Dynamics of Bacterial Communities and Their Relationship with Nutrients in a Full-Scale Shrimp Recirculating Aquaculture System in Brackish Water
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture System Setup
2.2. Sample Collection and Water Quality Analysis
2.3. Processing of DNA Sequencing Data
2.4. Statistical Analysis
3. Results
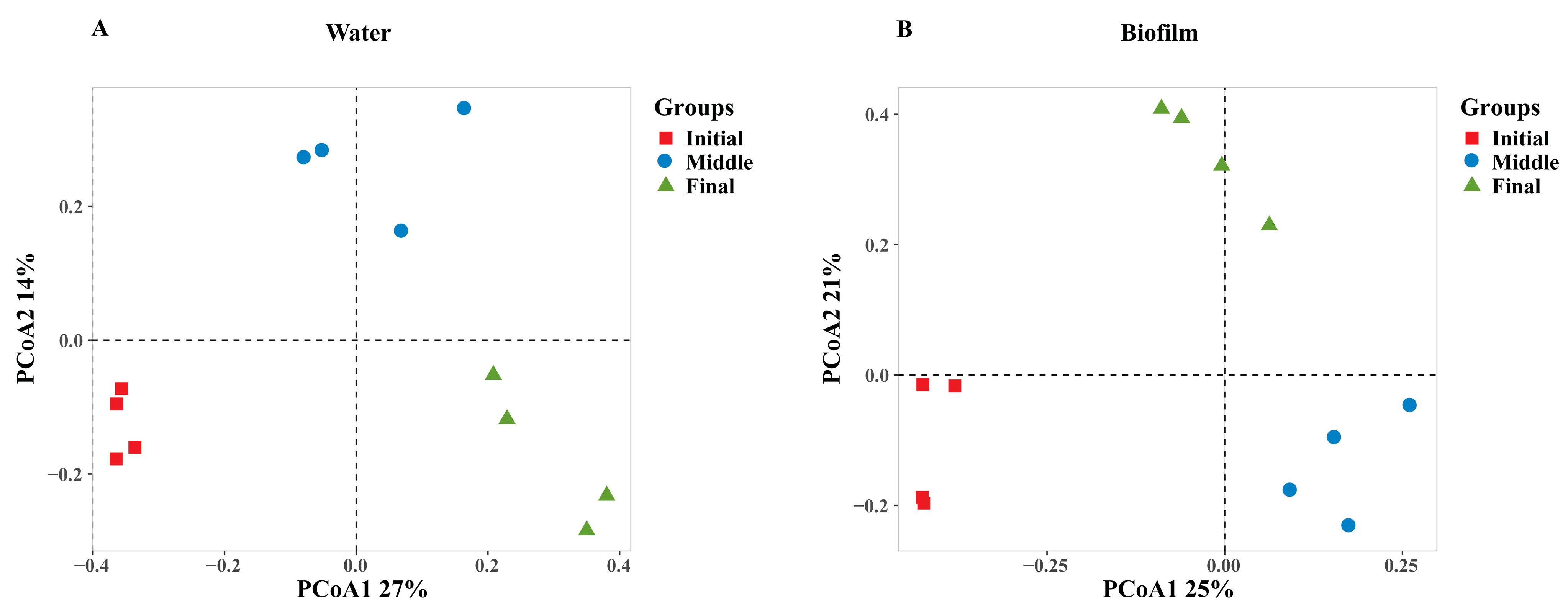
3.1. Temporal Change in Bacterial Communities in Water and Biofilm Environments
3.2. Bacterial Community Composition in Water and Biofilms
3.3. Shrimp Growth Performance
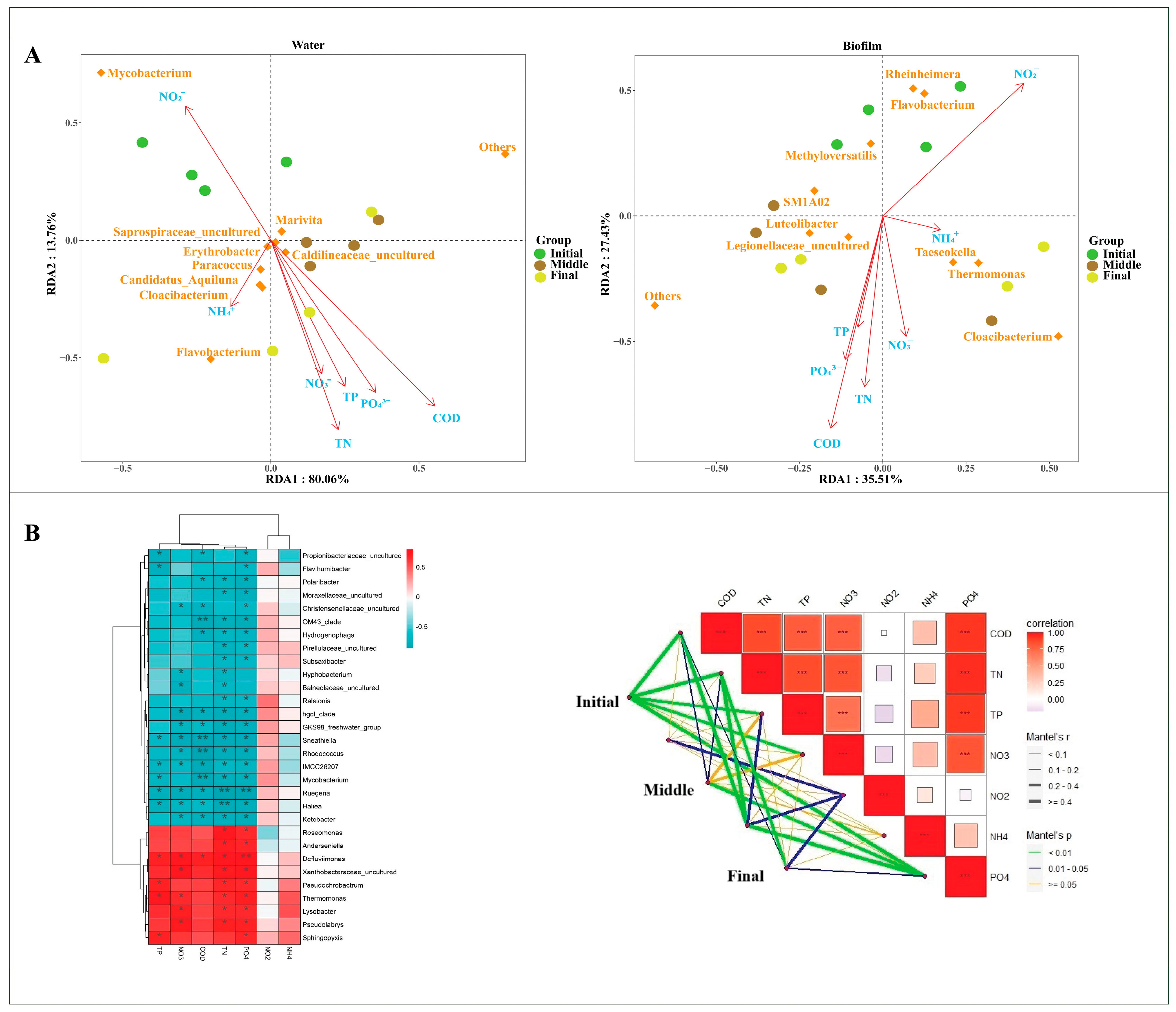
3.4. Relationships Between Microbial Communities and Environmental Factors
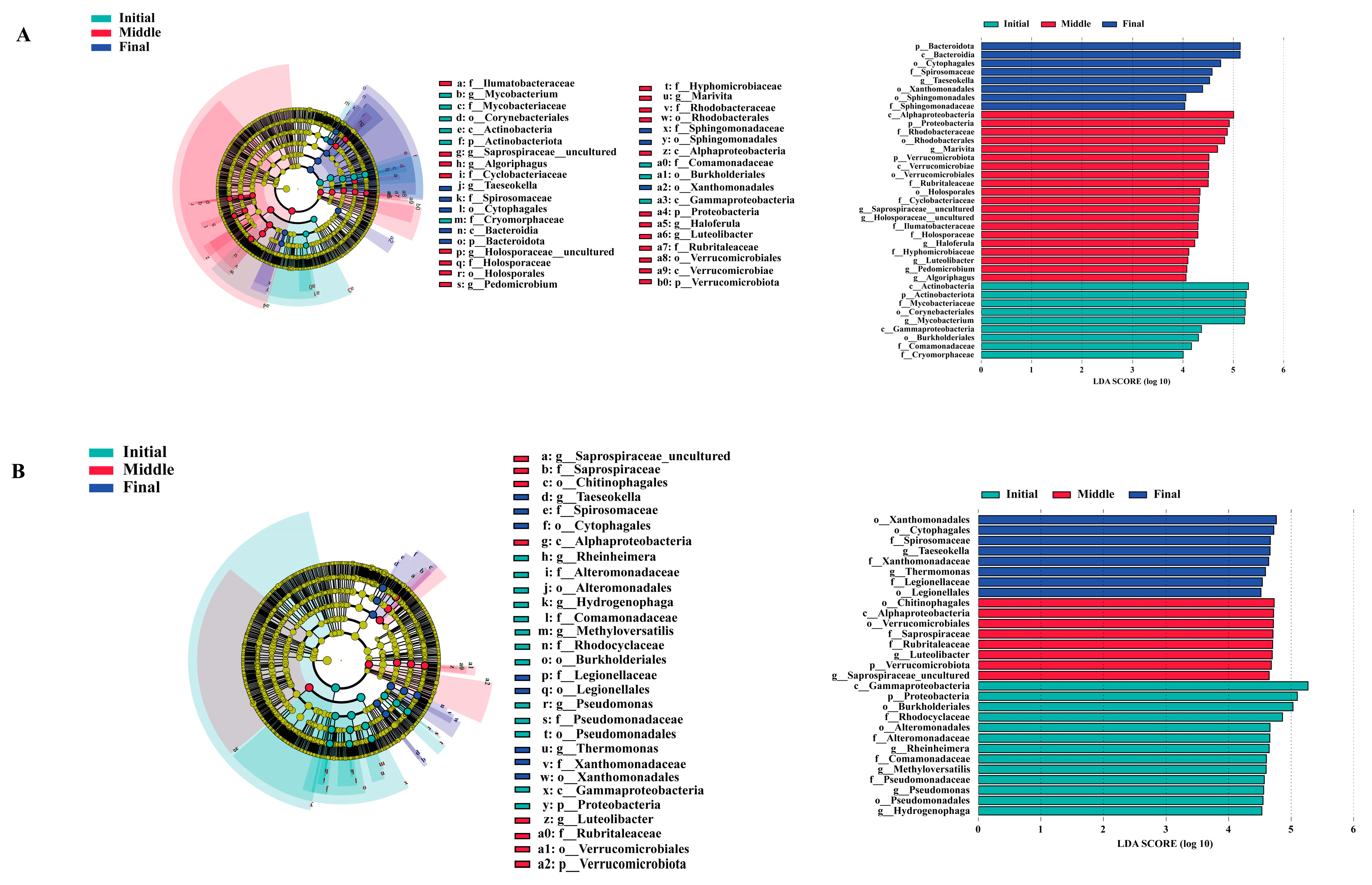
3.5. Identification of Biomarkers
4. Discussion
4.1. Temporal Dynamics of Microbial Communities
4.2. Association of Bacterial Community with Physiochemical Parameters
4.3. Identification of Biomarkers and Function Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Initial | Final | |||||||
|---|---|---|---|---|---|---|---|---|
| Stocking Density (ind m−3) | Weight (g) | Number of Shrimps per Tank | Total Weight (kg) per Tank | Stocking Density (kg m−3) | Weight (g) | Number of Shrimps per Tank | Total Weight (kg) per Tank | |
| RAS | 658 ± 0.0032 | 0.02 ± 0.005 | 25,000 | 0.5 ± 0.125 | 9.14 ± 1.53 | 13.89 ± 2.71 | 18,053 | 250.7 ± 48.92 |
| Ingredients | Postlarvae (Length < 3 cm) | Juveniles (Length 3~6 cm) | Adults (Length > 6 cm) |
|---|---|---|---|
| Crude protein | 48.0 | 43.0 | 43.0 |
| Crude fat | 6.0 | 6.0 | 6.0 |
| Crude fibre | 4.0 | 6.0 | 6.0 |
| Crude ash | 17.0 | 18.0 | 18.0 |
| Total phosphate | 1.4 | 1.0 | 1.0 |
| Lysine | 3.2 | 2.3 | 2.4 |
| water | 10.0 | 12.0 | 12.0 |
| Parameters | Value | Remarks |
|---|---|---|
| Daily Growth Rate | 0.154 g | Per day |
