Simple Summary
Shrimp farming in brackish water faces challenges in maintaining water quality and shrimp health, often due to changes in bacterial communities and nutrient levels. This study explored how bacterial communities shift over time in a full-scale shrimp recirculating aquaculture system (RAS), which reuses water to reduce environmental impact. The goal was to understand how these bacteria interact with nutrients like nitrogen and phosphorus, which are critical for shrimp health but can be harmful in excess. By monitoring water samples throughout a shrimp farming cycle, the researchers found that bacterial communities changed in response to nutrient levels, with certain beneficial bacteria becoming more dominant during stable water conditions. These findings suggest that managing bacterial balance can help control nutrient buildup and improve shrimp health and productivity. This study highlights the importance of understanding microbial dynamics in aquaculture systems, offering insights that can lead to more sustainable and efficient shrimp farming practices. This research benefits society by supporting food security, reducing environmental pollution, and promoting liable aquaculture development.
Abstract
Microbial communities in RASs play a critical role in maintaining water quality and supporting shrimp growth, development, and health. However, their dynamics, particularly in commercial systems, remain poorly understood. This study aimed to improve the understanding of bacterial community dynamics during shrimp culture in RASs. High-throughput amplicon sequencing of the 16S rRNA, PERMANOVA, PCoA, and other statistical analyses were used to investigate the bacterial dynamics. The entire succession process was categorized into three distinct phases, the initial, middle, and final phases, during the shrimp rearing in RASs to elucidate the spatial–temporal dynamics of the bacterial communities. Alpha diversity indicates the evenness of the bacterial community increased in the initial phase, while richness peaked in the middle phase. Notable taxonomic and functional groups within the bacterial community contributed to significant variations in the relative abundance of community composition across these phases. The dominant bacterial phyla in both water and biofilm included Proteobacteria, Actinobacteriota, Bacteroidota, and Patescibacteria. The dominant orders in both environments were Corynebacteriales, Burkholderiales, Rhodobacterales, Flavobacteriales, Saccharimonadales, and Micrococcales. Key bacterial taxa such as Pseudomonas, Mycobacterium, and Hydrogenophaga were critical for microbial community assembly, nutrient cycling, biodegradation, and water quality monitoring. Nitrite, ammonium, and nitrate were positively correlated with Mycobacterium, Rheinheimera, Taeseokela, and Thermomonas, while negatively correlated with the Cloacibacterium community composition. These findings expand our understanding of the underlying mechanisms of bacterial community succession in RASs with intensive rearing of shrimp and suggest that stabilizing environmental variables could be a useful management tool for promoting and maintaining healthy aquaculture environments.
1. Introduction
Recirculating aquaculture systems (RASs) represent advanced aquaculture technologies that have the potential to support the cultivation of a diverse range of seafood species but not limited to prawns, salmon, sea bass, shrimp, trout, and tuna across various environments [1]. These systems use a closed-loop filtration process to optimize water quality (temperature, salinity, pH, oxygen) for species growth [2] while minimizing effluent discharge, such as ammonium, solid waste, and carbon dioxide [3], converted into non-toxic compounds that can be safely reused within the aquaculture system [4]. Previous studies have demonstrated that RASs can reduce water consumption by 90–99% and significantly decrease ammonia concentrations [5]. Additionally, the adoption of RASs has been found to enhance growth performance, survival rates, and overall production efficiency [6].
Bio-filters, which rely on microbial communities for nutrient processing and maintaining water quality, play a critical role in the effectiveness of RASs, making them essential for sustainable shrimp farming practices [7]. Furthermore, the flexibility of RASs in supporting a diverse range of species highlights their potential as a sustainable solution for expanding global seafood production, especially in regions with restricted access to conventional marine environments [8]. Consequently, RASs are often referred to as “hyper-intensive” or “super-intensive” farming [9]. Moreover, it has been involved in the cultivation of Litopenaeus vannamei, commonly known as white-leg shrimp, which are economically significant worldwide [10]. By 2018, production of this species had reached 3.5 million tons, establishing it as one of the most crucial species in aquaculture [11]. The L. vannamei is particularly favored for its superior disease resistance, rapid growth rates, and high tolerance to stressful conditions [12]. These attributes have contributed to the increasing interest in utilizing RAS technology to optimize its cultivation [13]. This intensive farming approach can generate significant yields, often reaching up to 500 tons of shrimp per hectare annually, all within a relatively small water volume [14].
Microbial communities in RASs have been extensively studied for aquaculture systems [15], but shrimp RAS microbial communities remain rarely explored. Microorganisms play a pivotal role in maintaining the stability of the aquaculture environment within RASs [1]. The bio-filter, a core component of RASs, functions as a microbial system specifically designed to remove nitrogenous waste byproducts generated during the breakdown and oxidation of shrimp proteins [16]. Despite its essential function, the bio-filter remains a complex system with a limited understanding of its underlying mechanisms. The efficiency of the bio-filter is primarily influenced by the composition and abundance of functional microbial communities within it, which directly affect its capacity for water purification [17]. These microbial communities frequently form complex biofilms that play a critical role in biotransformation processes, converting toxic substances into less harmful compounds, thus improving water quality and promoting the health of cultured organisms [18] whereas, denitrifying bacteria can be used in another treatment step to reduce water usage even further by converting nitrate into nitrogen gas that can be removed from the system [19]. Different bacteria in nitrifying biofilters of RASs have been reviewed [20,21,22,23], but the knowledge of temporal dynamics in full-scale RASs is under-reviewed.
The microbial communities in RASs can respond rapidly to changes in the environment, with different selection pressures acting on the microbial communities [24]. The different forces driving these selection pressures include feed and feeding regimes, the make-up water, management routines, system design, physicochemical water quality, and the shrimp itself [25,26]. In addition to the bio-filter, microorganisms present within the culture water itself contribute to environmental stability and support the overall well-being of the cultured species [27]. These microbes directly interact with aquaculture organisms, influencing their health, growth, and productivity [28]. Consequently, a comprehensive understanding of the bacterial community composition and its fluctuations within biofilters and culture water is vital for optimizing the design, operation, and functionality of RASs [29]. Solutions to maintain beneficial microbial communities in RASs, which is important for system management and control, are practically lacking [24,30].
Previous research has shown that during shrimp culture in RASs, different bacterial communities were involved in improving the water quality and the health of shrimp [31] while also focusing on the relationship between bacterioplankton community stability and eutrophication levels [32]. Taking this scenario, in this study, we observed the following: (1) the successional changes in the bacterial community in water during different phases of shrimp culture in RASs; (2) the bacterial community shift in biofilm during shrimp culture in RASs; and (3) the relationship between bacterial communities and environmental factors during shrimp culture in RASs. This study will explore the fundamental mechanism of specific bacterial taxa behind the water quality management during the shrimp growth period, specifically during the last stage of cultivation.
2. Materials and Methods
2.1. Culture System Setup
The field experiment was conducted at Jufu Agriculture Farm, located in Beilun District, Zhejiang Province, Ningbo, China. Four full-scale RASs on the farm were selected for this study. Each RAS setup consisted of four replicated rearing tanks, each with a volume of 38 m3, a water pump, a microfiltration unit, and a bio-filter tank with a volume of 40 m3. On 1 May 2023, juvenile L. vannamei shrimp were stocked in the rearing tanks at a density of 25,000 individuals per tank, equivalent to approximately 658 individuals per cubic meter. The bio-filter used moving-bed biofilm reactor (MBBR) technology, utilizing polyethylene elastic media submerged in water. The bio-filter was inoculated with rearing water one month in advance to facilitate biofilm formation. All of the RASs were housed within a greenhouse to maintain a relatively stable temperature. The salinity of the rearing water was approximately 5 ppt, and the shrimp were cultured for a duration of three months and harvested on 18 July 2023. Each day, the shrimps were fed three times (at 9:00, 14:00, and 20:00) using commercial pellets (48% proteins; Table A2). The daily feeding rate was 6% of the biomass at the beginning and adjusted according to the actual feeding condition. The total feed, residual feed, and feces, as well as water exchange quantity and nitrogen concentration, were monitored to calculate nitrogen input and output. Furthermore, water temperature and turbidity were measured daily in each system before feeding to assess the culture condition and estimate the effect of bacteria on the nitrogen budget.
