Enhancing Laying Hen Productivity and Health: Influence of Dietary Probiotic Bacillus Strains and Prebiotic Saccharomyces cerevisiae Yeast Cell Wall on Production Performance, Egg Quality, and Inflammatory Responses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Husbandry Practices
2.2. Sampling and Measurements
2.3. Data Analysis
3. Results
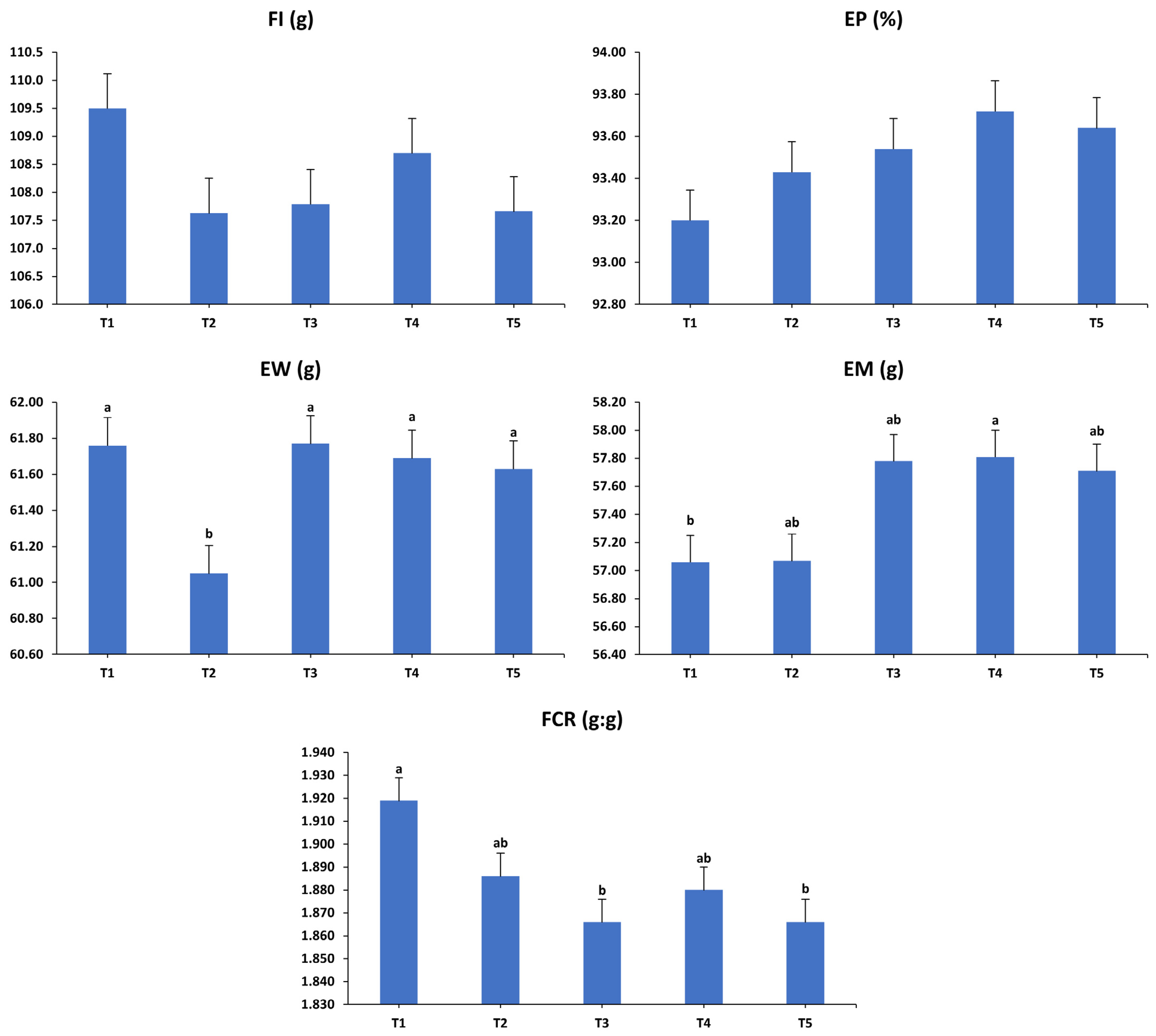
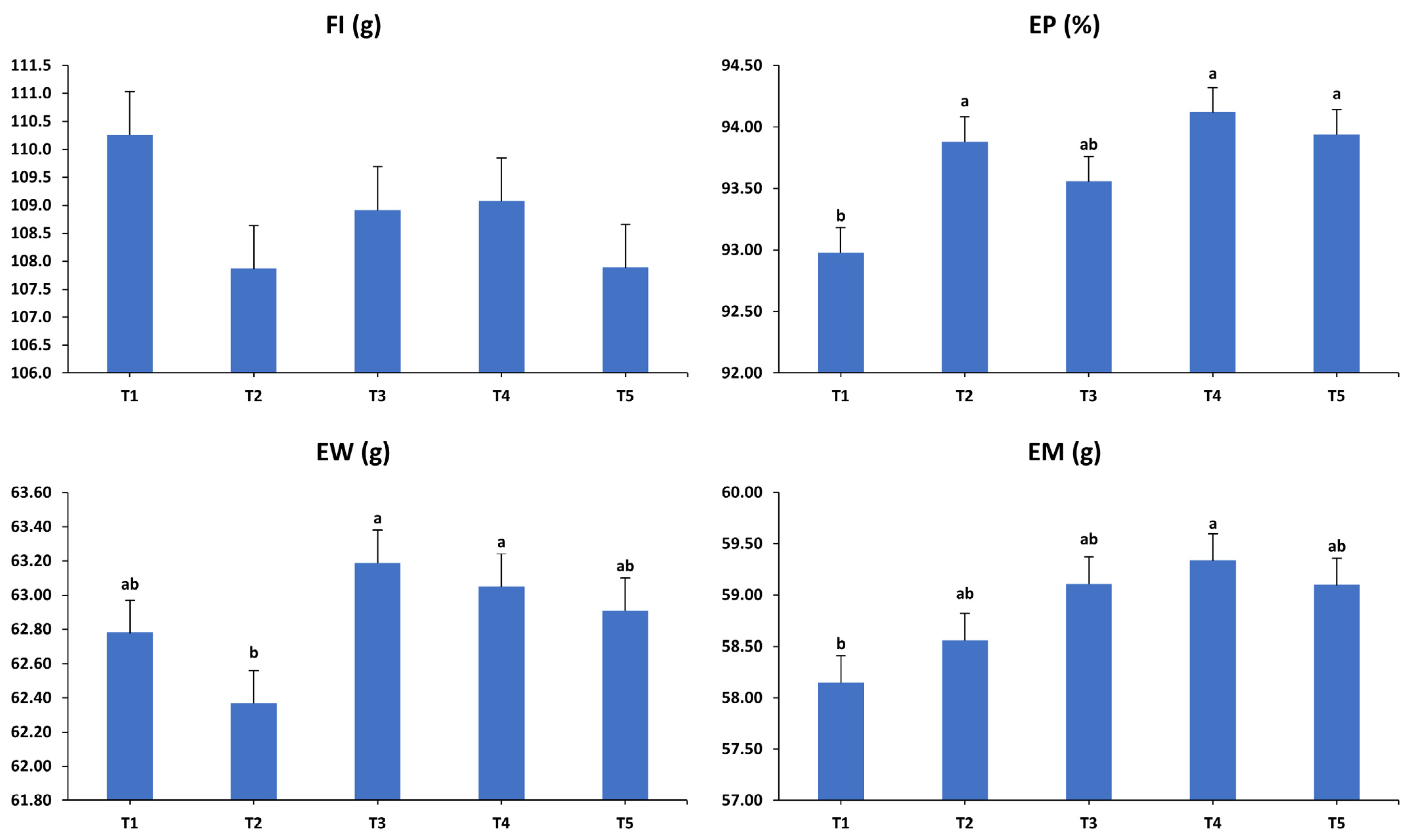
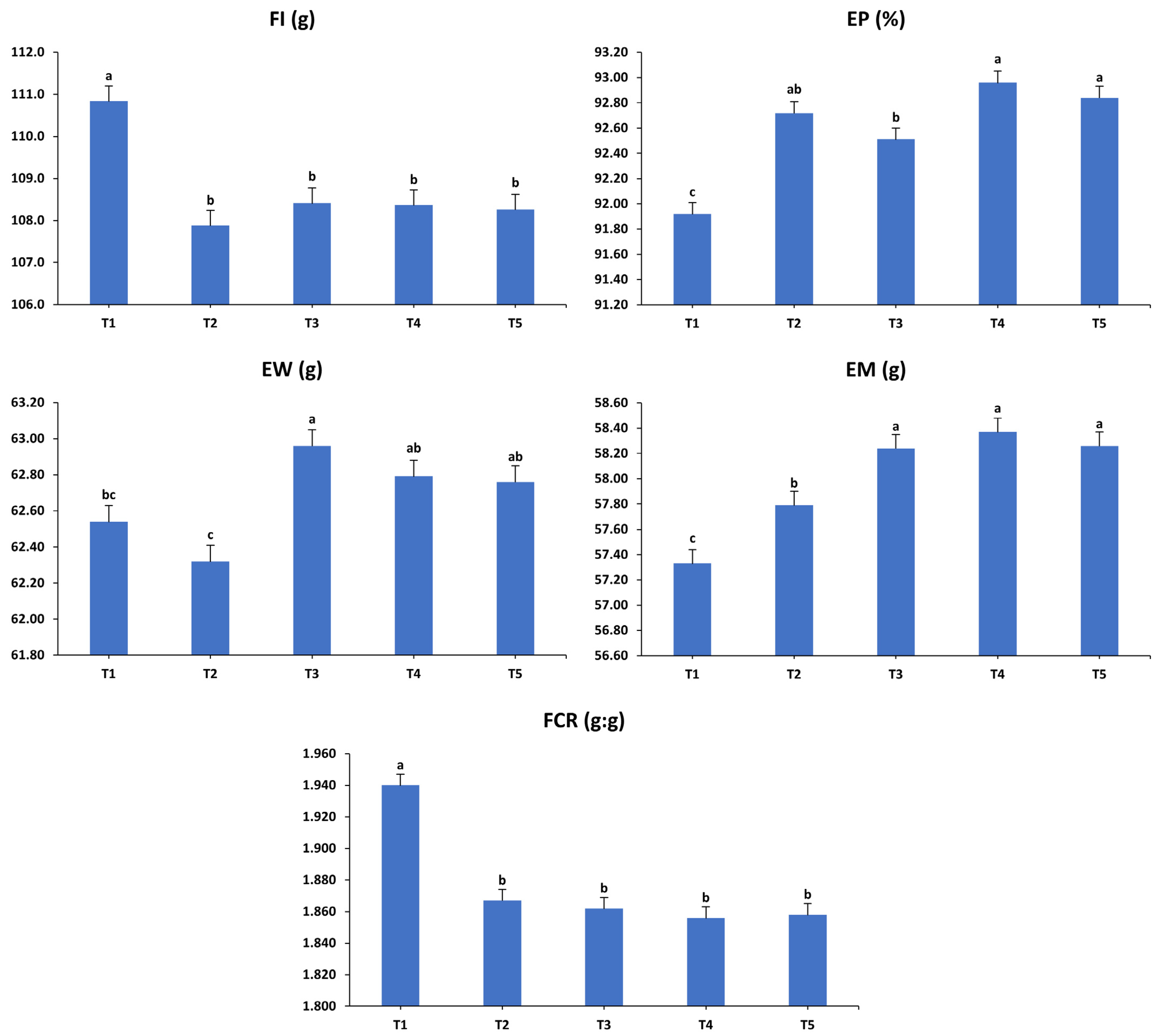
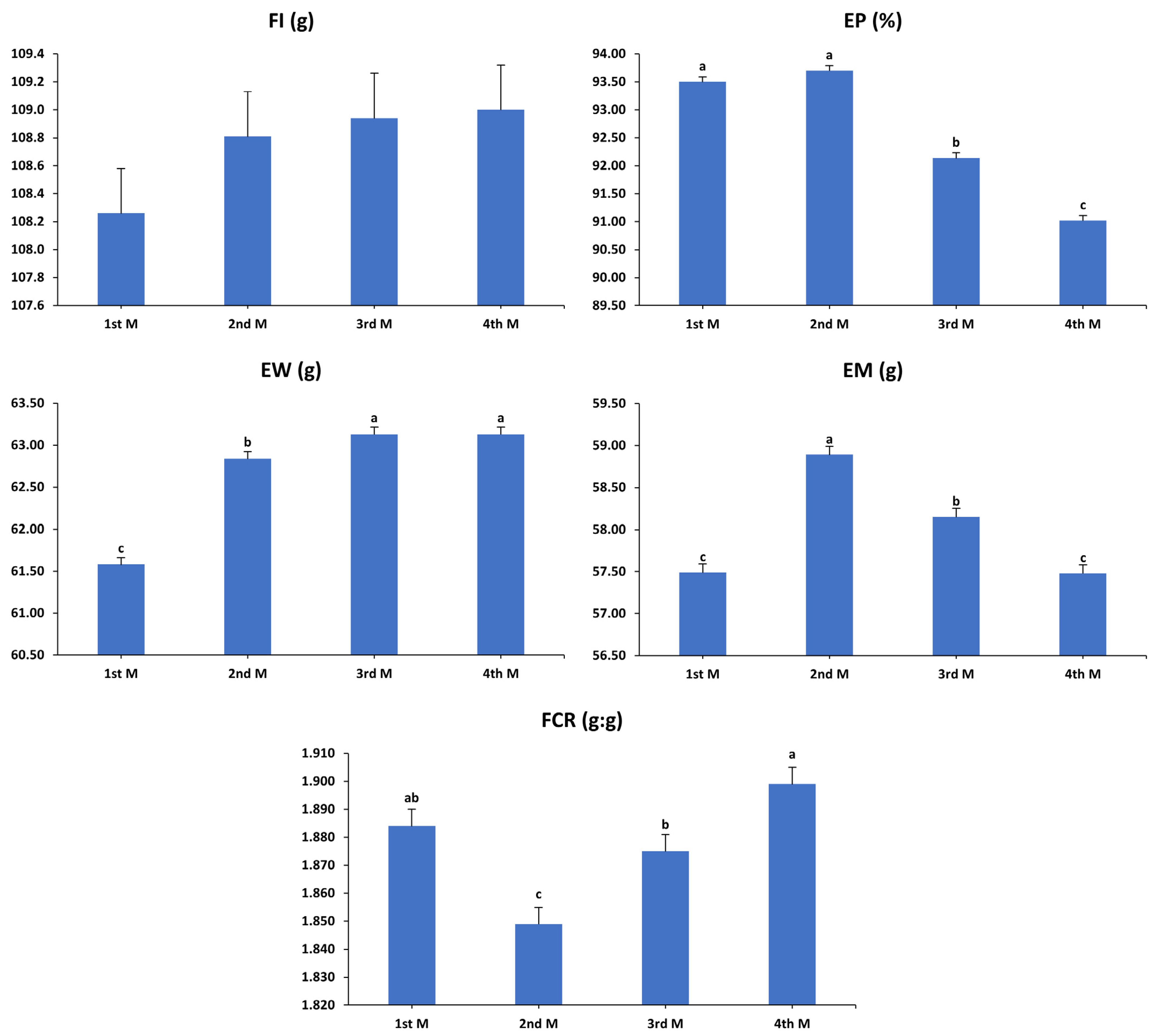
3.1. Production Performance
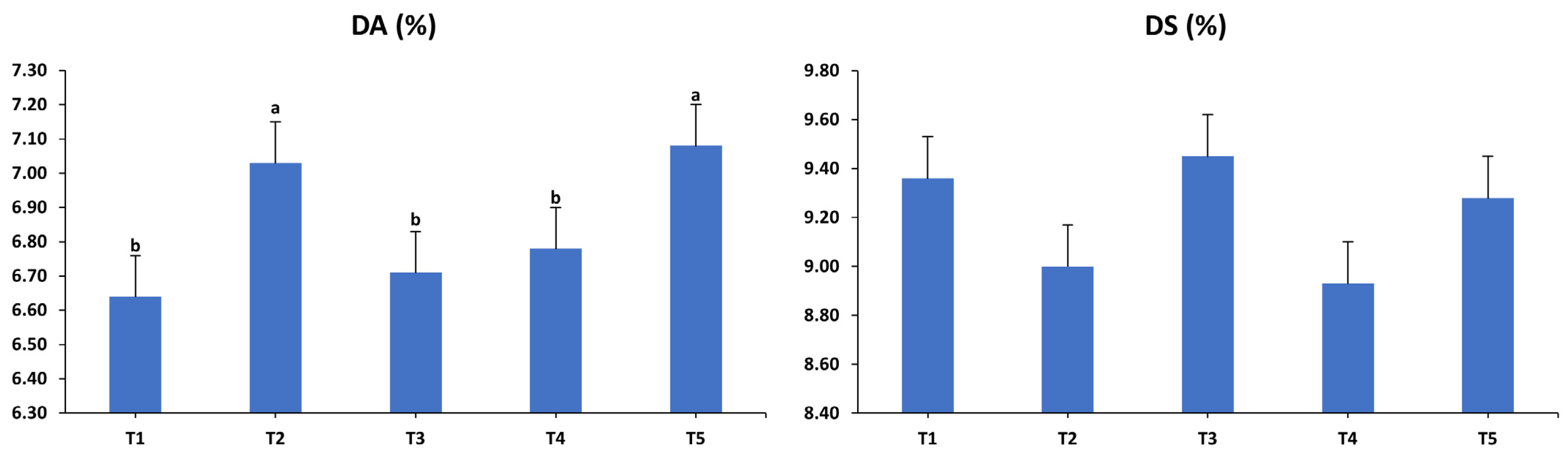
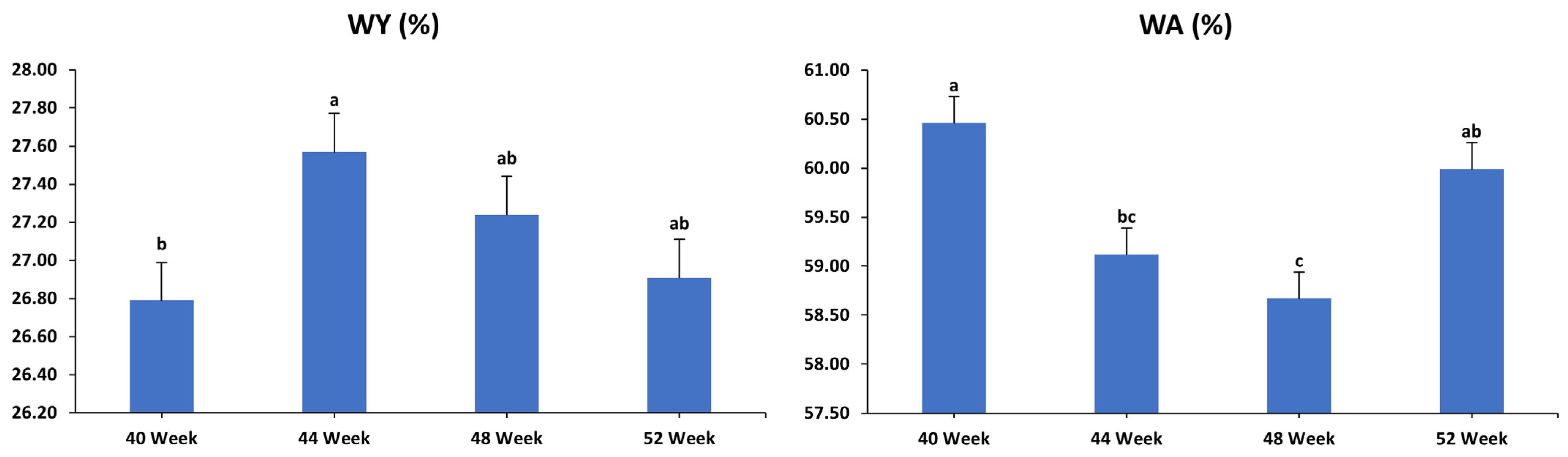
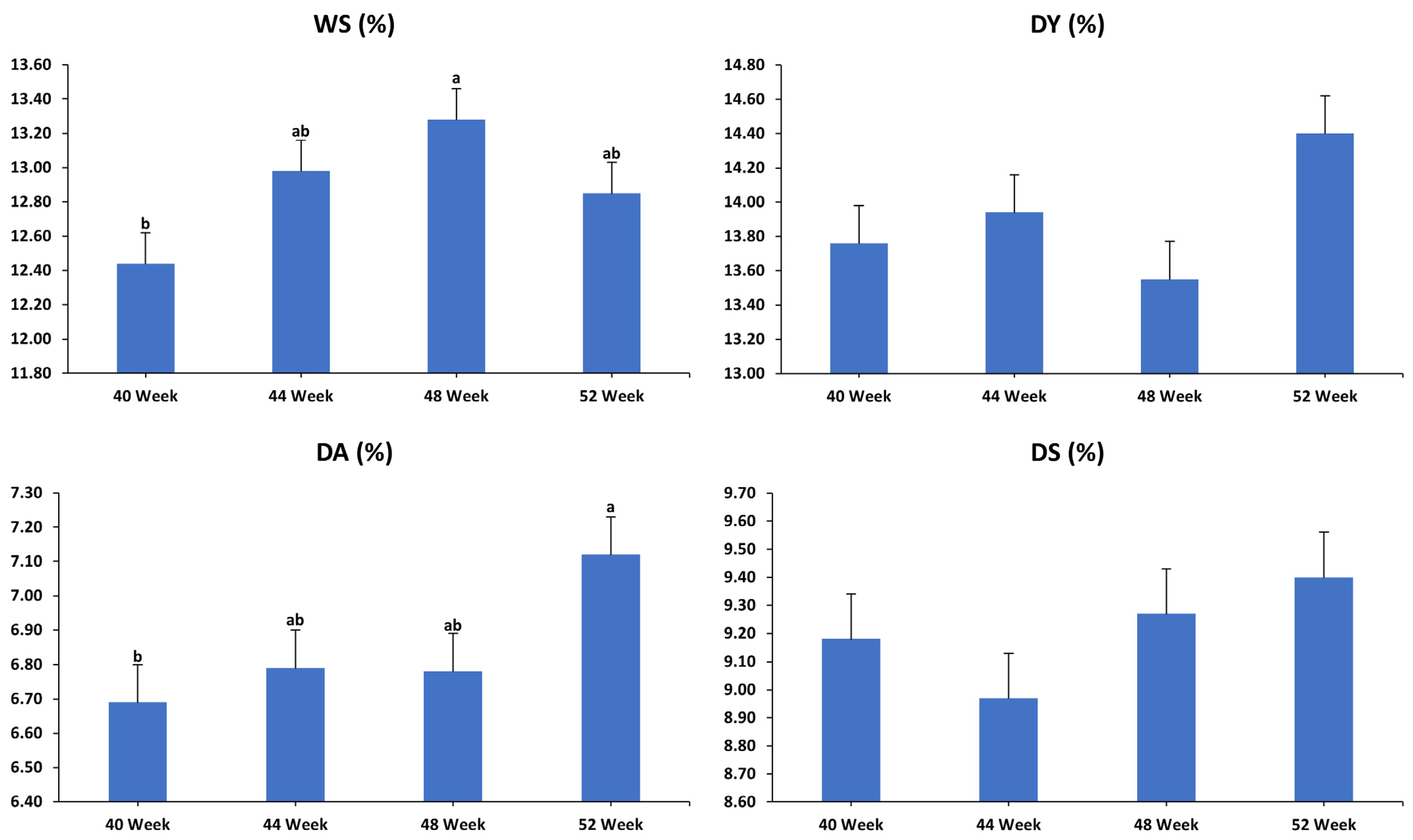
3.2. Egg Quality Characteristics
3.3. Immune-Related Inflammatory Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to Antibiotics for Maximizing Growth Performance and Feed Efficiency in Poultry: A Review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Low, C.X.; Tan, L.T.H.; Mutalib, N.S.A.; Pusparajah, P.; Goh, B.H.; Chan, K.G.; Letchumanan, V.; Lee, L.H. Unveiling the Impact of Antibiotics and Alternative Methods for Animal Husbandry: A Review. Antibiotics 2021, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Ares, I.; Martínez-Larrañaga, M.R.; Martínez, M.A. Prebiotics and Probiotics in Feed and Animal Health. In Nutraceuticals in Veterinary Medicine; Gupta, R., Srivastava, A., Lall, R., Eds.; Springer: Cham, Switzerland, 2019; pp. 261–285. [Google Scholar] [CrossRef]
- Ramlucken, U.; Lalloo, R.; Roets, Y.; Moonsamy, G.; van Rensburg, C.J.; Thantsha, M.S. Advantages of Bacillus-Based Probiotics in Poultry Production. Livest. Sci. 2020, 241, 104215. [Google Scholar] [CrossRef]
- Bahaddad, S.A.; Almalki, M.H.K.; Alghamdi, O.A.; Sohrab, S.S.; Yasir, M.; Azhar, E.I.; Chouayekh, H. Bacillus Species as Direct-Fed Microbial Antibiotic Alternatives for Monogastric Production. Probiotics Antimicrob. Proteins 2022, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Gay, C.G.; Lillehoj, H.S. Bacillus spp. as Direct-Fed Microbial Antibiotic Alternatives to Enhance Growth, Immunity, and Gut Health in Poultry. Avian Pathol. 2018, 47, 339–351. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Aljumaah, M.R.; Alkhulaifi, M.M.; Alabdullatif, A.; Suliman, G.M.; Al Sulaiman, A.R. Comparative Effects of Bacillus subtilis and Bacillus licheniformis on Live Performance, Blood Metabolites and Intestinal Features in Broiler Inoculated with Salmonella Infection during the Finisher Phase. Microb. Pathog. 2020, 139, 103870. [Google Scholar] [CrossRef]
- Neijat, M.; Shirley, R.B.; Barton, J.; Thiery, P.; Welsher, A.; Kiarie, E. Effect of Dietary Supplementation of Bacillus subtilis DSM29784 on Hen Performance, Egg Quality Indices, and Apparent Retention of Dietary Components in Laying Hens from 19 to 48 Weeks of Age. Poult. Sci. 2019, 98, 5622–5635. [Google Scholar] [CrossRef]
- Guo, J.R.; Dong, X.F.; Liu, S.; Tong, J.M. Effects of Long-Term Bacillus Subtilis CGMCC 1.921 Supplementation on Performance, Egg Quality, and Fecal and Cecal Microbiota of Laying Hens. Poult. Sci. 2017, 96, 1280–1289. [Google Scholar] [CrossRef]
- Elisashvili, V.; Kachlishvili, E.; Chikindas, M.L. Recent Advances in the Physiology of Spore Formation for Bacillus Probiotic Production. Probiotics Antimicrob. Proteins 2019, 11, 731–747. [Google Scholar] [CrossRef]
- Popov, I.V.; Algburi, A.; Prazdnova, E.V.; Mazanko, M.S.; Elisashvili, V.; Bren, A.B.; Chistyakov, V.A.; Tkacheva, E.V.; Trukhachev, V.I.; Donnik, I.M.; et al. A Review of the Effects and Production of Spore-Forming Probiotics for Poultry. Animals 2021, 11, 1941. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wu, C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-Biotics, and Post-Biotics. Front. Nutr. 2022, 8, 634897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, W.; Lei, K.; Wang, B.; Wang, Y.; Zhou, Y.; Li, W. Effects of Dietary Bacillus Licheniformis on Gut Physical Barrier, Immunity, and Reproductive Hormones of Laying Hens. Probiotics Antimicrob. Proteins 2017, 9, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Luise, D.; Bosi, P.; Raff, L.; Amatucci, L.; Virdis, S.; Trevisi, P. Bacillus spp. Probiotic Strains as a Potential Tool for Limiting the Use of Antibiotics, and Improving the Growth and Health of Pigs and Chickens. Front. Microbiol. 