| Survival Rate | 72.21% | At harvesting |
| Specific Growth Rate (SGR) | 7.27% | Per day based on log weight change |
| Initial Biomass | 0.5 kg | Per tank |
| Final Biomass | 250.7 kg | Per tank |
| Weight Gain (Biomass) | 250.2 kg | Per tank |
| Total Feed Given | 452.16 kg | Estimated over 90 days |
| Feed Conversion Ratio (FCR) | 1.81 | Feed used per kg weight gain |
| Protein in Feed | 48% | Commercial pellet composition |
| Protein Intake | 216.93 kg | 48% of total feed |
| Protein Efficiency Ratio (PER) | 1.15 | Weight gain per kg protein intake |
| Chao1 | Richness | Shannon | Simpson | ||
|---|---|---|---|---|---|
| p-value | Water | 0.8 | 0.7 | 0.3 | 0.22 |
| Biofilm | 0.06 | 0.01 ** | 0.04 * | 0.12 |
| Days | Factors | Days × Factors | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Community composition | 109.4 | 0.000 | 180.2 | 0.000 | 18.6 | 0.000 |
References
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.J.; Heinsbroek, L.T.N.; Schneider, O.; Blancheton, J.P.; d’Orbcastel, E.R.; Verreth, J.A.J. New Developments in Recirculating Aquaculture Systems in Europe: A Perspective on Environmental Sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Xiao, R.; Wei, Y.; An, D.; Li, D.; Ta, X.; Wu, Y.; Ren, Q. A Review on the Research Status and Development Trend of Equipment in Water Treatment Processes of Recirculating Aquaculture Systems. Rev. Aquac. 2019, 11, 863–895. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Lund, I.; Thorarinsdottir, R.; Drengstig, A.; Arvonen, K.; Pedersen, P.B. Farming Different Species in RAS in Nordic Countries: Current Status and Future Perspectives. Aquacult. Eng. 2013, 53, 2–13. [Google Scholar] [CrossRef]