2.2. Sample Collection and Water Quality Analysis
Sampling was conducted on days 15, 31, 46, 61, and 76 of the experimental period. A total of 80 samples, including both water and biofilm, were collected from four replicate random sampling positions designated within the bio-filters (at 9:00) to monitor succession processes. The shrimp samples’ average wet weight was recorded on both the first day (0.02 ± 0.005 g) and the last day (13.89 ± 2.71 g) of the experiment, i.e., the 90th day (Table A1). Water samples were filtered using 0.45 μm polycarbonate membrane filters and stored at −20 °C until analysis. Microbial water and biofilm samples were filtered using a 0.22 μm membrane filter until the membrane surface became visibly obscured. The filtered membranes were then stored in sterile Eppendorf tubes at −80 °C and transported on dry ice for subsequent DNA extraction and high-throughput sequencing analysis. Prior to analysis, the biofilm samples were immersed in sterile water for 5 min, followed by ultrasonic treatment at a specified temperature (<30 °C) for 3 min using an ultrasonic vibration instrument model XM-3200UVF (Shenzhen Xiangmin Instrument and Equipment Co., Ltd., Shenzhen, China) at 50 Hz. Water quality parameters such as pH, salinity (S), dissolved oxygen (DO), temperature (T), chemical oxygen demand (CODMn), active phosphate (PO43−-P), nitrite (NO2−-N), nitrate (NO3−-N), ammonia nitrogen (NH4+-N), total nitrogen (TN), and total phosphorus (TP) were measured on a weekly basis. Temperature, salinity, pH, and dissolved oxygen were measured with a water quality analyzer. Nutrient concentrations, including NH4⁺-N, NO₂−-N, NO3−-N, PO43−-P, TN, and TP, were determined by SmartChem450 (KPM Analytics, Westborough, MA, USA). The content of NH₄⁺-N was analyzed using hypobromite oxidation, while NO3−-N was reduced through a zinc–cadmium column. Nitrate concentrations were measured spectrophotometrically using naphthalene ethylenediamine for NO2−-N detection. The concentration of PO43−-P was determined spectrophotometrically using the phosphomolybdenum blue method. TN and TP were analyzed by potassium persulfate oxidation. COD was measured using a modified permanganate method with an alkaline solution, followed by titration with potassium permanganate.
2.3. Processing of DNA Sequencing Data
The bacterial DNA was extracted in this study using the Minkgene Water DNA kit (Guangdong Magigene Biotechnology Co., Ltd., Guangzhou, China) for 16S rRNA sequencing. The yield and quality of the extracted DNA were assessed using a NanoDrop One spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Universal primers 338F (forward; 5∲-ACTCCTACGGAGGCAGCA-3∲) and 806R (reverse; 5∲-GGACTACHVGGGTWTCTAAT-3∲) were utilized for PCR amplification of the bacterial genome. The PCR products were sequenced on the Illumina HiSeq 2500 platform (Guangdong Magigene Biotechnology Co., Ltd., located in Guangzhou, China) using paired-end sequencing with a read length of 250 bp. Sequence assembly and analysis were conducted on the raw sequencing data using the USEARCH program, version 11.0.667_I8. The UNOISE3 algorithm parameters unoise_alpha = 2, minsize = 8, and default settings were utilized for denoising, error correction, chimeric sequence removal, and generation of zero-radius operational taxonomic units (ZOTUs). Taxonomic classification of the ZOTUs was performed by aligning them against the SILVA database version SSU Ref NR 99 138.1 to obtain taxonomic information.
2.4. Statistical Analysis
Statistical analyses were conducted using R software version 4.3.2. Visualizations were generated with the ‘ggplot2’ package unless otherwise stated. A one-way analysis of variance (ANOVA; p < 0.05) was conducted to assess differences among samples with respect to water quality parameters, as well as the α-diversity (p < 0.05) indices using SPSS version 20, whereas R version 4.3.2 was used to analyze β-diversity. Additionally, the Adonis function() from the ‘vegan’ package in R (version 4.3.2) was used to perform permutation multivariate analysis of variance (PERMANOVA; p < 0.05). This analysis was conducted to examine differences in community composition between groups and to assess the influence of environmental factors on these differences. Principal coordinate analysis (PCoA) [33] was performed using the ‘cmdscale()’ function from the ‘ape’ package, along with the ‘ddply()’ function from the ‘plyr’ package in R version 4.3.2. The analysis aimed to evaluate the bacterial community structure, which was then visualized for interpretation. A stacked histogram of relative abundance percentages was generated using the ‘ggplot2’ package in R version 4.3.2 for effective visualization. A differential analysis cladogram of bacterial genera present in water and biofilms was constructed using linear discriminant analysis effect size (LEfSe) to identify potential biomarkers at the genus level. Heatmap visualization was generated in R version 4.3.2 using the ‘pheatmap’ package for data representation. Redundancy analysis (RDA) was performed to explore the relationship between environmental factors and microbial community structure, utilizing the ‘vegan’ package in R version 4.3.2. Bray–Curtis dissimilarity was calculated at different time points between groups, and statistical differences were assessed using the Kruskal–Wallis test (p < 0.05) in R version 4.3.2 [34].
3. Results
3.1. Temporal Change in Bacterial Communities in Water and Biofilm Environments
The PERMANOVA test was applied to assess the significance of differences between groups. A significant difference was observed among the groups (Table 1; p < 0.05). Pairwise comparisons indicated the presence of three significantly distinct groups (initial, middle, and final), suggesting the existence of three successional stages in the bacterial communities of both environments (Table 1; p < 0.05).

Table 1.
Pairwise differences between bacterial communities in the water column and biofilm at different times—PERMANOVA analysis based on adonis function (* p < 0.05, ** p < 0.01, *** p < 0.001).
Furthermore, principal coordinate analysis (PCoA) revealed significant temporal effects on the bacterial communities over the rearing period in both the water and biofilm (Figure 1A,B). In the water column, PCoA1 and PCoA2 accounted for 27% and 14% of the total variation (Figure 1A), respectively, while in biofilms, these axes explained 25% and 21% of the variation (Figure 1B).
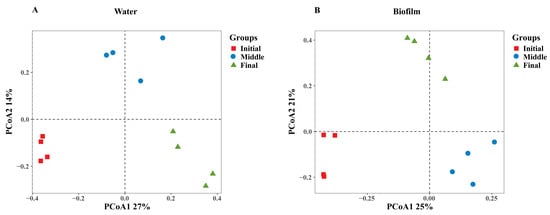
Figure 1.
PCoA based on Bray–Curtis’s matrix indicates differences in bacterial community composition in the system. Bacterial communities in water (A) and biofilm (B). Initial, days 15–31; middle, days 46–61; and final, day 76.
The diversity indices indicated overall stability in diversity, with notable temporal fluctuations, aligning with three distinct phases: the initial phase (days 15–31), the middle phase (days 46–61), and the final phase (day 76). The p-values indicated that biofilm diversity increased significantly over time while water diversity remained stable (Table A4, p < 0.05). In the water samples, the Shannon diversity index peaked initially and, thereafter, declined in the middle and final phases. Conversely, the Simpson diversity index was highest in the middle phase compared to the other sampling times. In the biofilm samples, the Shannon diversity index reached its peak in the middle, followed by a significant decrease in subsequent phases. The Simpson diversity index exhibited substantial variation across different time points (Table 2, p < 0.05).

Table 2.
α-diversity of bacterial communities (mean ± SD) in water and biofilm. Different letters specify the significant difference between groups (days) at the same column and same medium (water or biofilm) (p < 0.05, one-way ANOVA; Tukey’s HSD).
3.2. Bacterial Community Composition in Water and Biofilms
A total of 4221 operational taxonomic units (OTUs) were derived from 2,339,952 high-quality sequences collected from 20 samples. Over the course of a 90-day experimental period, significant shifts in the composition of dominant bacterial taxa were observed, emphasizing the dynamic nature of microbial communities. The taxonomic composition of the bacterial community revealed distinct shifts in microbial phyla across the experimental groups. Among the top most abundant phyla, Proteobacteria emerged as the dominant phylum in the final phase, comprising 49.5 ± 0.04% in water, whereas during the middle phase, it accounted for 40.5 ± 0.01% in the biofilm. Furthermore, Bacteroidota was the second predominant group in the final phase, accounting for 21.8 ± 0.12% in the water and 27.4 ± 0.04% in the biofilm. A notable decline in Actinobacteriota, the third most abundant phyla, was observed during the final phase, with its relative abundance of 7.9 ± 0.05% in the water. Meanwhile, in the biofilm, Patescibacteria, categorized as the third most abundant phyla, declined to 12.8 ± 0.06% (Figure 2A). Order-level analysis revealed that Corynebacteriales and Burkholderiales were the most abundant taxa in the initial phase. This was followed by Rhodobacterales and Flavobacteriales, which dominated in the middle phase, whereas, in the final phase, Saccharimonadales and Micrococcales emerged as the most prevalent orders in both water and biofilm (Figure 2B).
