Metabolite Profiling Under Dietary Myo-Inositol Supplementation in Laying Hens from Two High-Performing Strains
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Diets
2.3. Plasma Sampling
2.4. Tissue Sampling
2.5. Myo-Inositol Determination in Plasma
2.6. Insulin Determination in Plasma
2.7. Triglyceride Determination in Liver
2.8. Protein Quantification
2.9. Targeted Metabolomics Approach
2.10. Bioinformatic and Statistical Analyses
3. Results
3.1. Effects of Dietary Myo-Inositol Supplementation on Myo-Inositol and Insulin Concentrations in Plasma and Triglyceride Concentrations in Liver
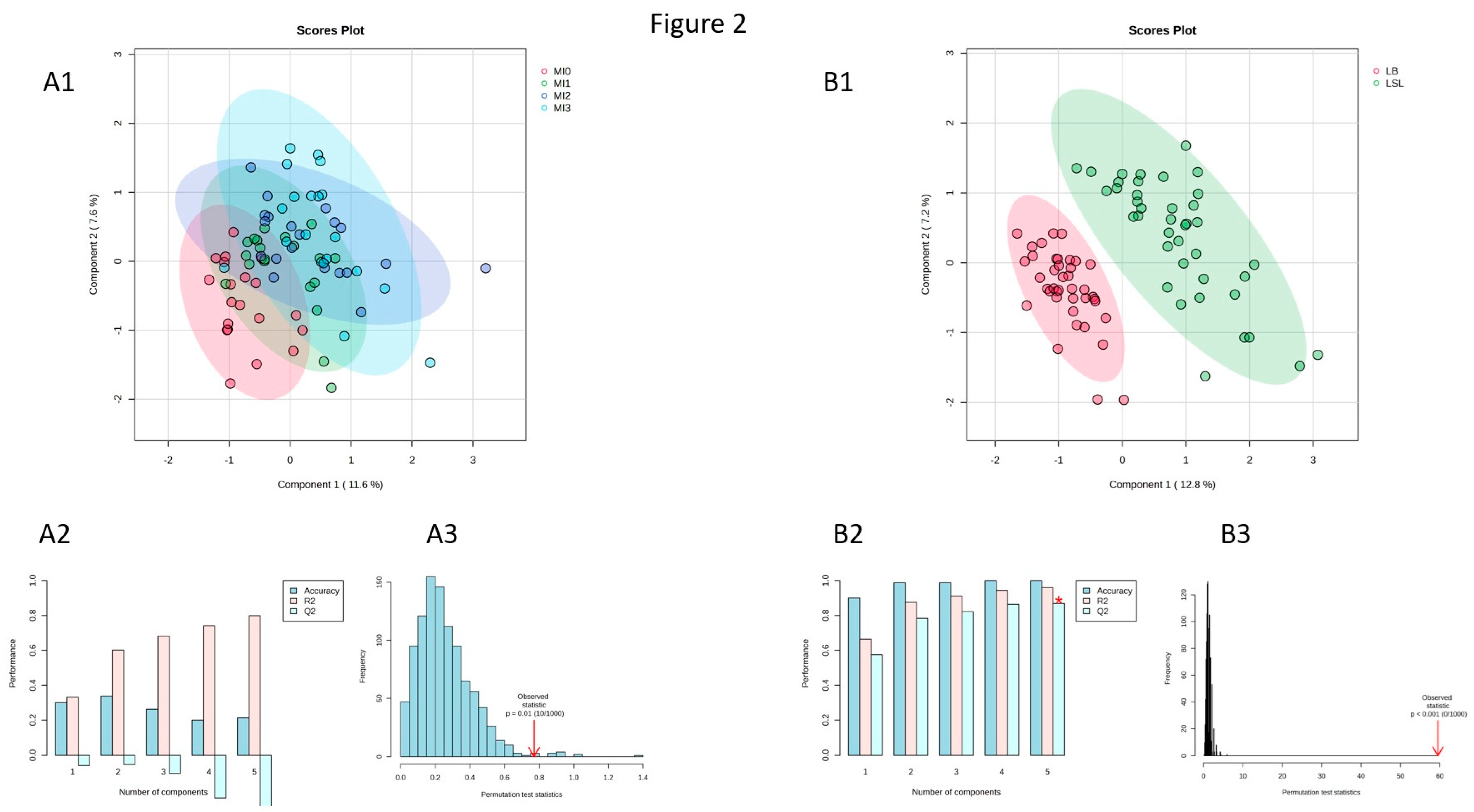
3.2. Effects of Dietary MI Supplementation on Metabolite Profiles of Laying Hens
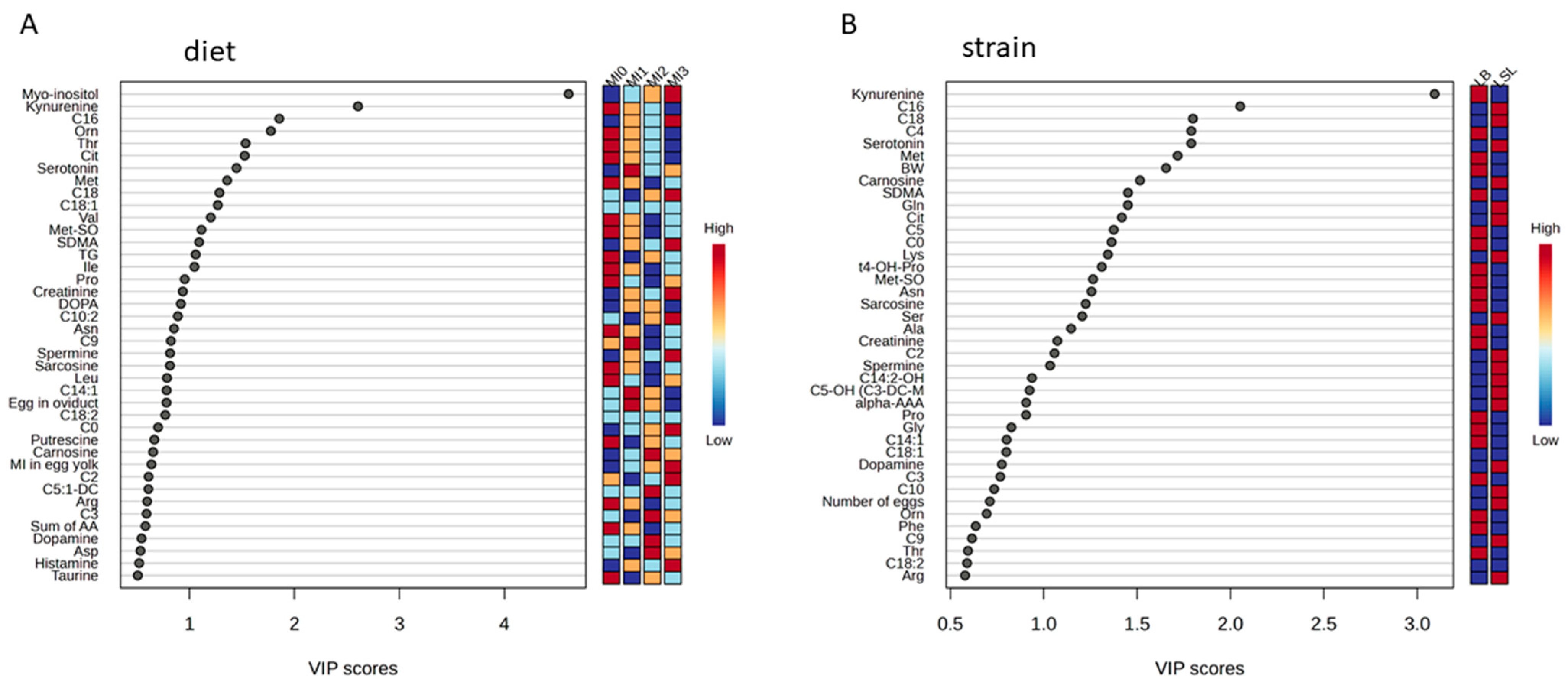
3.3. Identification of Discriminating Metabolites Due to Myo-Inositol Supplementation
3.4. Identification of Discriminating Metabolites and Traits Due to Genetic Background
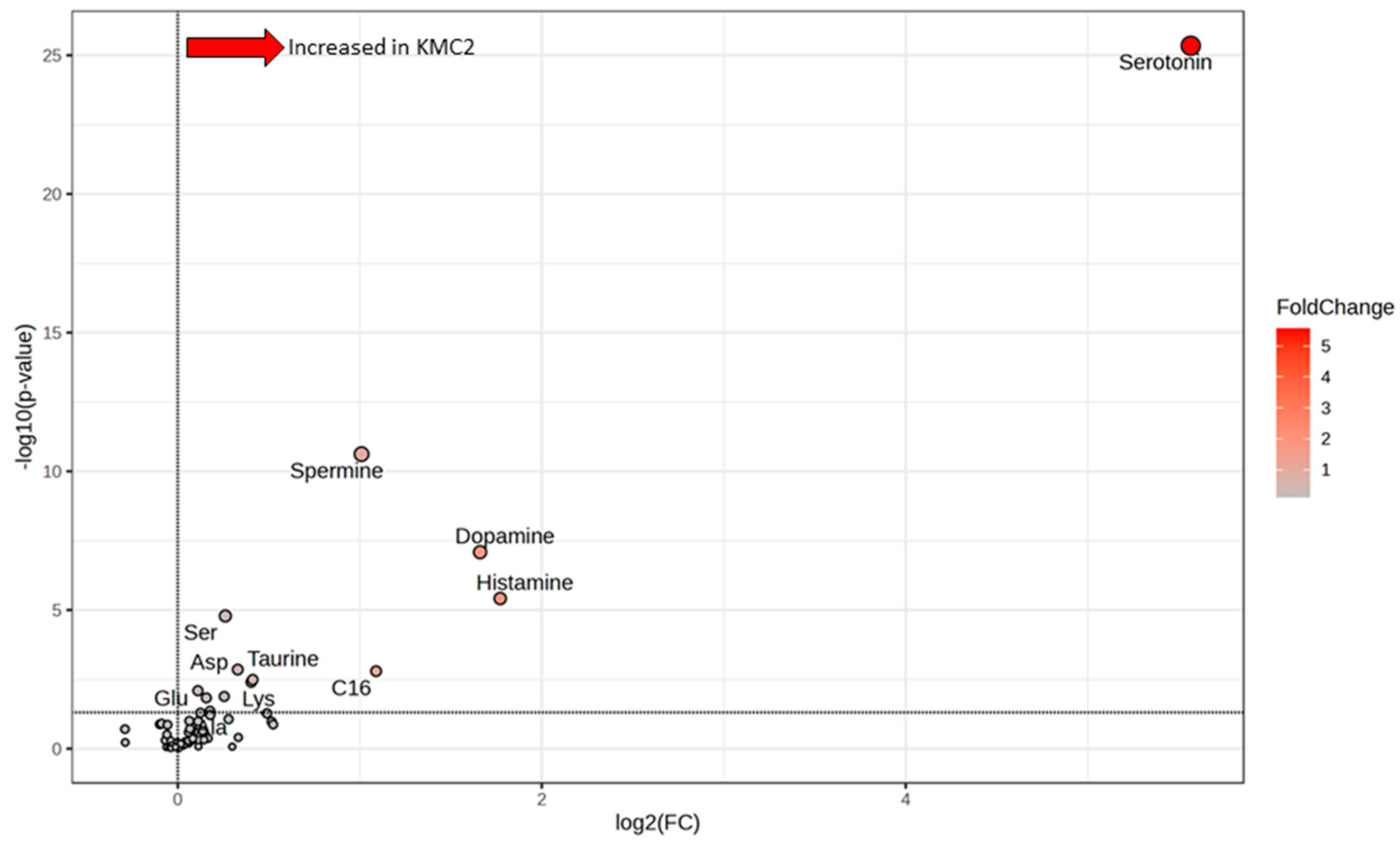
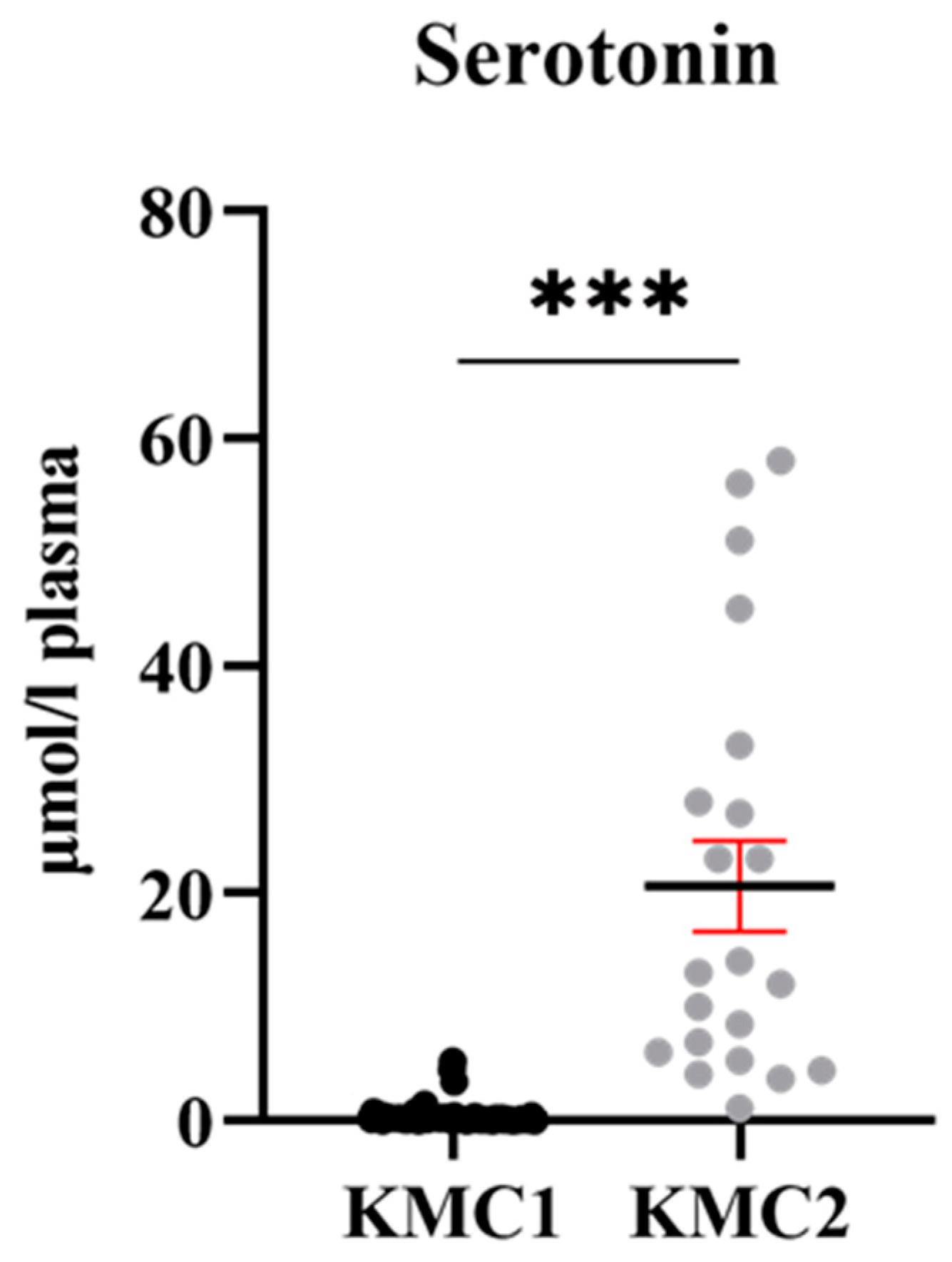
3.5. Grouping of Laying Hens by Cluster Analysis
4. Discussion
4.1. Myo-Inositol Supplementation and Metabolite Profiles in Laying Hens
4.2. Strain-Related Differences of Metabolite Profiles in Laying Hens
4.3. Potential Meaning of Newly Identified Clusters of Laying Hens
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Amino acid |
| AC | Acylcarnitine |
| Ala | Alanine |
| Arg | Arginine |
| Asn | Asparagine |
| Asp | Aspartate |
| BW | Body weight |
| Cit | Citrulline |
| C0 | Carnitine |
| C2 | Acetylcarnitine |
| C4 | Butyrylcarnitine |
| C5 | Valerylcarnitine |
| C16 | Hexadecanoylcarnitine |
| Glu | Glutamate |
| Gln | Glutamine |
| KMC | K-means cluster |
| Kyn | Kynurenine |
| Kyn/Trp | Kynurenine/Tryptophan |
| LB | Lohmann Brown-Classic |
| LPC | lysophosphatidylcholine |
| LSL | Lohmann LSL-Classic |
| Lys | Lys |
| Met | Methionine |
| Met-SO | Methionine sulfoxide |
| MI | Myo-inositol |
| MOI | Metabolites of interest |