- Indriastuti, C.E.; Ratnawati, B.; Budiharto, I.W. Survival and Growth Performance of the Catfish Clarias gariepinus in High Density Nurseries Using Recirculating Aquaculture System (RAS). E3S Web Conf. 2022, 348, 00013. [Google Scholar] [CrossRef]
- Gichana, Z.M.; Liti, D.; Waidbacher, H.; Zollitsch, W.; Drexler, S.; Waikibia, J. Waste Management in Recirculating Aquaculture System through Bacteria Dissimulation and Plant Assimilation. Aquac. Int. 2018, 26, 1541–1572. [Google Scholar] [CrossRef]
- Wang, Y.; Chi, L.; Liu, Q.; Xiao, Y.; Ma, D.; Xiao, Z.; Xu, S.; Li, J. Effects of Stocking Density on the Growth and Immunity of Atlantic Salmon Salmo salar Reared in Recirculating Aquaculture System (RAS). J. Oceanol. 2019, 37, 350–360. [Google Scholar] [CrossRef]
- Lorgen-Ritchie, M.; Clarkson, M.; Chalmers, L.; Taylor, J.F.; Migaud, H.; Martin, S.A. A Temporally Dynamic Gut Microbiome in Atlantic Salmon during Freshwater Recirculating Aquaculture System (RAS) Production and Post-Seawater Transfer. Front. Mar. Sci. 2021, 8, 711797. [Google Scholar] [CrossRef]
- Chang, B.V.; Liao, C.S.; Chang, Y.T.; Chao, W.L.; Yeh, S.L.; Kuo, D.L.; Yang, C.W. Investigation of a Farm-Scale Multitrophic Recirculating Aquaculture System with the Addition of Rhodovulum sulfidophilum for Milkfish (Chanos chanos) Coastal Aquaculture. Sustainability 2019, 11, 1880. [Google Scholar] [CrossRef]
- O’Shea, T.; Jones, R.; Markham, A.; Norell, E.; Scott, J.; Theuerkauf, S.; Waters, T. Towards a Blue Revolution: Catalyzing Private Investment in Sustainable Aquaculture Production Systems; The Nature Conservancy: Arlington, VA, USA; Encourage Capital: New York, NY, USA, 2019. [Google Scholar]
- Global Aquaculture Alliance (GOAL). Shrimp Production Review. 2018. Available online: https://www.globalseafood.org/wp-content/uploads/2017/06/Day1_JimAnderson.pdf (accessed on 18 October 2022).
