | 29.06 (21.53–36.6) | 19.37 (11.85–26.9) | 15.44 (7.81–23.07) | 11.77 (4.03–19.49) | 10.95 (3.11–18.81) | 11.86 (3.74–19.99) |
| p-value | 0.02 | 3 × 10−4 | 3.8 × 10−6 | 2.2 × 10−6 | 2.5 × 10−5 | ||
| Effect size (95%CI) | 1.15 (0.43–1.86) | 1.61 (0.86–2.36) | 2.04 (1.27–2.82) | 2.14 (1.34–2.94) | 2.03 (1.2–2.87) | ||
| Control group | |||||||
| Baseline | 2mo | 3mo | 4mo | 5mo | 6mo | ||
| Emmean (95%CI) | 28.00 (18.48–37.52) | 23.00 (13.48–32.52) | 24.5 (14.98–34.02) | 22.51 (12.58–32.45)) | 19.33 (9.1–29.57) | 32.78 (22.18–43.38) | |
| p-value | 0.77 | 0.94 | 0.76 | 0.33 | 0.89 | ||
| Effect size (95%CI) | 0.59 (−0.3–1.48) | 0.41 (−0.47–1.3) | 0.65 (−0.31–1.61) | 1.02 (0.02–2.03) | 0.57 (−1.62–0.49) | ||
| Diet | Test | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Timepoint (mo) | 2 | 3 | 4 | 5 | 6 | 2 | 3 | 4 | 5 | 6 |
| FeDESI | 47% | 50% | 30% | 67% | ||||||
| VAScat | 40% | 45% | 10% | 14% | ||||||
| Medication score | 20% | 53% | 57% | 69% | 73% | 20% | 20% | 50% | 29% | 17% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watson, A.; Laxalde, J.; Brément, T.; Drevon-Gaillot, E.V.; Mosca, M.; Maina, E.; Langon, X. A Randomised-Controlled Study Demonstrates That Diet Can Contribute to the Clinical Management of Feline Atopic Skin Syndrome (FASS). Animals 2025, 15, 1429. https://doi.org/10.3390/ani15101429
Watson A, Laxalde J, Brément T, Drevon-Gaillot EV, Mosca M, Maina E, Langon X. A Randomised-Controlled Study Demonstrates That Diet Can Contribute to the Clinical Management of Feline Atopic Skin Syndrome (FASS). Animals. 2025; 15(10):1429. https://doi.org/10.3390/ani15101429
Chicago/Turabian StyleWatson, Adrian, Jeremy Laxalde, Thomas Brément, Emilie Vidémont Drevon-Gaillot, Marion Mosca, Elisa Maina, and Xavier Langon. 2025. "A Randomised-Controlled Study Demonstrates That Diet Can Contribute to the Clinical Management of Feline Atopic Skin Syndrome (FASS)" Animals 15, no. 10: 1429. https://doi.org/10.3390/ani15101429
APA StyleWatson, A., Laxalde, J., Brément, T., Drevon-Gaillot, E. V., Mosca, M., Maina, E., & Langon, X. (2025). A Randomised-Controlled Study Demonstrates That Diet Can Contribute to the Clinical Management of Feline Atopic Skin Syndrome (FASS). Animals, 15(10), 1429. https://doi.org/10.3390/ani15101429