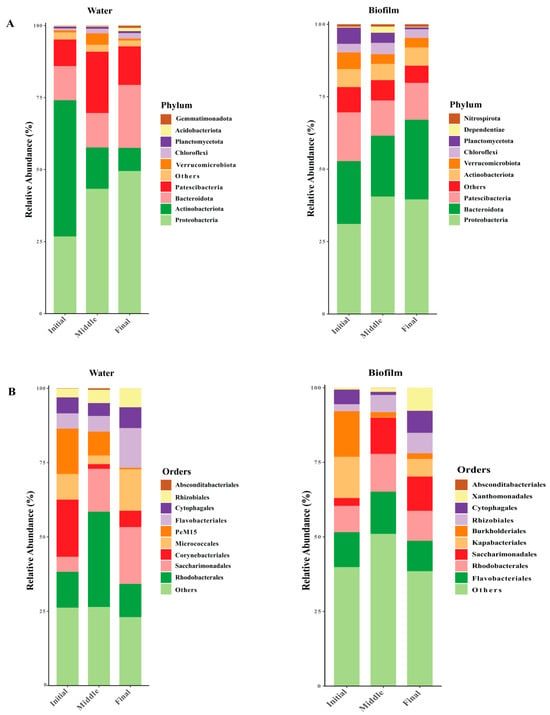
Figure 2.
Relative abundance of bacterial communities’ dominant phylum and order levels (top 10) at different times. Bacterial composition in water and biofilm at the phylum level (A). Bacterial composition in water and biofilm at the order level (B).
3.3. Shrimp Growth Performance
3.4. Relationships Between Microbial Communities and Environmental Factors
Redundancy analysis of bacterial communities and nutrient concentrations revealed significant correlations between TP, TN, NO2−-N, NH4+, and NO3−-N. In the water column, Mycobacterium exhibited a strong positive correlation with NO2−-N, whereas Cloacibacterium and Flavobacterium showed negative correlations with NH4+ (Figure 3A). In the biofilm samples, Rheinheimera and Flavobacterium showed a positive correlation with NO2−-N, while Taeseokela and Thermomonas were positively correlated with NH4+. Additionally, Cloacibacterium demonstrated a positive correlation with NO3−-N (Figure 3A). Furthermore, the heat map revealed significant correlations between various environmental factors and the distribution of microorganisms within a RAS (Figure 3B). A Mantel test revealed a strong association between PO₄3⁻-P and microbial communities (r > 0.4, p < 0.001), emphasizing the influence of phosphorus on microbial community structure. TP, TN, CODMn, NO3−-N, and NH4+-N exhibited positive correlations with microbial communities (r > 0.3, p < 0.05), whereas NH4+-N and NO2−-N displayed negative associations (Figure 3B).
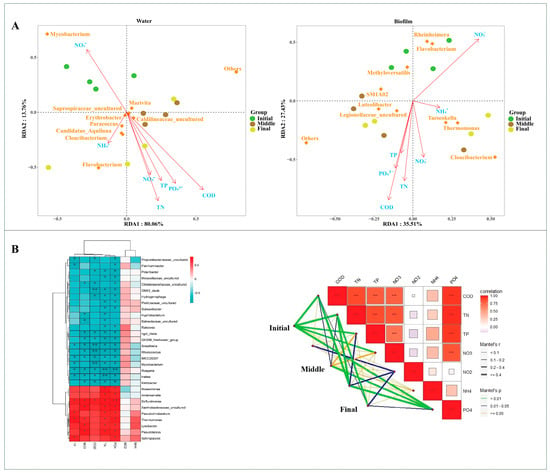
Figure 3.
Exploring the relationship between microbial communities and environmental factors using RDA (A). RDA explores the relationship between water and biofilm microbial community genera and total nitrogen (TN), chemical oxygen demand (CODMn), nitrite (NO2−-N), phosphate (PO43−-P), total phosphorus (TP), nitrate (NO3−-N), and ammonia (NH4+-N). Environmental correlation of microbial communities, as evaluated by a heat map (* p < 0.05, ** p < 0.01, *** p < 0.001) and Mantel tests (B).
Nutrient analysis demonstrated a consistent increase in water nutrient concentrations throughout the study period: initial (days 15–31), middle (days 46–61), and final (day 76). Bacterial community composition changed significantly over time, whereas different environmental factors had a significant impact on the bacterial communities (Table A5, p < 0.05), particularly total nitrogen (TN), total phosphorus (TP), and nitrate (NO₃⁻-N), with significant increases observed from the middle to the final phase. This period indicates nitrogen enrichment and enhanced nitrification. Additionally, organic pollution levels showed an upward trend, as evidenced by the increasing chemical oxygen demand (CODMn) from initial to final. Nitrite (NO2−-N) concentrations exhibited a complex temporal pattern, peaking initially before decreasing, while ammonia (NH4⁺-N) levels fluctuated, with initially high concentrations that decreased in the middle phase before reaching their peak in the final stage. Phosphate (PO43−-P) concentrations also increased substantially, particularly from middle to final, correlating with the trend in TP and suggesting potential nutrient enrichment with ecological implications (Table 3, p < 0.05).

Table 3.
Nutrient content of water at different times (mean ± SD, mg/L). Different letters specify the significant difference between groups at the same row (p < 0.05, one-way ANOVA; Tukey’s HSD).
3.5. Identification of Biomarkers
The LEfSe analysis identified significant potential biomarkers across three experimental groups: initial, middle, and final. A total of seven genera in both water and biofilm samples were identified as potential biomarkers, with an LDA threshold value of 4. In the water column of the initial group, Mycobacterium emerged as the key biomarker. In the middle group, Marivita, Haloferula, Luteolibacter, Pedomicrobium, and Algoriphagus were identified as the dominant biomarkers, while in the final group, Taeseokella was identified as the predominant biomarker (Figure 4A). Conversely, in the biofilm community, the initial group was characterized by potential biomarkers such as Rheinheimera, Methyloversatilis, Pseudomonas, and Hydrogenophaga. The middle group included Luteolibacter, whereas, in the final group, Taeseokella and Thermomonas were identified as the dominant biomarkers (Figure 4B).
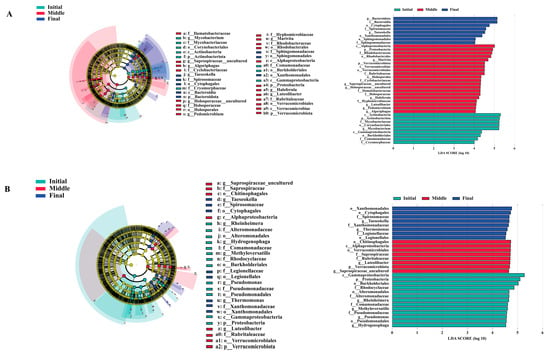
Figure 4.
Biomarkers identified during the bacterial community succession process. Water cladogram of bacterial communities and LDA scores with a threshold value of 4 identified the size of differentiation among the three different times (A). Biofilm cladogram of bacterial communities and LDA scores with a threshold value of 4.5 identified the size of differentiation among the three different times (B).