| Orn | Ornithine |
| PC | Phosphatidylcholines |
| PLS-DA | Partial Least-Squares Discriminant Analysis |
| SDMA | Symmetric dimethylarginine |
| Ser | Serine |
| SM | sphingomyelin |
| TG | Triglyceride |
| Thr | Threonine |
| t4-OH-Pro | Trans-4-hydroxyproline |
| VIP | Variable Importance in Projection |
References
- Lewin, L.M.; Yannai, Y.; Sulimovici, S.; Kraicer, P.F. Studies on the Metabolic Role of Myo -Inositol. Distribution of Radioactive Myo -Inositol in the Male Rat. Biochem. J. 1976, 156, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Aouameur, R.; Da Cal, S.; Bissonnette, P.; Coady, M.J.; Lapointe, J.-Y. SMIT2 Mediates All Myo-Inositol Uptake in Apical Membranes of Rat Small Intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G1300–G1307. [Google Scholar] [CrossRef]
- Croze, M.L.; Soulage, C.O. Potential Role and Therapeutic Interests of Myo-Inositol in Metabolic Diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- Donà, G.; Sabbadin, C.; Fiore, C.; Bragadin, M.; Giorgino, F.L.; Ragazzi, E.; Clari, G.; Bordin, L.; Armanini, D. Inositol Administration Reduces Oxidative Stress in Erythrocytes of Patients with Polycystic Ovary Syndrome. Eur. J. Endocrinol. 2012, 166, 703–710. [Google Scholar] [CrossRef]
- Ortmeyer, H.K. Dietary Myoinositol Results in Lower Urine Glucose and in Lower Postprandial Plasma Glucose in Obese Insulin Resistant Rhesus Monkeys. Obes. Res. 1996, 4, 569–575. [Google Scholar] [CrossRef]
- Pirgozliev, V.; Brearley, C.A.; Rose, S.P.; Mansbridge, S.C. Manipulation of Plasma Myo-Inositol in Broiler Chickens: Effect on Growth Performance, Dietary Energy, Nutrient Availability, and Hepatic Function. Poult. Sci. 2019, 98, 260–268. [Google Scholar] [CrossRef]
- Sommerfeld, V.; Huber, K.; Bennewitz, J.; Camarinha-Silva, A.; Hasselmann, M.; Ponsuksili, S.; Seifert, J.; Stefanski, V.; Wimmers, K.; Rodehutscord, M. Phytate Degradation, Myo-Inositol Release, and Utilization of Phosphorus and Calcium by Two Strains of Laying Hens in Five Production Periods. Poult. Sci. 2020, 99, 6797–6808. [Google Scholar] [CrossRef] [PubMed]