2022, 13, 801827. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, Z.; Xu, Y.; Ying, J.; Wang, B.; Majeed, M.; Majeed, S.; Pande, A.; Li, W. Application of Bacillus coagulans in Animal Husbandry and Its Underlying Mechanisms. Animals 2020, 10, 454. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Yu, Y.; Zhang, R.; Wu, Y.; Yue, M.; Yang, C. Effects of Bacillus coagulans on Growth Performance, Antioxidant Capacity, Immunity Function, and Gut Health in Broilers. Poult. Sci. 2021, 100, 101168. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Y.; Zhan, Z.C.; Zhang, W.; Fu, D.B.; Zhao, R.; Chen, X.D. Research Note: Effects of Bacillus coagulans X26 on the Production Performance, Intestinal Structure, Short-Chain Fatty Acids and Flora Composition of Laying Hens during the Peak Laying Period. Poult. Sci. 2022, 101, 101835. [Google Scholar] [CrossRef]
- Yang, J.; Zhan, K.; Zhang, M. Effects of the Use of a Combination of Two Bacillus Species on Performance, Egg Quality, Small Intestinal Mucosal Morphology, and Cecal Microbiota Profile in Aging Laying Hens. Probiotics Antimicrob. Proteins 2020, 12, 204–213. [Google Scholar] [CrossRef]
- Perricone, V.; Sandrini, S.; Irshad, N.; Savoini, G.; Comi, M.; Agazzi, A. Yeast-Derived Products: The Role of Hydrolyzed Yeast and Yeast Culture in Poultry Nutrition—A Review. Animals 2022, 12, 1426. [Google Scholar] [CrossRef]
- Fathima, S.; Shanmugasundaram, R.; Sifri, M.; Selvaraj, R. Yeasts and Yeast-Based Products in Poultry Nutrition. J. Appl. Poult. Res. 2023, 32, 100345. [Google Scholar] [CrossRef]
- Bar-Dagan, H.; Gover, O.; Cohen, N.A.; Vetvicka, V.; Rozenboim, I.; Schwartz, B. Beta-Glucans Induce Cellular Immune Training and Changes in Intestinal Morphology in Poultry. Front. Vet. Sci. 2023, 9, 1092812. [Google Scholar] [CrossRef]