4. Discussion
4.1. Temporal Dynamics of Microbial Communities
RASs for shrimp culture have gained increasing popularity due to their ability to recycle water, minimize waste discharge, and provide stable environmental conditions for optimal shrimp growth [35]. In RASs, water quality is closely monitored, and biofilm, which forms on surfaces within the system, plays a vital role in the biological filtration process by hosting beneficial microorganisms that break down organic matter and nitrogenous waste [36]. The integration of biofilm into RASs has been shown to enhance water quality, reduce disease risk, and improve shrimp health and growth [37]. The efficiency of biofilm in RASs is crucial for achieving sustainable shrimp farming with minimal environmental impact [38]. In our study, we examined that bacterial communities in both water and biofilm environments of the shrimp RAS undergo distinct successional changes over time. The shifts are closely linked to environmental fluctuations, highlighting the dynamic nature of microbial ecosystems in aquaculture systems as confirmed by PCoA and PERMANOVA analyses (Figure 1, Table 1). This aligns with previous findings, which show that as the environments fluctuate, different bacterial taxa may become more or less competitive, leading to the observed temporal restructuring of the microbial community [39]. This pattern of microbial succession is a well-documented phenomenon wherein communities progressively shift through different stages, often culminating in a more stable community structure as the ecosystem reaches an equilibrium; such changes are indicative of the community’s adaptive responses to dynamic ecological pressures [40]. Previous research supports the significance of temporal dynamics in bacterial communities, particularly in water and biofilm ecosystems [41]. The significant fluctuations in nutrient concentrations, particularly nitrogen and phosphorus, in this study are consistent with the findings of Dmitrijs et al. [42], who observed that nutrient enrichment induces shifts in microbial community structure, often favoring taxa capable of nitrification or organic matter degradation. A comparison of α-diversity indices between water and biofilm environments reveals significant differences in bacterial community dynamics. Notably, biofilms exhibited higher diversity indices (Chao1, Richness, and Shannon) in the initial days (Table 2) compared to water, suggesting that biofilms may provide a more stable and favorable environment for bacterial growth and colonization in the early stages [43]. These findings align with previous research, which has demonstrated that biofilm habitats typically support more diverse microbial communities due to factors such as surface attachment, nutrient gradients, and protection from environmental stresses [44]. However, both environments displayed a decline in diversity at later stages, which may reflect an ecological shift toward fewer, more competitive species that thrive in stable biofilm or water conditions. This reduction in diversity over time is a well-documented phenomenon in microbial community studies, wherein successional processes and competitive exclusion play a significant role in community structuring [45]. Nutrient analysis revealed increasing concentrations of total nitrogen (TN), total phosphorus (TP), and nitrate nitrogen (NO3−-N) in the water over the study period, which aligns with similar research focused on aquaculture environments [24]. The significant rise in TN levels from the initial to the final phase highlights the potential for progressive nitrogen enrichment (Table 3) and the importance of effective nutrient management strategies in aquaculture systems to mitigate the risks associated with organic pollution and eutrophication [46]. The upward trend in TP levels and phosphate (PO43−-P) concentrations (Table 3) aligns with previous observations that indicate nutrient loading can profoundly influence microbial community structure and function [47]. Additionally, the fluctuations in NO2−-N levels offer insights into the dynamic nature of nutrient cycling within the system (Table 3). This pattern is consistent with findings suggesting that fluctuating nitrite levels are indicative of shifting nitrification rates and microbial community responses to changing nutrient availability [48]. Similarly, the fluctuations in ammonium nitrogen (NH4⁺-N) concentrations, which peaked in the final stage, further illustrate the complexity of nutrient interactions and microbial processing. These observations reinforce the intricate relationships between microbial communities and nutrient dynamics in aquaculture systems [49].
This study revealed the bacterial community composition in both water and biofilm column samples to examine the temporal variations and ecological interactions within these microbial communities associated with shrimp cultivation. The results align with the previous findings and provide valuable insights into the shifts in bacterial diversity and composition, enhancing our understanding of the microbial dynamics in RAS systems and their potential implications for shrimp health and system performance [50]. The observed shifts in bacterial community composition align with previous studies examining microbial dynamics in response to environmental perturbations [51]. The dominance of Proteobacteria in the final phase of our experiment likely reflects a response to increased nutrients or other ecological factors that favor the proliferation of this phylum (Figure 2). This aligns with findings from similar studies that highlight the resilience of Proteobacteria to environmental fluctuations such as nutrient availability, temperature fluctuations, oxygen levels [52], nitrogen removal, and water quality management [53]. Many nitrogen-removing bacteria, including ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB), belong to the phylum Proteobacteria [54]. The decline in Actinobacteriota in the final phase was particularly noteworthy (Figure 2). This aligns with previous research indicating that Actinobacteria, especially those within the Corynebacteriales order, are sensitive to changes in environmental conditions such as pH and organic matter availability [55]. The observed rise in Bacteroidota, which was associated with the degradation of complex organic compounds and an increase in oxygen availability, supports the notion that the final phase of our study was marked by shifts in resource utilization and microbial metabolism [56]. At the order level, Corynebacteriales (Phylum Actinobacteria) and Burkholderiales (Phylum Pseudomonadota) were the most abundant taxa in the initial phase (Figure 2B), which could suggest a shift in the microbial community from early colonizers to more stable, mature communities as the environmental conditions over time [57]. The dominance of Rhodobacterales and Flavobacteriales in the middle phase (Figure 2B) of this study is consistent with previous research that has recognized these taxa as significant players in biofilm maturation and organic matter degradation [58] facilitating COD removal [59]. The members of the order Saccharimonadales (Phylum Patescibacteria) and Micrococcales (Phylum Actinobacteria) demonstrated a significant increase in the final phase (Figure 2B), which is consistent with studies that have identified members of this order as key contributors to biofilm formation and organic matter cycling in aquatic environments [60]. This suggests that Saccharimonadales and Micrococcales may play a significant role in biofilm development and stabilization under the experimental conditions. The rise in unclassified taxa in the later phases of the experiment also supports findings from microbial diversity studies, which often report the rise in low-abundance taxa as environmental conditions change [61]. This phenomenon may reflect microbial niche specialization or the adaptation of previously rare organisms to new ecological opportunities [62].
4.2. Association of Bacterial Community with Physiochemical Parameters
The interaction between microorganisms and environmental variables is essential for optimizing aquaculture practices and enhancing ecosystem health [63,64]. During this study, RDA analysis revealed that environmental factors significantly influence microbial community composition (Figure 3A,B). NH4+-N and NO2−-N exhibited distinct correlations with microbial communities (Figure 3A), suggesting that these factors influence community structure in unique ways. The positive correlation between Mycobacterium and nitrite (NO2−-N) in the water column aligns with previous research suggesting that certain bacteria are specialized in utilizing nitrogen compounds during the nitrogen cycle [65]. Similarly, Cloacibacterium and Flavobacterium exhibited negative correlations (Figure 3A) with ammonia (NH4⁺-N), indicating a preference for environments with lower levels of this compound, in line with observations by Chun et al. [66], where these genera were associated with organic matter degradation in low-nutrient environments. The positive correlations between Rheinheimera and Flavobacterium with NO₂⁻-N in biofilms (Figure 3A) further support their roles in nitrogen cycling, as these genera contribute to organic matter degradation and the nitrogen cycle [67,68]. The positive correlations of Taeseokela and Thermomonas with NH4⁺-N (Figure 3A) suggest that these genera potentially participate in ammonium oxidation or utilization, consistent with research linking them to nitrogen cycling in biofilm environments [69].
4.3. Identification of Biomarkers and Function Analysis
LEfSe analysis results in our study provided critical insights into the temporal dynamics and bacterial succession within the microbial communities of the RAS. In the initial stage of the water column, Mycobacterium emerged as a dominant biomarker (Figure 4A). Previous research has highlighted that this genus is known for its resilience in oligotrophic conditions and its potential roles in biodegradation, making it a prominent taxon in aquatic systems [70]. During the mid-successional phase, Marivita, Haloferula, Luteolibacter, Pedomicrobium, and Algoriphagus were identified as the dominant biomarkers (Figure 4A). Previous findings indicate that genera Marivita and Haloferula, both belonging to the Alphaproteobacteria, are associated with organic matter degradation, nutrient cycling, improved water quality, and enhanced bioremediation potential [71]. Additionally, Luteolibacter enhanced microbial mineralization, whereas Pedomicrobium, known for biofilm formation and metal oxidation [72], likely contributed to the development of beneficial biofilms and trace metal regulation in the RAS [73]. Furthermore, the presence of Algoriphagus, adept at degrading complex polysaccharides, suggests a role in organic matter breakdown, promoting system cleanliness and reducing pathogenic risks [74,75]. In the final stage, Taeseokella was identified as the predominant biomarker (Figure 4A). Previous research indicated that though relatively less studied, this genus has been reported in marine and aquaculture environments, with potential roles in nutrient cycling and antimicrobial compound production [76]. Its dominance in later stages suggests the establishment of a mature and stable microbial community capable of sustaining homeostasis and resisting pathogenic invasions [77]. In the biofilm column, the initial stage was characterized by Rheinheimera, Methyloversatilis, and Pseudomonas as key biomarkers (Figure 4B). Previous research indicated that Rheinheimera is noted for its antimicrobial properties and potential as a biological control agent [78], while Pseudomonas is a well-documented biofilm former in aquatic systems [79]. Methyloversatilis plays a pivotal role in carbon and nitrogen cycling, facilitating detoxification during early biofilm development [80], whereas Hydrogenophaga is known for its hydrogen oxidation and nitrate reduction capabilities. Its activity under low-oxygen conditions supports nitrogen removal, contributing to reduced total ammonia nitrogen (TAN) and nitrite concentrations [81,82]. In the mid-successional stage, the concurrent presence of Luteolibacter reflects increased metabolic diversity and biofilm complexity, promoting organic matter degradation and reducing the risk of eutrophication [83,84]. In the final stage, Taeseokella and Thermomonas dominated the biofilm communities (Figure 4B). Previous research indicated that these genera are linked to organic matter degradation, stress tolerance, and thermo-tolerance, which are critical for long-term biofilm stability under fluctuating environmental conditions [85]. Their enrichment suggests a functionally stable and resilient microbial consortium that enhances nitrification and denitrification processes, improves nutrient recycling, and supports overall system stability and shrimp health while minimizing the need for external interventions [86].