- Cowieson, A.J.; Zhai, H.-X. Research Note: The Effect of Sequential Displacement of Dietary Dextrose with Myo-Inositol on Broiler Chicken Growth Performance, Bone Characteristics, Ileal Nutrient Digestibility, and Total Tract Nutrient Retention. Poult. Sci. 2021, 100, 993–997. [Google Scholar] [CrossRef]
- Sprigg, C.; Whitfield, H.; Burton, E.; Scholey, D.; Bedford, M.R.; Brearley, C.A. Phytase Dose-Dependent Response of Kidney Inositol Phosphate Levels in Poultry. PLoS ONE 2022, 17, e0275742. [Google Scholar] [CrossRef]
- Gonzalez-Uarquin, F.; Rodehutscord, M.; Huber, K. Myo-Inositol: Its Metabolism and Potential Implications for Poultry Nutrition—A Review. Poult. Sci. 2020, 99, 893–905. [Google Scholar] [CrossRef]
- Thorsell, A.-G.; Persson, C.; Voevodskaya, N.; Busam, R.D.; Hammarström, M.; Gräslund, S.; Gräslund, A.; Hallberg, B.M. Structural and Biophysical Characterization of Human Myo-Inositol Oxygenase. J. Biol. Chem. 2008, 283, 15209–15216. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Uarquin, F.; Kenéz, Á.; Rodehutscord, M.; Huber, K. Dietary Phytase and Myo-Inositol Supplementation Are Associated with Distinct Plasma Metabolome Profile in Broiler Chickens. Animal 2020, 14, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Żyła, K.; Mika, M.; Duliński, R.; Świątkiewicz, S.; Koreleski, J.; Pustkowiak, H.; Piironen, J. Effects of Inositol, Inositol-Generating Phytase B Applied Alone, and in Combination with 6-Phytase A to Phosphorus-Deficient Diets on Laying Performance, Eggshell Quality, Yolk Cholesterol, and Fatty Acid Deposition in Laying Hens. Poult. Sci. 2012, 91, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Uarquin, F.; Sommerfeld, V.; Rodehutscord, M.; Huber, K. Interrelationship of Myo-Inositol Pathways with Systemic Metabolic Conditions in Two Strains of High-Performance Laying Hens during Their Productive Life Span. Sci. Rep. 2021, 11, 4641. [Google Scholar] [CrossRef]