- Schwartz, B.; Vetvicka, V. Review: β-Glucans as Effective Antibiotic Alternatives in Poultry. Molecules 2021, 26, 3560. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Mondragon, A.d.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-Origin Prebiotics Based on Chitin: An Alternative for the Future? A Critical Review. Foods 2020, 9, 782. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, M.U.; El-Hack, M.E.A.; Hassan, F.; El-Saadony, M.T.; Khafaga, A.F.; Batiha, G.E.; Yehia, N.; Elnesr, S.S.; Alagawany, M.; El-Tarabily, K.A.; et al. The Potential Mechanistic Insights and Future Implications for the Effect of Prebiotics on Poultry Performance, Gut Microbiome, and Intestinal Morphology. Poult. Sci. 2021, 100, 101143. [Google Scholar] [CrossRef]
- Koiyama, N.T.G.; Utimi, N.B.P.; Santos, B.R.L.; Bonato, M.A.; Barbalho, R.; Gameiro, A.H.; Araújo, C.S.S.; Araújo, L.F. Effect of Yeast Cell Wall Supplementation in Laying Hen Feed on Economic Viability, Egg Production, and Egg Quality. J. Appl. Poult. Res. 2018, 27, 116–123. [Google Scholar] [CrossRef]
- Zhou, J.; Fu, Y.; Qi, G.; Dai, J.; Zhang, H.; Wang, J.; Wu, S. Yeast Cell-Wall Polysaccharides Improve Immunity and Attenuate Inflammatory Response via Modulating Gut Microbiota in LPS-Challenged Laying Hens. Int. J. Biol. Macromol. 2023, 224, 407–421. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Poultry: Ninth Revised Edition; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. Official Methods of Analysis of AOAC INTERNATIONAL, 22nd ed.; AOAC Publications: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Khomayezi, R.; Adewole, D. Probiotics, Prebiotics, and Synbiotics: An Overview of Their Delivery Routes and Effects on Growth and Health of Broiler Chickens. Worlds Poult. Sci. J. 2022, 78, 57–81. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Y.; Li, D.; Yan, C.; Jiang, Y.; Hu, Z.; Zhang, Z.; Du, R.; Zhao, X.; Zhang, Y.; et al. Probiotic Mediated Intestinal Microbiota and Improved Performance, Egg Quality and Ovarian Immune Function of Laying Hens at Different Laying Stage. Front. Microbiol. 2023, 14, 1041072. [Google Scholar] [CrossRef] [PubMed]
- Youssef, I.M.; Khalil, H.A.; Jaber, F.A.; Alhazzaa, R.A.; Alkholy, S.O.; Almehmadi, A.M.; Alhassani, W.E.; Al-Shehri, M.; Hassan, H.; Hassan, M.S.; et al. Influence of Dietary Mannan-Oligosaccharides Supplementation on Hematological Characteristics, Blood Biochemical Parameters, Immune Response and Histological State of Laying Hens. Poult. Sci. 2023, 102, 103071. [Google Scholar] [CrossRef]