- Bardera, G.; Usman, N.; Owen, M.; Pountney, D.; Sloman, K.A.; Alexander, M.E. The Importance of Behaviour in Improving the Production of Shrimp in Aquaculture. Rev. Aquac. 2019, 11, 1104–1132. [Google Scholar] [CrossRef]
- Li, H.; Tian, X.; Zhao, K.; Jiang, W.; Dong, S. Effect of Clostridium butyricum in Different Forms on Growth Performance, Disease Resistance, Expression of Genes Involved in Immune Responses and mTOR Signaling Pathway of Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 13–21. [Google Scholar] [CrossRef]
- Bauer, J.; Teitge, F.; Neffe, L.; Adamek, M.; Jung, A.; Peppler, C.; Steinhagen, D.; Jung-Schroers, V. Impact of a Reduced Water Salinity on the Composition of Vibrio spp. in Recirculating Aquaculture Systems for Pacific White Shrimp (Litopenaeus vannamei) and Its Possible Risks for Shrimp Health and Food Safety. J. Fish Dis. 2021, 44, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Das, R.R.; Sarkar, S.; Saranya, C.; Esakkiraj, P.; Aravind, R.; Saraswathy, R.; Rekha, P.N.; Muralidhar, M.; Panigrahi, A. Co-Culture of Indian White Shrimp, Penaeus indicus, and Seaweed, Gracilaria tenuispitata, in Amended Biofloc and Recirculating Aquaculture System (RAS). Aquaculture 2022, 548, 737432. [Google Scholar] [CrossRef]
- Roques, J.A.; Micolucci, F.; Hosokawa, S.; Sundell, K.; Kindaichi, T. Effects of Recirculating Aquaculture System Wastewater on Anammox Performance and Community Structure. Processes 2021, 9, 1183. [Google Scholar] [CrossRef]
- Bartelme, R.P.; McLellan, S.L.; Newton, R.J. Freshwater Recirculating Aquaculture System Operations Drive Biofilter Bacterial Community Shifts Around a Stable Nitrifying Consortium of Ammonia-Oxidizing Archaea and Comammox Nitrospira. Front. Microbiol. 2017, 8, 101. [Google Scholar] [CrossRef]
- Suantika, G.; Situmorang, M.L.; Nurfathurahmi, A.; Taufik, I.; Aditiawati, P.; Yusuf, N.; Aulia, R. Application of Indoor Recirculating Aquaculture System for White Shrimp (Litopenaeus vannamei) Growout Super-Intensive Culture at Low Salinity Condition. J. Aquac. Res. Dev. 2018, 9, 1000530. [Google Scholar] [CrossRef]
- Ramzan, M.N.; Shen, D.; Wei, Y.; Emmanuel, A.; Yang, W.; Zhu, J.; Wang, Y.; Zheng, Z. Performance and Microbial Community Analysis of Integrated Bioremediation Systems with Photosynthetic Bacteria in Treating Mariculture Tailwater. J. Ocean Univ. China 2025, 24, 515–524. [Google Scholar] [CrossRef]
- Ramzan, M.N.; Shen, D.; Wei, Y.; Emmanuel, A.; Nicholaus, R.; Yang, W.; Zheng, Z. Nitrogen and Phosphorus-Related Functional Genes Enhance Nutrient Removal in the Integrated Aquaculture Wastewater Bioremediation System in the Presence of Photosynthetic Bacteria. Aquac. Int. 2025, 33, 131. [Google Scholar] [CrossRef]
- Navada, S.; Vadstein, O.; Gaumet, F.; Tveten, A.-K.; Spanu, C.; Mikkelsen, Ø.; Kolarevic, J. Biofilms Remember: Osmotic Stress Priming as a Microbial Management Strategy for Improving Salinity Acclimation in Nitrifying Biofilms. Water Res. 2020, 176, 115732. [Google Scholar] [CrossRef]
- Navada, S.; Sebastianpillai, M.; Kolarevic, J.; Fossmark, R.O.; Tveten, A.K.; Gaumet, F.; Mikkelsen, Ø.; Vadstein, O. A Salty Start: Brackish Water Start-Up as a Microbial Management Strategy for Nitrifying Bioreactors with Variable Salinity. Sci. Total Environ. 2020, 739, 139934. [Google Scholar] [CrossRef]
- Roalkvam, I.; Drønen, K.; Dahle, H.; Wergeland, H.I. A Case Study of Biofilter Activation and Microbial Nitrification in a Marine Recirculation Aquaculture System for Rearing Atlantic Salmon (Salmo salar L.). Aquac. Res. 2020, 52, 94–104. [Google Scholar] [CrossRef]
- Rojas-Tirado, P.; Pedersen, P.B.; Vadstein, O.; Pedersen, L.-F. Microbial Dynamics in RAS Water: Effects of Adding Acetate as a Biodegradable Carbon-Source. Aquac. Eng. 2019, 84, 106–116. [Google Scholar] [CrossRef]
- Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L. Monitoring and Managing Microbes in Aquaculture—Towards a Sustainable Industry. Microb. Biotechnol. 2016, 9, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Fossmark, R.O.; Attramadal, K.J.K.; Nordøy, K.; Østerhus, S.W.; Vadstein, O. A Comparison of Two Seawater Adaption Strategies for Atlantic Salmon Post-Smolt (Salmo salar) Grown in Recirculating Aquaculture Systems (RAS): Nitrification, Water and Gut Microbiota, and Performance of Fish. Aquaculture 2021, 532, 735973. [Google Scholar] [CrossRef]