5. Conclusions
This study emphasizes the dynamic nature of bacterial communities within brackish water RASs during shrimp culture, highlighting their close association with temporal changes in environmental parameters. Successional shifts in microbial composition were strongly influenced by nutrient concentrations, particularly phosphorus and nitrogen, which emerged as key environmental drivers. The identification of distinct bacterial taxa, such as Mycobacterium, Flavobacterium, and Rheinheimera, linked to nutrient cycling, organic matter degradation, and biofilm formation, highlights their ecological significance within RAS environments. Our results revealed clear phase-specific microbial assemblages and biomarkers reflecting the influence of environmental gradients on microbial succession. These findings contribute valuable insights into microbial community dynamics and their functional roles in nutrient processing and ecological stability. Future research should focus on elucidating the specific metabolic functions of key microbial taxa to better inform microbial management strategies and enhance the sustainability and performance of RAS-based shrimp aquaculture systems.
Author Contributions
A.E.: writing—original draft preparation. Y.W.: writing—review and editing. M.N.R.: writing—review and editing. W.Y.: directed writing. Z.Z.: project administration and directed writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was partially (or fully) sponsored by the National Key R&D Program of China (grant number 2020YFD0900201), the Ningbo Public Welfare Technology Application Research Project (grant number 2022S164), and the K.C. Wong Magna Fund at Ningbo University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Growth performance of shrimp in RAS.
Table A1.
Growth performance of shrimp in RAS.
| Initial | Final | |||||||
|---|---|---|---|---|---|---|---|---|
| Stocking Density (ind m−3) | Weight (g) | Number of Shrimps per Tank | Total Weight (kg) per Tank | Stocking Density (kg m−3) | Weight (g) | Number of Shrimps per Tank | Total Weight (kg) per Tank | |
| RAS | 658 ± 0.0032 | 0.02 ± 0.005 | 25,000 | 0.5 ± 0.125 | 9.14 ± 1.53 | 13.89 ± 2.71 | 18,053 | 250.7 ± 48.92 |

Table A2.
The percentages of shrimp feed ingredients over cultivation.
Table A2.
The percentages of shrimp feed ingredients over cultivation.
| Ingredients | Postlarvae (Length < 3 cm) | Juveniles (Length 3~6 cm) | Adults (Length > 6 cm) |
|---|---|---|---|
| Crude protein | 48.0 | 43.0 | 43.0 |
| Crude fat | 6.0 | 6.0 | 6.0 |
| Crude fibre | 4.0 | 6.0 | 6.0 |
| Crude ash | 17.0 | 18.0 | 18.0 |
| Total phosphate | 1.4 | 1.0 | 1.0 |
| Lysine | 3.2 | 2.3 | 2.4 |
| water | 10.0 | 12.0 | 12.0 |

Table A3.
Shrimp growth performance indicators.
Table A3.
Shrimp growth performance indicators.
| Parameters | Value | Remarks |
|---|---|---|
| Daily Growth Rate | 0.154 g | Per day |
| Survival Rate | 72.21% | At harvesting |
| Specific Growth Rate (SGR) | 7.27% | Per day based on log weight change |
| Initial Biomass | 0.5 kg | Per tank |
| Final Biomass | 250.7 kg | Per tank |
| Weight Gain (Biomass) | 250.2 kg | Per tank |
| Total Feed Given | 452.16 kg | Estimated over 90 days |
| Feed Conversion Ratio (FCR) | 1.81 | Feed used per kg weight gain |
| Protein in Feed | 48% | Commercial pellet composition |
| Protein Intake | 216.93 kg | 48% of total feed |
| Protein Efficiency Ratio (PER) | 1.15 | Weight gain per kg protein intake |

Table A4.
α-diversity of bacterial communities (mean ± SD) in water and biofilm. Significant differences between groups (days) within the same column are indicated (* p < 0.05, ** p < 0.01, one-way ANOVA; Tukey’s HSD).
Table A4.
α-diversity of bacterial communities (mean ± SD) in water and biofilm. Significant differences between groups (days) within the same column are indicated (* p < 0.05, ** p < 0.01, one-way ANOVA; Tukey’s HSD).
| Chao1 | Richness | Shannon | Simpson | ||
|---|---|---|---|---|---|
| p-value | Water | 0.8 | 0.7 | 0.3 | 0.22 |
| Biofilm | 0.06 | 0.01 ** | 0.04 * | 0.12 |

Table A5.
Quantitative effects of sampling time and environmental factors on variation in bacterial community structure using one-way ANOVA (p < 0.05).
Table A5.
Quantitative effects of sampling time and environmental factors on variation in bacterial community structure using one-way ANOVA (p < 0.05).
| Days | Factors | Days × Factors | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Community composition | 109.4 | 0.000 | 180.2 | 0.000 | 18.6 | 0.000 |
References
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.J.; Heinsbroek, L.T.N.; Schneider, O.; Blancheton, J.P.; d’Orbcastel, E.R.; Verreth, J.A.J. New Developments in Recirculating Aquaculture Systems in Europe: A Perspective on Environmental Sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Xiao, R.; Wei, Y.; An, D.; Li, D.; Ta, X.; Wu, Y.; Ren, Q. A Review on the Research Status and Development Trend of Equipment in Water Treatment Processes of Recirculating Aquaculture Systems. Rev. Aquac. 2019, 11, 863–895. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Lund, I.; Thorarinsdottir, R.; Drengstig, A.; Arvonen, K.; Pedersen, P.B. Farming Different Species in RAS in Nordic Countries: Current Status and Future Perspectives. Aquacult. Eng. 2013, 53, 2–13. [Google Scholar] [CrossRef]
- Indriastuti, C.E.; Ratnawati, B.; Budiharto, I.W. Survival and Growth Performance of the Catfish Clarias gariepinus in High Density Nurseries Using Recirculating Aquaculture System (RAS). E3S Web Conf. 2022, 348, 00013. [Google Scholar] [CrossRef]
- Gichana, Z.M.; Liti, D.; Waidbacher, H.; Zollitsch, W.; Drexler, S.; Waikibia, J. Waste Management in Recirculating Aquaculture System through Bacteria Dissimulation and Plant Assimilation. Aquac. Int. 2018, 26, 1541–1572. [Google Scholar] [CrossRef]
- Wang, Y.; Chi, L.; Liu, Q.; Xiao, Y.; Ma, D.; Xiao, Z.; Xu, S.; Li, J. Effects of Stocking Density on the Growth and Immunity of Atlantic Salmon Salmo salar Reared in Recirculating Aquaculture System (RAS). J. Oceanol. 2019, 37, 350–360. [Google Scholar] [CrossRef]
- Lorgen-Ritchie, M.; Clarkson, M.; Chalmers, L.; Taylor, J.F.; Migaud, H.; Martin, S.A. A Temporally Dynamic Gut Microbiome in Atlantic Salmon during Freshwater Recirculating Aquaculture System (RAS) Production and Post-Seawater Transfer. Front. Mar. Sci. 2021, 8, 711797. [Google Scholar] [CrossRef]
- Chang, B.V.; Liao, C.S.; Chang, Y.T.; Chao, W.L.; Yeh, S.L.; Kuo, D.L.; Yang, C.W. Investigation of a Farm-Scale Multitrophic Recirculating Aquaculture System with the Addition of Rhodovulum sulfidophilum for Milkfish (Chanos chanos) Coastal Aquaculture. Sustainability 2019, 11, 1880. [Google Scholar] [CrossRef]
- O’Shea, T.; Jones, R.; Markham, A.; Norell, E.; Scott, J.; Theuerkauf, S.; Waters, T. Towards a Blue Revolution: Catalyzing Private Investment in Sustainable Aquaculture Production Systems; The Nature Conservancy: Arlington, VA, USA; Encourage Capital: New York, NY, USA, 2019. [Google Scholar]
- Global Aquaculture Alliance (GOAL). Shrimp Production Review. 2018. Available online: https://www.globalseafood.org/wp-content/uploads/2017/06/Day1_JimAnderson.pdf (accessed on 18 October 2022).