- Laganà, A.S.; Rossetti, P.; Buscema, M.; La Vignera, S.; Condorelli, R.A.; Gullo, G.; Granese, R.; Triolo, O. Metabolism and Ovarian Function in PCOS Women: A Therapeutic Approach with Inositols. Int. J. Endocrinol. 2016, 2016, 6306410. [Google Scholar] [CrossRef]
- Sommerfeld, V.; Hanauska, A.; Huber, K.; Bennewitz, J.; Camarinha-Silva, A.; Feger, M.; Föller, M.; Oster, M.; Ponsuksili, S.; Schmucker, S.; et al. Effects of Myo-Inositol Supplementation in the Diet on Myo-Inositol Concentrations in the Intestine, Blood, Eggs, and Excreta of Laying Hens. Poult. Sci. 2025, 104, 104545. [Google Scholar] [CrossRef]
- Sommerfeld, V.; Künzel, S.; Schollenberger, M.; Kühn, I.; Rodehutscord, M. Influence of Phytase or Myo-Inositol Supplements on Performance and Phytate Degradation Products in the Crop, Ileum, and Blood of Broiler Chickens. Poult. Sci. 2018, 97, 920–929. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.P.A.; Di Tomo, P.; Centorame, G.; Pandolfi, A.; Di Pietro, N.; Consoli, A.; Formoso, G. Myoinositol Reduces Inflammation and Oxidative Stress in Human Endothelial Cells Exposed In Vivo to Chronic Hyperglycemia. Nutrients 2021, 13, 2210. [Google Scholar] [CrossRef]
- Joffin, N.; Jaubert, A.-M.; Durant, S.; Barouki, R.; Forest, C.; Noirez, P. Citrulline Counteracts Overweight- and Aging-Related Effects on Adiponectin and Leptin Gene Expression in Rat White Adipose Tissue. Biochim. Open 2015, 1, 1–5. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, T.; Chen, S.; Shen, H.; Wang, J. Dietary Supplementation with L-Citrulline Improves Amino Acid Composition and Broiler Performance, and Modulates Gut Microbiota. Front. Microbiol. 2025, 16, 1551012. [Google Scholar] [CrossRef]
- Morris, S.M. Arginine Metabolism: Boundaries of Our Knowledge. J. Nutr. 2007, 137, 1602S–1609S. [Google Scholar] [CrossRef]