- Alqhtani, A.H.; Al Sulaiman, A.R.; Alharthi, A.S.; Abudabos, A.E. Dietary Supplementation of Prebiotic Yeast Saccharomyces cerevisiae Cell Wall Promotes Growth Performance and Intestinal Health in Broiler Chickens Challenged with Clostridium Perfringens. Br. Poult. Sci. 2024, 65, 129–136. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Rudeaux, F.; Kim, I.H. Efficacy of Dietary Bacillus subtilis and Bacillus licheniformis Supplementation Continuously in Pullet and Lay Period on Egg Production, Excreta Microflora, and Egg Quality of Hyline-Brown Birds. Poult. Sci. 2019, 98, 4722–4728. [Google Scholar] [CrossRef]
- Shini, S.; Bryden, W.L. Probiotics and Gut Health: Linking Gut Homeostasis and Poultry Productivity. Anim. Prod. Sci. 2021, 62, 1090–1112. [Google Scholar] [CrossRef]
- Pan, X.; Cai, Y.; Kong, L.; Xiao, C.; Zhu, Q.; Song, Z. Probiotic Effects of Bacillus licheniformis DSM5749 on Growth Performance and Intestinal Microecological Balance of Laying Hens. Front. Nutr. 2022, 9, 868093. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jian, H.; Zhao, W.; Li, J.; Zou, X.; Dong, X. Effects of Dietary Bacillus coagulans on the Productive Performance, Egg Quality, Serum Parameters, and Intestinal Morphology of Laying Hens during the Late Laying Period. Ital. J. Anim. Sci. 2023, 22, 95–105. [Google Scholar] [CrossRef]
- Hao, E.Y.; Wang, D.H.; Chen, Y.F.; Zhou, R.Y.; Chen, H.; Huang, R.L. The Relationship between the MTOR Signaling Pathway and Ovarian Aging in Peak-Phase and Late-Phase Laying Hens. Poult. Sci. 2021, 100, 334–347. [Google Scholar] [CrossRef]
- Samiullah, S.; Omar, A.S.; Roberts, J.; Chousalkar, K. Effect of Production System and Flock Age on Eggshell and Egg Internal Quality Measurements. Poult. Sci. 2017, 96, 246–258. [Google Scholar] [CrossRef]
- Mfoundou, J.D.L.; Guo, Y.J.; Liu, M.M.; Ran, X.R.; Fu, D.H.; Yan, Z.Q.; Li, M.N.; Wang, X.R. The Morphological and Histological Study of Chicken Left Ovary during Growth and Development among Hy-Line Brown Layers of Different Ages. Poult. Sci. 2021, 100, 101191. [Google Scholar] [CrossRef]