- Bardera, G.; Usman, N.; Owen, M.; Pountney, D.; Sloman, K.A.; Alexander, M.E. The Importance of Behaviour in Improving the Production of Shrimp in Aquaculture. Rev. Aquac. 2019, 11, 1104–1132. [Google Scholar] [CrossRef]
- Li, H.; Tian, X.; Zhao, K.; Jiang, W.; Dong, S. Effect of Clostridium butyricum in Different Forms on Growth Performance, Disease Resistance, Expression of Genes Involved in Immune Responses and mTOR Signaling Pathway of Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 13–21. [Google Scholar] [CrossRef]
- Bauer, J.; Teitge, F.; Neffe, L.; Adamek, M.; Jung, A.; Peppler, C.; Steinhagen, D.; Jung-Schroers, V. Impact of a Reduced Water Salinity on the Composition of Vibrio spp. in Recirculating Aquaculture Systems for Pacific White Shrimp (Litopenaeus vannamei) and Its Possible Risks for Shrimp Health and Food Safety. J. Fish Dis. 2021, 44, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Das, R.R.; Sarkar, S.; Saranya, C.; Esakkiraj, P.; Aravind, R.; Saraswathy, R.; Rekha, P.N.; Muralidhar, M.; Panigrahi, A. Co-Culture of Indian White Shrimp, Penaeus indicus, and Seaweed, Gracilaria tenuispitata, in Amended Biofloc and Recirculating Aquaculture System (RAS). Aquaculture 2022, 548, 737432. [Google Scholar] [CrossRef]
- Roques, J.A.; Micolucci, F.; Hosokawa, S.; Sundell, K.; Kindaichi, T. Effects of Recirculating Aquaculture System Wastewater on Anammox Performance and Community Structure. Processes 2021, 9, 1183. [Google Scholar] [CrossRef]
- Bartelme, R.P.; McLellan, S.L.; Newton, R.J. Freshwater Recirculating Aquaculture System Operations Drive Biofilter Bacterial Community Shifts Around a Stable Nitrifying Consortium of Ammonia-Oxidizing Archaea and Comammox Nitrospira. Front. Microbiol. 2017, 8, 101. [Google Scholar] [CrossRef]
- Suantika, G.; Situmorang, M.L.; Nurfathurahmi, A.; Taufik, I.; Aditiawati, P.; Yusuf, N.; Aulia, R. Application of Indoor Recirculating Aquaculture System for White Shrimp (Litopenaeus vannamei) Growout Super-Intensive Culture at Low Salinity Condition. J. Aquac. Res. Dev. 2018, 9, 1000530. [Google Scholar] [CrossRef]
- Ramzan, M.N.; Shen, D.; Wei, Y.; Emmanuel, A.; Yang, W.; Zhu, J.; Wang, Y.; Zheng, Z. Performance and Microbial Community Analysis of Integrated Bioremediation Systems with Photosynthetic Bacteria in Treating Mariculture Tailwater. J. Ocean Univ. China 2025, 24, 515–524. [Google Scholar] [CrossRef]
- Ramzan, M.N.; Shen, D.; Wei, Y.; Emmanuel, A.; Nicholaus, R.; Yang, W.; Zheng, Z. Nitrogen and Phosphorus-Related Functional Genes Enhance Nutrient Removal in the Integrated Aquaculture Wastewater Bioremediation System in the Presence of Photosynthetic Bacteria. Aquac. Int. 2025, 33, 131. [Google Scholar] [CrossRef]
- Navada, S.; Vadstein, O.; Gaumet, F.; Tveten, A.-K.; Spanu, C.; Mikkelsen, Ø.; Kolarevic, J. Biofilms Remember: Osmotic Stress Priming as a Microbial Management Strategy for Improving Salinity Acclimation in Nitrifying Biofilms. Water Res. 2020, 176, 115732. [Google Scholar] [CrossRef]
- Navada, S.; Sebastianpillai, M.; Kolarevic, J.; Fossmark, R.O.; Tveten, A.K.; Gaumet, F.; Mikkelsen, Ø.; Vadstein, O. A Salty Start: Brackish Water Start-Up as a Microbial Management Strategy for Nitrifying Bioreactors with Variable Salinity. Sci. Total Environ. 2020, 739, 139934. [Google Scholar] [CrossRef]
- Roalkvam, I.; Drønen, K.; Dahle, H.; Wergeland, H.I. A Case Study of Biofilter Activation and Microbial Nitrification in a Marine Recirculation Aquaculture System for Rearing Atlantic Salmon (Salmo salar L.). Aquac. Res. 2020, 52, 94–104. [Google Scholar] [CrossRef]
- Rojas-Tirado, P.; Pedersen, P.B.; Vadstein, O.; Pedersen, L.-F. Microbial Dynamics in RAS Water: Effects of Adding Acetate as a Biodegradable Carbon-Source. Aquac. Eng. 2019, 84, 106–116. [Google Scholar] [CrossRef]
- Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L. Monitoring and Managing Microbes in Aquaculture—Towards a Sustainable Industry. Microb. Biotechnol. 2016, 9, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Fossmark, R.O.; Attramadal, K.J.K.; Nordøy, K.; Østerhus, S.W.; Vadstein, O. A Comparison of Two Seawater Adaption Strategies for Atlantic Salmon Post-Smolt (Salmo salar) Grown in Recirculating Aquaculture Systems (RAS): Nitrification, Water and Gut Microbiota, and Performance of Fish. Aquaculture 2021, 532, 735973. [Google Scholar] [CrossRef]
- Dahle, S.W.; Bakke, I.; Birkeland, M.; Nordøy, K.; Dalum, A.S.; Attramadal, K.J.K. Production of Lumpfish (Cyclopterus lumpus L.) in RAS with Distinct Water Treatments: Effects on Fish Survival, Growth, Gill Health and Microbial Communities in Rearing Water and Biofilm. Aquaculture 2020, 552, 735097. [Google Scholar] [CrossRef]
- Almeida, D.B.; Magalhaes, C.; Sousa, Z.; Borges, M.T.; Silva, E.; Blanquet, I.; Mucha, A.P. Microbial Community Dynamics in a Hatchery Recirculating Aquaculture System (RAS) of Sole (Solea senegalensis). Aquaculture 2021, 539, 736592. [Google Scholar] [CrossRef]
- Vadstein, O.; Attramadal, K.J.K.; Bakke, I.; Olsen, Y. K-Selection as Microbial Community Management Strategy: A Method for Improved Viability of Larvae in Aquaculture. Front. Microbiol. 2018, 9, 2730. [Google Scholar] [CrossRef]
- Ruan, Y.J.; Guo, X.S.; Ye, Z.Y.; Liu, Y.; Zhu, S.M. Bacterial Community Analysis of Different Sections of a Biofilter in a Full-Scale Marine Recirculating Aquaculture System. N. Am. J. Aquac. 2015, 77, 318–326. [Google Scholar] [CrossRef]
- Blancheton, J.P.; Attramadal, K.J.K.; Michaud, L.; Roque D’orbcastel, E.; Vadstein, O. Insight into Bacterial Population in Aquaculture Systems and Its Implication. Aquac. Eng. 2013, 53, 30–39. [Google Scholar] [CrossRef]
- Chen, Z.; Chang, Z.; Zhang, L.; Jiang, Y.; Ge, H.; Song, X.; Chen, S.; Zhao, F.; Li, J. Effects of Water Recirculation Rate on the Microbial Community and Water Quality in Relation to the Growth and Survival of White Shrimp (Litopenaeus vannamei). BMC Microbiol. 2019, 19, 192. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, C.; Zheng, Z.; Wei, Y.; Lu, K.; Zhu, J. Nutrient Enrichment during Shrimp Cultivation Alters Bacterioplankton Assemblies and Destroys Community Stability. Ecotoxicol. Environ. Saf. 2018, 156, 366–374. [Google Scholar] [CrossRef]