- Dahle, S.W.; Bakke, I.; Birkeland, M.; Nordøy, K.; Dalum, A.S.; Attramadal, K.J.K. Production of Lumpfish (Cyclopterus lumpus L.) in RAS with Distinct Water Treatments: Effects on Fish Survival, Growth, Gill Health and Microbial Communities in Rearing Water and Biofilm. Aquaculture 2020, 552, 735097. [Google Scholar] [CrossRef]
- Almeida, D.B.; Magalhaes, C.; Sousa, Z.; Borges, M.T.; Silva, E.; Blanquet, I.; Mucha, A.P. Microbial Community Dynamics in a Hatchery Recirculating Aquaculture System (RAS) of Sole (Solea senegalensis). Aquaculture 2021, 539, 736592. [Google Scholar] [CrossRef]
- Vadstein, O.; Attramadal, K.J.K.; Bakke, I.; Olsen, Y. K-Selection as Microbial Community Management Strategy: A Method for Improved Viability of Larvae in Aquaculture. Front. Microbiol. 2018, 9, 2730. [Google Scholar] [CrossRef]
- Ruan, Y.J.; Guo, X.S.; Ye, Z.Y.; Liu, Y.; Zhu, S.M. Bacterial Community Analysis of Different Sections of a Biofilter in a Full-Scale Marine Recirculating Aquaculture System. N. Am. J. Aquac. 2015, 77, 318–326. [Google Scholar] [CrossRef]
- Blancheton, J.P.; Attramadal, K.J.K.; Michaud, L.; Roque D’orbcastel, E.; Vadstein, O. Insight into Bacterial Population in Aquaculture Systems and Its Implication. Aquac. Eng. 2013, 53, 30–39. [Google Scholar] [CrossRef]
- Chen, Z.; Chang, Z.; Zhang, L.; Jiang, Y.; Ge, H.; Song, X.; Chen, S.; Zhao, F.; Li, J. Effects of Water Recirculation Rate on the Microbial Community and Water Quality in Relation to the Growth and Survival of White Shrimp (Litopenaeus vannamei). BMC Microbiol. 2019, 19, 192. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, C.; Zheng, Z.; Wei, Y.; Lu, K.; Zhu, J. Nutrient Enrichment during Shrimp Cultivation Alters Bacterioplankton Assemblies and Destroys Community Stability. Ecotoxicol. Environ. Saf. 2018, 156, 366–374. [Google Scholar] [CrossRef]
- Raza, B.; Ke, J.; Chen, L.; Shi, Y.; Zhu, J.; Shao, Z.; Zheng, Z.; Lu, K.; Yang, W. Adding Glucose Combined with Microalgae to Water Improves the Benefits of the Fungal Community on the Whiteleg Shrimp (Litopenaeus vannamei) Culture. Aquac. Rep. 2025, 40, 102580. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Aust. J. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Dahle, S.W.; Attramadal, K.J.K.; Vadstein, O.; Hestdahl, H.I.; Bakke, I. Microbial Community Dynamics in a Commercial RAS for Production of Atlantic Salmon Fry (Salmo salar). Aquaculture 2022, 546, 737382. [Google Scholar] [CrossRef]
- Chen, Z.; Chang, Z.; Zhang, L.; Wang, J.; Qiao, L.; Song, X.; Li, J. Effects of Carbon Source Addition on Microbial Community and Water Quality in Recirculating Aquaculture Systems for Litopenaeus vannamei. Fish. Sci. 2020, 86, 507–517. [Google Scholar] [CrossRef]
- Abubakar, S.; Liu, G.; Muhammad, I.A.; Zhang, Y.; Tadda, M.A.; Qi, W.; Liu, D.; Ye, Z.; Zhu, S. Recent Advances in Application of Moving Bed Bioreactors for Wastewater Treatment from Recirculating Aquaculture Systems: A Review. Aquac. Fish. 2022, 7, 244–258. [Google Scholar]
- Kinyage, J.P.H.; Pedersen, P.B.; Pedersen, L.F. Effects of Abrupt Salinity Increase on Nitrification Processes in a Freshwater Moving Bed Biofilter. Aquac. Eng. 2019, 84, 91–98. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.X.; Chai, B.F.; Luo, Z.M.; Zhao, P.Y.; Bao, J.B. Spatio-Temporal Patterns of Microbial Communities and Their Driving Mechanisms in Subalpine Lakes, Ningwu, Shanxi. Huan Jing Ke Xue 2019, 40, 3285–3294. [Google Scholar]
- Jiang, W.W.; Tian, X.L.; Li, L.; Dong, S.L.; Zhao, K.; Li, H.D.; Cai, Y. Temporal Bacterial Community Succession during the Start-Up Process of Biofilters in a Cold Freshwater Recirculating Aquaculture System. Bioresour. Technol. 2019, 287, 121441. [Google Scholar] [CrossRef]
- Gautam, A.; Lear, G.; Lewis, G.D. Analysis of Spatial and Temporal Variations in Bacterial Community Dynamics within Stream Biofilms. N. Z. J. Mar. Freshw. Res. 2021, 55, 505–523. [Google Scholar] [CrossRef]
- Dmitrijs, F.; Guo, J.; Huang, Y.; Liu, Y.; Fang, X.; Jiang, K.; Zha, L.; Cai, J.; Fu, X. Bacterial Succession in Microbial Biofilm as a Potential Indicator for Postmortem Submersion Interval Estimation. Front. Microbiol. 2022, 13, 951707. [Google Scholar] [CrossRef]
- Sprong, P.A.A.; Fofonova, V.; Wiltshire, K.H.; Neuhaus, S.; Ludwichowski, K.U.; Käse, L.; Androsov, A.; Metfies, K. Spatial Dynamics of Eukaryotic Microbial Communities in the German Bight. J. Sea Res. 2020, 163, 101914. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, Z.; Xiaodong, L.; Shengxian, Y.; Xin, C.; Huiqiu, L.; Sang, B. Distribution Patterns and Community Assembly Processes of Eukaryotic Microorganisms along an Altitudinal Gradient in the Middle Reaches of the Yarlung Zangbo River. Water Res. 2023, 239, 120047. [Google Scholar] [CrossRef] [PubMed]