- Tůmová, E.; Uhlířová, L.; Tůma, R.; Chodová, D.; Máchal, L. Age Related Changes in Laying Pattern and Egg Weight of Different Laying Hen Genotypes. Anim. Reprod. Sci. 2017, 183, 21–26. [Google Scholar] [CrossRef]
- Alkhulaifi, M.M.; Alqhtani, A.H.; Alharthi, A.S.; Al Sulaiman, A.R.; Abudabos, A.M. Influence of Prebiotic Yeast Cell Wall Extracts on Growth Performance, Carcase Attributes, Biochemical Metabolites, and Intestinal Morphology and Bacteriology of Broiler Chickens Challenged with Salmonella typhimurium and Clostridium perfringens. Ital. J. Anim. Sci. 2022, 21, 1190–1199. [Google Scholar] [CrossRef]
- Bilal, R.M.; Elwan, H.A.M.; Elnesr, S.S.; Farag, M.R.; El-Shall, N.A.; Ismail, T.A.; Alagawany, M. Use of Yeast and Its Derived Products in Laying Hens: An Updated Review. Worlds Poult. Sci. J. 2022, 78, 1087–1104. [Google Scholar] [CrossRef]
- Obianwuna, U.E.; Oleforuh-Okoleh, V.U.; Wang, J.; Zhang, H.J.; Qi, G.H.; Qiu, K.; Wu, S.G. Natural Products of Plants and Animal Origin Improve Albumen Quality of Chicken Eggs. Front. Nutr. 2022, 9, 875270. [Google Scholar] [CrossRef]
- Bilal, R.M.; Hassan, F.U.; Saeed, M.; Rafeeq, M.; Zahra, N.; Fraz, A.; Saeed, S.; Khan, M.A.; Mahgoub, H.A.M.; Farag, M.R.; et al. Role of Yeast and Yeast-Derived Products as Feed Additives in Broiler Nutrition. Anim. Biotechnol. 2023, 34, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Marzec, A.; Damaziak, K.; Kowalska, H.; Riedel, J.; Michalczuk, M.; Koczywąs, E.; Cisneros, F.; Lenart, A.; Niemiec, J. Effect of Hens Age and Storage Time on Functional and Physiochemical Properties of Eggs. J. Appl. Poult. Res. 2019, 28, 290–300. [Google Scholar] [CrossRef]
- Chang, X.Y.; Edna, O.U.; Wang, J.; Zhang, H.J.; Zhou, J.M.; Qiu, K.; Wu, S.G. Histological and Molecular Difference in Albumen Quality between Post-Adolescent Hens and Aged Hens. Poult. Sci. 2024, 103, 103618. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The Crucial Roles of Inflammatory Mediators in Inflammation: A Review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef]