- Raza, B.; Ke, J.; Chen, L.; Shi, Y.; Zhu, J.; Shao, Z.; Zheng, Z.; Lu, K.; Yang, W. Adding Glucose Combined with Microalgae to Water Improves the Benefits of the Fungal Community on the Whiteleg Shrimp (Litopenaeus vannamei) Culture. Aquac. Rep. 2025, 40, 102580. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Aust. J. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Dahle, S.W.; Attramadal, K.J.K.; Vadstein, O.; Hestdahl, H.I.; Bakke, I. Microbial Community Dynamics in a Commercial RAS for Production of Atlantic Salmon Fry (Salmo salar). Aquaculture 2022, 546, 737382. [Google Scholar] [CrossRef]
- Chen, Z.; Chang, Z.; Zhang, L.; Wang, J.; Qiao, L.; Song, X.; Li, J. Effects of Carbon Source Addition on Microbial Community and Water Quality in Recirculating Aquaculture Systems for Litopenaeus vannamei. Fish. Sci. 2020, 86, 507–517. [Google Scholar] [CrossRef]
- Abubakar, S.; Liu, G.; Muhammad, I.A.; Zhang, Y.; Tadda, M.A.; Qi, W.; Liu, D.; Ye, Z.; Zhu, S. Recent Advances in Application of Moving Bed Bioreactors for Wastewater Treatment from Recirculating Aquaculture Systems: A Review. Aquac. Fish. 2022, 7, 244–258. [Google Scholar]
- Kinyage, J.P.H.; Pedersen, P.B.; Pedersen, L.F. Effects of Abrupt Salinity Increase on Nitrification Processes in a Freshwater Moving Bed Biofilter. Aquac. Eng. 2019, 84, 91–98. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.X.; Chai, B.F.; Luo, Z.M.; Zhao, P.Y.; Bao, J.B. Spatio-Temporal Patterns of Microbial Communities and Their Driving Mechanisms in Subalpine Lakes, Ningwu, Shanxi. Huan Jing Ke Xue 2019, 40, 3285–3294. [Google Scholar]
- Jiang, W.W.; Tian, X.L.; Li, L.; Dong, S.L.; Zhao, K.; Li, H.D.; Cai, Y. Temporal Bacterial Community Succession during the Start-Up Process of Biofilters in a Cold Freshwater Recirculating Aquaculture System. Bioresour. Technol. 2019, 287, 121441. [Google Scholar] [CrossRef]
- Gautam, A.; Lear, G.; Lewis, G.D. Analysis of Spatial and Temporal Variations in Bacterial Community Dynamics within Stream Biofilms. N. Z. J. Mar. Freshw. Res. 2021, 55, 505–523. [Google Scholar] [CrossRef]
- Dmitrijs, F.; Guo, J.; Huang, Y.; Liu, Y.; Fang, X.; Jiang, K.; Zha, L.; Cai, J.; Fu, X. Bacterial Succession in Microbial Biofilm as a Potential Indicator for Postmortem Submersion Interval Estimation. Front. Microbiol. 2022, 13, 951707. [Google Scholar] [CrossRef]
- Sprong, P.A.A.; Fofonova, V.; Wiltshire, K.H.; Neuhaus, S.; Ludwichowski, K.U.; Käse, L.; Androsov, A.; Metfies, K. Spatial Dynamics of Eukaryotic Microbial Communities in the German Bight. J. Sea Res. 2020, 163, 101914. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, Z.; Xiaodong, L.; Shengxian, Y.; Xin, C.; Huiqiu, L.; Sang, B. Distribution Patterns and Community Assembly Processes of Eukaryotic Microorganisms along an Altitudinal Gradient in the Middle Reaches of the Yarlung Zangbo River. Water Res. 2023, 239, 120047. [Google Scholar] [CrossRef] [PubMed]
- Siponen, S.; Ikonen, J.; Gomez-Alvarez, V.; Hokajärvi, A.M.; Ruokolainen, M.; Jayaprakash, B.; Kolehmainen, M.; Miettinen, I.; Pitkänen, T.; Torvinen, E. Effect of Pipe Material and Disinfectant on Active Bacterial Communities in Drinking Water and Biofilms. J. Appl. Microbiol. 2025, 136, lxaf004. [Google Scholar] [CrossRef] [PubMed]
- Irhayyim, T.; Beliczky, G.; Bercsényi, M. Nutrient Bioremediation Efficiency of Bacterial Biofilms and Plant-Based Biofilters in a Recirculating Common Carp (Cyprinus carpio L.) Culture System. Iran. J. Fish. Sci. 2021, 20, 828–845. [Google Scholar]
- Wang, H.; He, W.; Zhang, Z.; Liu, X.; Yang, Y.; Xue, H. Spatio-Temporal Evolution Mechanism and Dynamic Simulation of Nitrogen and Phosphorus Pollution of the Yangtze River Economic Belt in China. Environ. Pollut. 2024, 357, 124402. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Liu, B.; Xuan, Y.; Jiang, M.; Pan, Y.; Zhang, Y.; Gong, Y.; Lu, X.; Yu, D.; et al. Dynamic Changes of Microbial Communities in Litopenaeus vannamei Cultures and the Effects of Environmental Factors. Aquaculture 2016, 455, 97–108. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Xiong, J.; Dai, W.; Li, C. Advances, Challenges, and Directions in Shrimp Disease Control: The Guidelines from an Ecological Perspective. Appl. Microbiol. Biotechnol. 2016, 100, 6947–6954. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Huang, L.; Yan, M.; Dong, P.; Chen, C.; Guo, H.; Zhang, D. Effects of Sucrose Addition on Water Quality and Bacterioplankton Community in the Pacific White Shrimp (Litopenaeus vannamei) Culture System. Aquac. Res. 2021, 52, 4184–4197. [Google Scholar] [CrossRef]
- Xu, W.; Xu, Y.; Su, H.; Hu, X.; Xu, Y.; Li, Z.; Wen, G.; Cao, Y. Production Performance, Inorganic Nitrogen Control and Bacterial Community Characteristics in a Controlled Biofloc-Based System for Indoor and Outdoor Super-Intensive Culture of Litopenaeus vannamei. Aquaculture 2021, 531, 735749. [Google Scholar] [CrossRef]
- Wang, D.P.; Li, T.; Huang, K.L.; He, X.W.; Zhang, X.X. Roles and Correlations of Functional Bacteria and Genes in the Start-Up of Simultaneous Anammox and Denitrification System for Enhanced Nitrogen Removal. Sci. Total Environ. 2019, 655, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; He, X.; Feng, Y.; Miao, M.S.; Wang, Q.; Du, Y.D.; Xu, F. Pollutant Removal and Microorganism Evolution of Activated Sludge under Ofloxacin Selection Pressure. Bioresour. Technol. 2017, 241, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Huang, Z.; Zeng, S.; Liu, J.; Wei, D.; Deng, X.; Weng, S.; He, Z.; He, J. Environmental Factors Shape Water Microbial Community Structure and Function in Shrimp Cultural Enclosure Ecosystems. Front. Microbiol. 2017, 8, 2359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Zhang, C.; Wen, H.; Guo, W.; Hao, H. Effect of Filling Fraction on the Performance of Sponge-Based Moving Bed Biofilm Reactor. Bioresour. Technol. 2016, 219, 762–767. [Google Scholar] [CrossRef]
- Yu, B.B.; Xie, G.J.; Shen, Z.; Shao, K.Q.; Tang, X.M. Spatiotemporal Variations, Assembly Processes, and Co-Occurrence Patterns of Particle-Attached and Free-Living Bacteria in a Large Drinking Water Reservoir in China. Front. Microbiol. 2023, 13, 1056147. [Google Scholar] [CrossRef]
- Gu, Y.; Wei, Y.; Xiang, Q.; Zhao, K.; Yu, X.; Zhang, X.; Li, C.; Chen, Q.; Xiao, H.; Zhang, X. C:N Ratio Shaped Both Taxonomic and Functional Structure of Microbial Communities in Livestock and Poultry Breeding Wastewater Treatment Reactor. Sci. Total Environ. 2019, 651, 625–633. [Google Scholar] [CrossRef]
- Shaw, J.L.; Monis, P.; Fabris, R.; Ho, L.; Braun, K.; Drikas, M.; Cooper, A. Assessing the Impact of Water Treatment on Bacterial Biofilms in Drinking Water Distribution Systems Using High-Throughput DNA Sequencing. Chemosphere 2014, 117, 185–192. [Google Scholar] [CrossRef]