- Crenn, P.; Messing, B.; Cynober, L. Citrulline as a Biomarker of Intestinal Failure Due to Enterocyte Mass Reduction. Clin. Nutr. 2008, 27, 328–339. [Google Scholar] [CrossRef]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Haraguchi, A.; Kuwahara, M.; Nakamura, K.; Hamaguchi, Y.; Ikeda, Y.; Ishida, Y.; Wang, G.; Shirakawa, C.; Tanihata, Y.; et al. L-Ornithine Affects Peripheral Clock Gene Expression in Mice. Sci. Rep. 2016, 6, 34665. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Tan, P.; Ma, N.; Ma, X. Physiological Functions of Threonine in Animals: Beyond Nutrition Metabolism. Nutrients 2021, 13, 2592. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Smith, A. Methionine Sulfoxide and the Methionine Sulfoxide Reductase System as Modulators of Signal Transduction Pathways: A Review. Amino Acids 2021, 53, 1011–1020. [Google Scholar] [CrossRef]
- Kwaśny-Krochin, B.; Głuszko, P.; Undas, A. Plasma Asymmetric Dimethylarginine in Active Rheumatoid Arthritis: Links with Oxidative Stress and Inflammation. Pol. Arch. Med. Wewn. 2012, 122, 270–276. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s Metabolites in Exercise, Inflammation, and Mental Health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Robinson, C.M.; Hale, P.T.; Carlin, J.M. The Role of IFN-γ and TNF-α-Responsive Regulatory Elements in the Synergistic Induction of Indoleamine Dioxygenase. J. Interferon Cytokine Res. 2005, 25, 20–30. [Google Scholar] [CrossRef]
- Åkesson, K.; Pettersson, S.; Ståhl, S.; Surowiec, I.; Hedenström, M.; Eketjäll, S.; Trygg, J.; Jakobsson, P.-J.; Gunnarsson, I.; Svenungsson, E.; et al. Kynurenine Pathway Is Altered in Patients with SLE and Associated with Severe Fatigue. Lupus Sci. Med. 2018, 5, e000254. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R.; Brownson, C. A Possible New Role for the Anti-Ageing Peptide Carnosine. Cell. Mol. Life Sci. 2000, 57, 747–753. [Google Scholar] [CrossRef]