- Rhayat, L.; Maresca, M.; Nicoletti, C.; Perrier, J.; Brinch, K.S.; Christian, S.; Devillard, E.; Eckhardt, E. Effect of Bacillus subtilis Strains on Intestinal Barrier Function and Inflammatory Response. Front. Immunol. 2019, 10, 431111. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. Effects of Bacillus subtilis and Bacillus licheniformis on Growth Performance, Immunity, Short Chain Fatty Acid Production, Antioxidant Capacity, and Cecal Microflora in Broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef]
- Rawling, M.; Schiavone, M.; Apper, E.; Merrifield, D.L.; Castex, M.; Leclercq, E.; Foey, A. Yeast Cell Wall Extracts from Saccharomyces Cerevisiae Varying in Structure and Composition Differentially Shape the Innate Immunity and Mucosal Tissue Responses of the Intestine of Zebrafish (Danio rerio). Front. Immunol. 2023, 14, 1158390. [Google Scholar] [CrossRef]
- Lambo, M.T.; Chang, X.; Liu, D.; Balasubramanian, B.; Sureshkumar, S. The Recent Trend in the Use of Multistrain Probiotics in Livestock Production: An Overview. Animals 2021, 11, 2805. [Google Scholar] [CrossRef]
- Wlaźlak, S.; Pietrzak, E.; Biesek, J.; Dunislawska, A. Modulation of the Immune System of Chickens a Key Factor in Maintaining Poultry Production—A Review. Poult. Sci. 2023, 102, 102785. [Google Scholar] [CrossRef]
- Yosi, F.; Metzler-Zebeli, B.U. Dietary Probiotics Modulate Gut Barrier and Immune-Related Gene Expression and Histomorphology in Broiler Chickens under Non- and Pathogen-Challenged Conditions: A Meta-Analysis. Animals 2023, 13, 1970. [Google Scholar] [CrossRef]






| Treatment | Diet Description |
|---|---|
| T1 | Control diet without supplementation |
| T2 | Control diet + Bacillus subtilis (DSM17299) at 1.1 × 108 CFU/kg |
| T3 | Control diet + Bacillus subtilis (DSM5750) and Bacillus licheniformis (DSM5749) in a 1:1 ratio at 1.3 × 109 CFU/kg |
| T4 | Control diet + Bacillus coagulans (DSM 32016) at 1.0 × 109 CFU/kg |
| T5 | Control diet + Saccharomyces cerevisiae yeast cell wall at 0.25 g/kg |
| Items | Proportion, g/kg | Nutrients | Levels |
|---|---|---|---|