- Siponen, S.; Ikonen, J.; Gomez-Alvarez, V.; Hokajärvi, A.M.; Ruokolainen, M.; Jayaprakash, B.; Kolehmainen, M.; Miettinen, I.; Pitkänen, T.; Torvinen, E. Effect of Pipe Material and Disinfectant on Active Bacterial Communities in Drinking Water and Biofilms. J. Appl. Microbiol. 2025, 136, lxaf004. [Google Scholar] [CrossRef] [PubMed]
- Irhayyim, T.; Beliczky, G.; Bercsényi, M. Nutrient Bioremediation Efficiency of Bacterial Biofilms and Plant-Based Biofilters in a Recirculating Common Carp (Cyprinus carpio L.) Culture System. Iran. J. Fish. Sci. 2021, 20, 828–845. [Google Scholar]
- Wang, H.; He, W.; Zhang, Z.; Liu, X.; Yang, Y.; Xue, H. Spatio-Temporal Evolution Mechanism and Dynamic Simulation of Nitrogen and Phosphorus Pollution of the Yangtze River Economic Belt in China. Environ. Pollut. 2024, 357, 124402. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Liu, B.; Xuan, Y.; Jiang, M.; Pan, Y.; Zhang, Y.; Gong, Y.; Lu, X.; Yu, D.; et al. Dynamic Changes of Microbial Communities in Litopenaeus vannamei Cultures and the Effects of Environmental Factors. Aquaculture 2016, 455, 97–108. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Xiong, J.; Dai, W.; Li, C. Advances, Challenges, and Directions in Shrimp Disease Control: The Guidelines from an Ecological Perspective. Appl. Microbiol. Biotechnol. 2016, 100, 6947–6954. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Huang, L.; Yan, M.; Dong, P.; Chen, C.; Guo, H.; Zhang, D. Effects of Sucrose Addition on Water Quality and Bacterioplankton Community in the Pacific White Shrimp (Litopenaeus vannamei) Culture System. Aquac. Res. 2021, 52, 4184–4197. [Google Scholar] [CrossRef]
- Xu, W.; Xu, Y.; Su, H.; Hu, X.; Xu, Y.; Li, Z.; Wen, G.; Cao, Y. Production Performance, Inorganic Nitrogen Control and Bacterial Community Characteristics in a Controlled Biofloc-Based System for Indoor and Outdoor Super-Intensive Culture of Litopenaeus vannamei. Aquaculture 2021, 531, 735749. [Google Scholar] [CrossRef]
- Wang, D.P.; Li, T.; Huang, K.L.; He, X.W.; Zhang, X.X. Roles and Correlations of Functional Bacteria and Genes in the Start-Up of Simultaneous Anammox and Denitrification System for Enhanced Nitrogen Removal. Sci. Total Environ. 2019, 655, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; He, X.; Feng, Y.; Miao, M.S.; Wang, Q.; Du, Y.D.; Xu, F. Pollutant Removal and Microorganism Evolution of Activated Sludge under Ofloxacin Selection Pressure. Bioresour. Technol. 2017, 241, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Huang, Z.; Zeng, S.; Liu, J.; Wei, D.; Deng, X.; Weng, S.; He, Z.; He, J. Environmental Factors Shape Water Microbial Community Structure and Function in Shrimp Cultural Enclosure Ecosystems. Front. Microbiol. 2017, 8, 2359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Zhang, C.; Wen, H.; Guo, W.; Hao, H. Effect of Filling Fraction on the Performance of Sponge-Based Moving Bed Biofilm Reactor. Bioresour. Technol. 2016, 219, 762–767. [Google Scholar] [CrossRef]
- Yu, B.B.; Xie, G.J.; Shen, Z.; Shao, K.Q.; Tang, X.M. Spatiotemporal Variations, Assembly Processes, and Co-Occurrence Patterns of Particle-Attached and Free-Living Bacteria in a Large Drinking Water Reservoir in China. Front. Microbiol. 2023, 13, 1056147. [Google Scholar] [CrossRef]
- Gu, Y.; Wei, Y.; Xiang, Q.; Zhao, K.; Yu, X.; Zhang, X.; Li, C.; Chen, Q.; Xiao, H.; Zhang, X. C:N Ratio Shaped Both Taxonomic and Functional Structure of Microbial Communities in Livestock and Poultry Breeding Wastewater Treatment Reactor. Sci. Total Environ. 2019, 651, 625–633. [Google Scholar] [CrossRef]
- Shaw, J.L.; Monis, P.; Fabris, R.; Ho, L.; Braun, K.; Drikas, M.; Cooper, A. Assessing the Impact of Water Treatment on Bacterial Biofilms in Drinking Water Distribution Systems Using High-Throughput DNA Sequencing. Chemosphere 2014, 117, 185–192. [Google Scholar] [CrossRef]
- Naik, A.; Smithers, M.; Moisander, P.H. Impacts of UV-C Irradiation on Marine Biofilm Community Succession. Appl. Environ. Microbiol. 2021, 88, e02642-21. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, K.X.; Li, K.H.; Jin, Y.; He, X.Q. Deciphering the Diversity Patterns and Community Assembly of Rare and Abundant Bacterial Communities in a Wetland System. Sci. Total Environ. 2022, 838, 156334. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, G.; Tang, X.; Shao, K. Impacts of Different Salinities on Bacterial Biofilm Communities in Fresh Water. Can. J. Microbiol. 2014, 60, 319–326. [Google Scholar] [CrossRef]