- Larqué, E.; Sabater-Molina, M.; Zamora, S. Biological Significance of Dietary Polyamines. Nutrition 2007, 23, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Schutte, J.E.; Longhurst, J.C.; Gaffney, F.A.; Bastian, B.C.; Blomqvist, C.G. Total Plasma Creatinine: An Accurate Measure of Total Striated Muscle Mass. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 762–766. [Google Scholar] [CrossRef]
- Gurgel, A.M.C.; Batista, A.L.; Cavalcanti, D.M.L.D.P.; Magalhães, A.; Zantut-Wittmann, D.E. Sarcosine, Trigonelline and Phenylalanine as Urinary Metabolites Related to Visceral Fat in Overweight and Obesity. Metabolites 2024, 14, 491. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Gauza-Włodarczyk, M.; Kubisz, L.; Włodarczyk, D. Amino Acid Composition in Determination of Collagen Origin and Assessment of Physical Factors Effects. Int. J. Biol. Macromol. 2017, 104, 987–991. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix Metalloproteinases (MMPs), the Main Extracellular Matrix (ECM) Enzymes in Collagen Degradation, as a Target for Anticancer Drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.; Spandidos, D.; Christodoulou, I.; Kyriakopoulos, A.; Zoumpourlis, V. Protective Role of Taurine against Oxidative Stress (Review). Mol. Med. Rep. 2021, 24, 605. [Google Scholar] [CrossRef]
- Goñi, F.M.; Requero, M.A.; Alonso, A. Palmitoylcarnitine, a Surface-active Metabolite. FEBS Lett. 1996, 390, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in Health and Disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [PubMed]
- Ebert-Zavos, E.; Horvat-Gordon, M.; Taylor, A.; Bartell, P.A. Biological Clocks in the Duodenum and the Diurnal Regulation of Duodenal and Plasma Serotonin. PLoS ONE 2013, 8, e58477. [Google Scholar] [CrossRef]
- Alberghina, D.; Biondi, V.; Passantino, A.; Giunta, F.; Panzera, M. Plasma Serotonin in Laying Hens (Gallus Gallus Domesticus) with and Without Foot Pad Dermatitis. Int. J. Tryptophan Res. 2020, 13, 1178646920927380. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.E.; Taylor, R.L.; Wideman, R.F. Analysis of Plasma Serotonin Levels and Hemodynamic Responses Following Chronic Serotonin Infusion in Broilers Challenged with Bacterial Lipopolysaccharide and Microparticles. Poult. Sci. 2008, 87, 116–124. [Google Scholar] [CrossRef]