| Maize | 556 | Metabolic energy, MJ/kg | 11.5 |
| Soybean meal | 276 | Crude protein, g/kg | 170 |
| Wheat bran | 25.0 | Available phosphorus, g/kg | 4.6 |
| Maize oil | 20.0 | Calcium, g/kg | 40 |
| Di-calcium phosphate | 16.0 | Lysine, g/kg | 7.6 |
| Limestone | 100 | Methionine + Cysteine, g/kg | 6.8 |
| Sodium chloride | 3.00 | Threonine, g/kg | 5.8 |
| DL-methionine | 2.00 | ||
| Vitamin–mineral mix 1 | 2.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakami, Z.M.; Alhotan, R.A.; Al Sulaiman, A.R.; Aljumaah, R.S.; Palombo, V.; D’Andrea, M.; Alharthi, A.S.; Abudabos, A.E. Enhancing Laying Hen Productivity and Health: Influence of Dietary Probiotic Bacillus Strains and Prebiotic Saccharomyces cerevisiae Yeast Cell Wall on Production Performance, Egg Quality, and Inflammatory Responses. Animals 2025, 15, 1398. https://doi.org/10.3390/ani15101398
Hakami ZM, Alhotan RA, Al Sulaiman AR, Aljumaah RS, Palombo V, D’Andrea M, Alharthi AS, Abudabos AE. Enhancing Laying Hen Productivity and Health: Influence of Dietary Probiotic Bacillus Strains and Prebiotic Saccharomyces cerevisiae Yeast Cell Wall on Production Performance, Egg Quality, and Inflammatory Responses. Animals. 2025; 15(10):1398. https://doi.org/10.3390/ani15101398
Chicago/Turabian StyleHakami, Zafar M., Rashed A. Alhotan, Ali R. Al Sulaiman, Riyadh S. Aljumaah, Valentino Palombo, Mariasilvia D’Andrea, Abdulrahman S. Alharthi, and Ala E. Abudabos. 2025. "Enhancing Laying Hen Productivity and Health: Influence of Dietary Probiotic Bacillus Strains and Prebiotic Saccharomyces cerevisiae Yeast Cell Wall on Production Performance, Egg Quality, and Inflammatory Responses" Animals 15, no. 10: 1398. https://doi.org/10.3390/ani15101398
APA StyleHakami, Z. M., Alhotan, R. A., Al Sulaiman, A. R., Aljumaah, R. S., Palombo, V., D’Andrea, M., Alharthi, A. S., & Abudabos, A. E. (2025). Enhancing Laying Hen Productivity and Health: Influence of Dietary Probiotic Bacillus Strains and Prebiotic Saccharomyces cerevisiae Yeast Cell Wall on Production Performance, Egg Quality, and Inflammatory Responses. Animals, 15(10), 1398. https://doi.org/10.3390/ani15101398