- Cai, W.; Huang, Q.; Li, H.; Cheng, H. Longitudinal Patterns of Microbial Communities in the Water Diversion Rivers of South-to-North Water Diversion Project. Clean-Soil Air Water 2022, 50, 2100303. [Google Scholar] [CrossRef]
- Xing, R.; Gao, Q.; Zhang, F.; Wang, J.; Chen, S. Environmental Filtering Affects Fungal Communities More than Dispersal Limitation in a High-Elevation Hyperarid Basin on Qinghai–Tibet Plateau. FEMS Microbiol. Lett. 2021, 368, fnab033. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.B.; Liu, X.X.; Shen, M.J.; She, G.L.; Ye, B.C. The Nitrogen Regulator GlnR Directly Controls Transcription of the prpDBC Operon Involved in Methylcitrate Cycle in Mycobacterium smegmatis. J. Bacteriol. 2019, 201, e00627-18. [Google Scholar] [CrossRef] [PubMed]
- Chun, B.H.; Lee, Y.; Jin, H.M.; Jeon, C.O. Cloacibacterium caeni sp. nov., Isolated from Activated Sludge. Int. J. Syst. Evol. Microbiol. 2017, 67, 1688–1692. [Google Scholar] [CrossRef]
- Sun, P.; Chen, Y.; Liu, J.; Xu, Y.; Zhou, L.; Wu, Y. Periphytic Biofilms Function as a Double-Edged Sword Influencing Nitrogen Cycling in Paddy Fields. Environ. Microbiol. 2022, 24, 6279–6289. [Google Scholar] [CrossRef]
- Lin, D.; Zhu, L.; Qiu, C.; Mu, X.; Zhang, S.; Ohore, O.E. Ammonium Loading Drives Bacterial Community Shifts in Biofilms Attached to the Submerged Macrophyte Hydrilla verticillata. Aquat. Microb. Ecol. 2020, 85, 59–69. [Google Scholar] [CrossRef]
- Kim, M.; Park, M.S.; Kang, I.; Cho, J.C. Thermomonas paludicola sp. nov., Isolated from a Lotus Wetland. Int. J. Syst. Evol. Microbiol. 2023, 73, 005737. [Google Scholar] [CrossRef]
- Lührig, K.; Canbäck, B.; Paul, C.J.; Johansson, T.; Persson, K.M.; Rådström, P. Bacterial Community Analysis of Drinking Water Biofilms in Southern Sweden. Microbes Environ. 2015, 30, 99–107. [Google Scholar] [CrossRef]
- Qiao, L.; Bao, J.; Li, T.; Sun, X.; Ze, W.; Li, J. Microbial Community Structure and Its Driving Factors in the Whiteleg Shrimp (Litopenaeus vannamei) Aquaculture System. Appl. Ecol. Environ. Res. 2025, 23, 671–686. [Google Scholar] [CrossRef]
- Chang, F.; He, S.; Dang, C. Assisted Selection of Biomarkers by Linear Discriminant Analysis Effect Size (LEfSe) in Microbiome Data. J. Visualized Exp. 2022, 183, e61715. [Google Scholar]
- Chen, Z.; Liu, Y.; Liu, L.Z.; Wang, X.J.; Liu, Z.P.; Liu, Y. Heterotrophic Bacterial Community Structure of Multistage Biofilters in a Commercial Pufferfish Takifugu rubripes RAS. Adv. Mater. Res. 2013, 726, 1621–1627. [Google Scholar] [CrossRef]
- Tang, B.; Chen, Q.Y.; Bin, L.Y.; Huang, S.S.; Zhang, W.X.; Fu, F.L.; Li, P. Insight into the Microbial Community and Its Succession of a Coupling Anaerobic-Aerobic Biofilm on Semi-Suspended Bio-Carriers. Bioresour. Technol. 2018, 247, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Nesje, J. Impacts of Organic Matter Removal Efficiency on the Microbial Carrying Capacity and Stability of Land-Based Recirculating Aquaculture Systems. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2018; pp. 1–58, NTNU Open. Available online: http://hdl.handle.net/11250/2495355 (accessed on 7 May 2025).
- Ganesan, G.; Velayudhan, S.S.; David, J.S.R. Statistical Optimization of Medium Constituents and Conditions for Improved Antimicrobial Compound Production by Marine Streptomyces sp. JRG-04. Arch. Biol. Sci. 2017, 69, 723–731. [Google Scholar] [CrossRef]
- Joung, Y.; Hong, S.; Kim, H.; Kang, H.; Farrance, C.E.; Joh, K. Taeseokella kangwonensis gen. nov., sp. nov., Isolated from a Freshwater Reservoir. Int. J. Syst. Evol. Microbiol. 2015, 65, 4309–4314. [Google Scholar] [CrossRef]
- Chen, W.M.; Lin, C.Y.; Young, C.C.; Sheu, S.Y. Rheinheimera aquatica sp. nov., Antimicrobial Activity-Producing Bacterium Isolated from Freshwater Culture Pond. J. Microbiol. Biotechnol. 2010, 20, 1386–1392. [Google Scholar] [CrossRef]
- Van Alst, N.E.; Picardo, K.F.; Iglewski, B.H.; Haidaris, C.G. Nitrate Sensing and Metabolism Modulate Motility, Biofilm Formation, and Virulence in Pseudomonas aeruginosa. Infect. Immun. 2007, 75, 3780–3790. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Chen, P.; Yuan, S.; Wang, K.; Wang, S.; Jiang, X. Role of Nitrogen Cycling Functional Genes and Their Key Influencing Factors in Eutrophic Aquatic Ecosystems. Environ. Rev. 2025, 33, 1–10. [Google Scholar] [CrossRef]