- Nocito, A.; Dahm, F.; Jochum, W.; Jang, J.H.; Georgiev, P.; Bader, M.; Renner, E.L.; Clavien, P. Serotonin Mediates Oxidative Stress and Mitochondrial Toxicity in a Murine Model of Nonalcoholic Steatohepatitis. Gastroenterology 2007, 133, 608–618. [Google Scholar] [CrossRef]


| Metabolite (µmol/L) | Strain | MI0 | MI1 | MI2 | MI3 | TWA 1 | p-Value |
|---|---|---|---|---|---|---|---|
| Cit 2 | LB 3 | 10.86 ± 1.03 a,x | 8.97 ± 1.03 ab,y | 7.89 ± 1.03 b,y | 9.95 ± 1.03 ab,x | Strain Diet Interaction | <0.01 0.098 0.013 |
| LSL 4 | 12.55 ± 1.03 a,x | 13.70 ± 1.03 a,x | 12.52 ± 1.03 a,x | 9.32 ± 1.03 b,x | |||
| Orn 5 | LB | 205.00 ± 15.71 a | 177.90 ± 15.71 a | 172.60 ± 15.71 a | 190.00 ± 15.71 a | Strain Diet Interaction | 0.107 0.017 0.292 |
| LSL | 209.70 ± 15.71 a | 167.20 ± 15.71 b | 160.80 ± 15.71 b | 139.30 ± 15.71 b | |||
| Thr 6 | LB | 376.70 ± 25.41 a | 345.10 ± 25.41 ab | 298.10 ± 25.41 b | 304.10 ± 25.41 b | Strain Diet Interaction | 0.087 0.036 0.846 |
| LSL | 327.40 ± 25.41 a | 297.10 ± 25.41 a | 280.30 ± 25.41 a | 284.00 ± 25.41 a | |||
| Met-SO 7 | LB | 13.20 ± 0.60 a,x | 11.46 ± 0.60 b | 11.81 ± 0.60 ab,x | 12.47 ± 0.60 ab,x | Strain Diet Interaction | <0.001 0.035 0.092 |
| LSL | 11.04 ± 0.60 a,y | 11.05 ± 0.60 a | 9.36 ± 0.60 b,y | 9.50 ± 0.60 b,y | |||
| SDMA8 | LB | 0.44 ± 0.01 a,x | 0.43 ± 0.01 a,x | 0.42 ± 0.01 a,x | 0.45 ± 0.01 a,x | Strain Diet Interaction | <0.001 0.044 0.052 |
| LSL | 0.32 ± 0.01 b,y | 0.37 ± 0.01 a,y | 0.37 ± 0.01 a,y | 0.38 ± 0.01 a,y | |||
| Kyn 9 | LB | 0.30 ± 0.03 a,x | 0.33 ± 0.03 a,x | 0.24 ± 0.03 a,x | 0.24 ± 0.03 a,x | Strain Diet Interaction | <0.001 0.039 0.700 |
| LSL | 0.19 ± 0.03 a,y | 0.15 ± 0.03 ab,y | 0.13 ± 0.03 ab,y | 0.08 ± 0.03 b,y | |||