- Naik, A.; Smithers, M.; Moisander, P.H. Impacts of UV-C Irradiation on Marine Biofilm Community Succession. Appl. Environ. Microbiol. 2021, 88, e02642-21. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, K.X.; Li, K.H.; Jin, Y.; He, X.Q. Deciphering the Diversity Patterns and Community Assembly of Rare and Abundant Bacterial Communities in a Wetland System. Sci. Total Environ. 2022, 838, 156334. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, G.; Tang, X.; Shao, K. Impacts of Different Salinities on Bacterial Biofilm Communities in Fresh Water. Can. J. Microbiol. 2014, 60, 319–326. [Google Scholar] [CrossRef]
- Cai, W.; Huang, Q.; Li, H.; Cheng, H. Longitudinal Patterns of Microbial Communities in the Water Diversion Rivers of South-to-North Water Diversion Project. Clean-Soil Air Water 2022, 50, 2100303. [Google Scholar] [CrossRef]
- Xing, R.; Gao, Q.; Zhang, F.; Wang, J.; Chen, S. Environmental Filtering Affects Fungal Communities More than Dispersal Limitation in a High-Elevation Hyperarid Basin on Qinghai–Tibet Plateau. FEMS Microbiol. Lett. 2021, 368, fnab033. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.B.; Liu, X.X.; Shen, M.J.; She, G.L.; Ye, B.C. The Nitrogen Regulator GlnR Directly Controls Transcription of the prpDBC Operon Involved in Methylcitrate Cycle in Mycobacterium smegmatis. J. Bacteriol. 2019, 201, e00627-18. [Google Scholar] [CrossRef] [PubMed]
- Chun, B.H.; Lee, Y.; Jin, H.M.; Jeon, C.O. Cloacibacterium caeni sp. nov., Isolated from Activated Sludge. Int. J. Syst. Evol. Microbiol. 2017, 67, 1688–1692. [Google Scholar] [CrossRef]
- Sun, P.; Chen, Y.; Liu, J.; Xu, Y.; Zhou, L.; Wu, Y. Periphytic Biofilms Function as a Double-Edged Sword Influencing Nitrogen Cycling in Paddy Fields. Environ. Microbiol. 2022, 24, 6279–6289. [Google Scholar] [CrossRef]
- Lin, D.; Zhu, L.; Qiu, C.; Mu, X.; Zhang, S.; Ohore, O.E. Ammonium Loading Drives Bacterial Community Shifts in Biofilms Attached to the Submerged Macrophyte Hydrilla verticillata. Aquat. Microb. Ecol. 2020, 85, 59–69. [Google Scholar] [CrossRef]
- Kim, M.; Park, M.S.; Kang, I.; Cho, J.C. Thermomonas paludicola sp. nov., Isolated from a Lotus Wetland. Int. J. Syst. Evol. Microbiol. 2023, 73, 005737. [Google Scholar] [CrossRef]
- Lührig, K.; Canbäck, B.; Paul, C.J.; Johansson, T.; Persson, K.M.; Rådström, P. Bacterial Community Analysis of Drinking Water Biofilms in Southern Sweden. Microbes Environ. 2015, 30, 99–107. [Google Scholar] [CrossRef]
- Qiao, L.; Bao, J.; Li, T.; Sun, X.; Ze, W.; Li, J. Microbial Community Structure and Its Driving Factors in the Whiteleg Shrimp (Litopenaeus vannamei) Aquaculture System. Appl. Ecol. Environ. Res. 2025, 23, 671–686. [Google Scholar] [CrossRef]
- Chang, F.; He, S.; Dang, C. Assisted Selection of Biomarkers by Linear Discriminant Analysis Effect Size (LEfSe) in Microbiome Data. J. Visualized Exp. 2022, 183, e61715. [Google Scholar]
- Chen, Z.; Liu, Y.; Liu, L.Z.; Wang, X.J.; Liu, Z.P.; Liu, Y. Heterotrophic Bacterial Community Structure of Multistage Biofilters in a Commercial Pufferfish Takifugu rubripes RAS. Adv. Mater. Res. 2013, 726, 1621–1627. [Google Scholar] [CrossRef]
- Tang, B.; Chen, Q.Y.; Bin, L.Y.; Huang, S.S.; Zhang, W.X.; Fu, F.L.; Li, P. Insight into the Microbial Community and Its Succession of a Coupling Anaerobic-Aerobic Biofilm on Semi-Suspended Bio-Carriers. Bioresour. Technol. 2018, 247, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Nesje, J. Impacts of Organic Matter Removal Efficiency on the Microbial Carrying Capacity and Stability of Land-Based Recirculating Aquaculture Systems. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2018; pp. 1–58, NTNU Open. Available online: http://hdl.handle.net/11250/2495355 (accessed on 7 May 2025).
- Ganesan, G.; Velayudhan, S.S.; David, J.S.R. Statistical Optimization of Medium Constituents and Conditions for Improved Antimicrobial Compound Production by Marine Streptomyces sp. JRG-04. Arch. Biol. Sci. 2017, 69, 723–731. [Google Scholar] [CrossRef]
- Joung, Y.; Hong, S.; Kim, H.; Kang, H.; Farrance, C.E.; Joh, K. Taeseokella kangwonensis gen. nov., sp. nov., Isolated from a Freshwater Reservoir. Int. J. Syst. Evol. Microbiol. 2015, 65, 4309–4314. [Google Scholar] [CrossRef]
- Chen, W.M.; Lin, C.Y.; Young, C.C.; Sheu, S.Y. Rheinheimera aquatica sp. nov., Antimicrobial Activity-Producing Bacterium Isolated from Freshwater Culture Pond. J. Microbiol. Biotechnol. 2010, 20, 1386–1392. [Google Scholar] [CrossRef]
- Van Alst, N.E.; Picardo, K.F.; Iglewski, B.H.; Haidaris, C.G. Nitrate Sensing and Metabolism Modulate Motility, Biofilm Formation, and Virulence in Pseudomonas aeruginosa. Infect. Immun. 2007, 75, 3780–3790. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Chen, P.; Yuan, S.; Wang, K.; Wang, S.; Jiang, X. Role of Nitrogen Cycling Functional Genes and Their Key Influencing Factors in Eutrophic Aquatic Ecosystems. Environ. Rev. 2025, 33, 1–10. [Google Scholar] [CrossRef]
- Orr, R.J.; Rombauts, S.; Van de Peer, Y.; Shalchian-Tabrizi, K. Draft Genome Sequences of Two Unclassified Bacteria, Hydrogenophaga sp. Strains IBVHS1 and IBVHS2, Isolated from Environmental Samples. Genome Announc. 2017, 5, 10-1128. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Sabaratnam, V.; Tan, G.Y.A.; Chong, V.C. Identification of Indigenous Bacteria Isolated from Shrimp Aquaculture Wastewater with Bioremediation Application: Total Ammoniacal Nitrogen (TAN) and Nitrite Removal. Sains Malaysiana 2015, 44, 1103–1110. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.N.; Zhang, G.Z.; Wang, B.S.; Zhang, X.; Li, J. Key Bacteria for the Microbial Degradation of Pollutants in Cellar Water. Huan Jing Ke Xue 2018, 39, 4766–4777. [Google Scholar]
- Ohshiro, T.; Harada, N.; Kobayashi, Y.; Miki, Y.; Kawamoto, H. Microbial Fucoidan Degradation by Luteolibacter algae H18 with Deacetylation. Biosci. Biotechnol. Biochem. 2012, 76, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Salcedo Moyano, A.J.; Delforno, T.P.; Subtil, E.L. Simultaneous Nitrification-Denitrification (SND) Using a Thermoplastic Gel as Support: Pollutants Removal and Microbial Community in a Pilot-Scale Biofilm Membrane Bioreactor. Environ. Technol. 2022, 43, 4411–4425. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.T.; He, Y.Q.; Li, G.X.; Xiao, H.; Dai, X.R.; Yang, M.R.; Bao, P. Genome Sequence of Sulfide-Dependent Denitrification Bacterium Thermomonas sp. Strain XSG, Isolated from Marine Sediment. Microbiol. Resour. Announc. 2021, 10, e00361-21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).