- Orr, R.J.; Rombauts, S.; Van de Peer, Y.; Shalchian-Tabrizi, K. Draft Genome Sequences of Two Unclassified Bacteria, Hydrogenophaga sp. Strains IBVHS1 and IBVHS2, Isolated from Environmental Samples. Genome Announc. 2017, 5, 10-1128. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Sabaratnam, V.; Tan, G.Y.A.; Chong, V.C. Identification of Indigenous Bacteria Isolated from Shrimp Aquaculture Wastewater with Bioremediation Application: Total Ammoniacal Nitrogen (TAN) and Nitrite Removal. Sains Malaysiana 2015, 44, 1103–1110. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.N.; Zhang, G.Z.; Wang, B.S.; Zhang, X.; Li, J. Key Bacteria for the Microbial Degradation of Pollutants in Cellar Water. Huan Jing Ke Xue 2018, 39, 4766–4777. [Google Scholar]
- Ohshiro, T.; Harada, N.; Kobayashi, Y.; Miki, Y.; Kawamoto, H. Microbial Fucoidan Degradation by Luteolibacter algae H18 with Deacetylation. Biosci. Biotechnol. Biochem. 2012, 76, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Salcedo Moyano, A.J.; Delforno, T.P.; Subtil, E.L. Simultaneous Nitrification-Denitrification (SND) Using a Thermoplastic Gel as Support: Pollutants Removal and Microbial Community in a Pilot-Scale Biofilm Membrane Bioreactor. Environ. Technol. 2022, 43, 4411–4425. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.T.; He, Y.Q.; Li, G.X.; Xiao, H.; Dai, X.R.; Yang, M.R.; Bao, P. Genome Sequence of Sulfide-Dependent Denitrification Bacterium Thermomonas sp. Strain XSG, Isolated from Marine Sediment. Microbiol. Resour. Announc. 2021, 10, e00361-21. [Google Scholar] [CrossRef] [PubMed]

| Water | Biofilm | |||
|---|---|---|---|---|
| Time Point | R2 | p | R2 | p |
| Overall (PERMANOVA) | 0.247 | 0.001 *** | 0.311 | 0.001 *** |
| Initial (days 15–31) | 0.207 | 0.002 ** | 0.241 | 0.002 ** |
| Middle (days 46–61) | 0.278 | 0.002 ** | 0.394 | 0.037 * |
| Final (day 76) | 0.137 | 0.001 *** | 0.187 | 0.001 *** |
| Medium | Time | Chao1 | Richness | Shannon | Simpson |
|---|---|---|---|---|---|
| Water | Initial | 2642.3 ± 186.5 | 2194.9 ± 152.6 | 6.93 ± 0.47 | 0.04 ± 0.02 a |
| Middle | 2599.7 ± 303.7 | 2118.5 ± 311.2 | 6.24 ± 1.01 | 0.07 ± 0.05 b | |
| Final | 2481.9 ± 124.6 | 1994 ± 161.3 | 6.17 ± 0.56 | 0.05 ± 0.01 ab | |
| Biofilm | Initial | 2807.3 ± 151.2 | 2363.3 ± 150.7 | 7.59 ± 0.61 | 0.03 ± 0.02 b |
| Middle | 2818.4 ± 158.7 | 2343.6 ± 142.5 | 7.66 ± 0.35 | 0.02 ± 0.01 a | |
| Final | 2511.8 ± 269.9 | 2012 ± 229.5 | 7.09 ± 0.20 | 0.03 ± 0.004 ab |
| Parameters (mg/L) | Day 15 | Day 31 | Day 46 | Day 61 | Day 76 |
|---|---|---|---|---|---|
| CODMn | 11.88 ± 2.54 b | 11.34 ± 2.06 b | 21.10 ± 1.84 a | 21.32 ± 2.73 a | 23.00 ± 5.71 a |
| TN | 16.88 ± 2.93 c | 18.97 ± 1.09 c | 37.50 ± 5.54 b | 40.83 ± 19.32 b | 72.96 ± 13.80 a |
| TP | 9.48 ± 7.68 b | 7.43 ± 4.86 b | 8.94 ± 3.18 b | 11.45 ± 6.25 b | 41.61 ± 26.39 a |
| NO3−-N | 4.46 ± 0.59 d | 7.63 ± 5.24 c | 4.51 ± 1.53 d | 14.17 ± 3.05 b | 30.08 ± 2.07 a |
| NO2−-N | 1.78 ± 0.25 a | 0.22 ± 0.07 c | 0.31 ± 0.22 bc | 0.44 ± 0.12 b | 0.31 ± 0.04 bc |
| NH4+-N | 0.79 ± 0.40 b | 0.44 ± 0.62 bc | 0.12 ± 0.10 c | 0.09 ± 0.11 c | 0.91 ± 0.16 a |
| PO43−-P | 1.00 ± 0.37 d | 1.00 ± 0.12 d | 3.17 ± 1.05 c | 5.58 ± 1.02 b | 8.59 ± 1.65 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmanuel, A.; Wei, Y.; Ramzan, M.N.; Yang, W.; Zheng, Z. Dynamics of Bacterial Communities and Their Relationship with Nutrients in a Full-Scale Shrimp Recirculating Aquaculture System in Brackish Water. Animals 2025, 15, 1400. https://doi.org/10.3390/ani15101400
Emmanuel A, Wei Y, Ramzan MN, Yang W, Zheng Z. Dynamics of Bacterial Communities and Their Relationship with Nutrients in a Full-Scale Shrimp Recirculating Aquaculture System in Brackish Water. Animals. 2025; 15(10):1400. https://doi.org/10.3390/ani15101400
Chicago/Turabian StyleEmmanuel, Arslan, Yingzhen Wei, Muhammad Naeem Ramzan, Wen Yang, and Zhongming Zheng. 2025. "Dynamics of Bacterial Communities and Their Relationship with Nutrients in a Full-Scale Shrimp Recirculating Aquaculture System in Brackish Water" Animals 15, no. 10: 1400. https://doi.org/10.3390/ani15101400
APA StyleEmmanuel, A., Wei, Y., Ramzan, M. N., Yang, W., & Zheng, Z. (2025). Dynamics of Bacterial Communities and Their Relationship with Nutrients in a Full-Scale Shrimp Recirculating Aquaculture System in Brackish Water. Animals, 15(10), 1400. https://doi.org/10.3390/ani15101400