| Kyn/Trp Ratio 10 | LB | 3.43 ± 0.38 ab,x | 3.93 ± 0.38 a,x | 2.88 ± 0.38 b,y | 2.83 ± 0.38 b,x | Strain Diet Interaction | <0.001 0.022 0.541 |
| LSL | 2.22 ± 0.38 a,y | 1.81 ± 0.38 ab,y | 1.55 ± 0.38 ab,y | 0.92 ± 0.38 b,y | |||
| Metabolite (µmol/L) | KMC1 1 | KMC2 2 | p-Value |
|---|---|---|---|
| Ala 3 | 435.62 ± 15.74 | 483.68 ± 22.05 | 0.035 |
| Asp 4 | 32.59 ± 1.33 | 40.81 ± 2.18 | <0.01 |
| Glu 5 | 210.11 ± 5.46 | 234.26 ± 8.82 | 0.019 |
| Lys 6 | 238.27 ± 13.28 | 276.82 ± 16.68 | 0.011 |
| Ser 7 | 749.89 ± 21.06 | 879.78 ± 26.99 | <0.001 |
| Dopamine 8 | No data | 0.29 ± 0.02 | - |
| Histamine | 0.03 ± 0.004 | 0.05 ± 0.004 | <0.001 |
| Serotonin | 0.43 ± 1.22 | 20.62 ± 2.08 | <0.001 |
| Spermidine | 0.32 ± 0.02 | 0.41 ± 0.03 | 0.010 |
| Spermine | 0.33 ± 0.03 | 0.64 ± 0.04 | <0.001 |
| Taurine | 92.62 ± 7.75 | 124.64 ± 10.69 | <0.01 |
| C16 9 | 0.01 ± 0.003 | 0.03 ± 0.004 | <0.001 |
| Sum of AA 10 | 5686.46 ± 110.35 | 6133.73 ± 155.79 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szentgyörgyi, Á.; Sommerfeld, V.; Rodehutscord, M.; Huber, K. Metabolite Profiling Under Dietary Myo-Inositol Supplementation in Laying Hens from Two High-Performing Strains. Animals 2025, 15, 1392. https://doi.org/10.3390/ani15101392
Szentgyörgyi Á, Sommerfeld V, Rodehutscord M, Huber K. Metabolite Profiling Under Dietary Myo-Inositol Supplementation in Laying Hens from Two High-Performing Strains. Animals. 2025; 15(10):1392. https://doi.org/10.3390/ani15101392
Chicago/Turabian StyleSzentgyörgyi, Ákos, Vera Sommerfeld, Markus Rodehutscord, and Korinna Huber. 2025. "Metabolite Profiling Under Dietary Myo-Inositol Supplementation in Laying Hens from Two High-Performing Strains" Animals 15, no. 10: 1392. https://doi.org/10.3390/ani15101392
APA StyleSzentgyörgyi, Á., Sommerfeld, V., Rodehutscord, M., & Huber, K. (2025). Metabolite Profiling Under Dietary Myo-Inositol Supplementation in Laying Hens from Two High-Performing Strains. Animals, 15(10), 1392. https://doi.org/10.3390/ani15101392





