Simple Summary
This investigation aimed to analyze the effect of medium-chain fatty acids (MCFAs) derived from black soldier fly larvae (BSFL-MCFAs) in male C57BL/6J mice fed a high-fat diet (HFD). The results suggest that BSFL-MCFAs may exert anti-obesity activity and contribute to improvements in blood lipid parameters in HFD-induced obese mal C57BL/6J mice. These findings demonstrate the potential of BSFL-MCFAs as feed and food ingredients for the prevention of obesity and related metabolic disorders in both animals and humans. However, further studies are needed to confirm these effects and to elucidate the underlying mechanisms.
Abstract
Obesity is a chronic disease associated with an increased dietary fat intake and reduced physical activity, posing significant health risks, including metabolic disorders, cardiovascular diseases, and diminished quality of life. This study investigated the anti-obesity potential of medium-chain fatty acids (MCFAs) derived from black soldier fly larvae (BSFL-MCFAs) in male C57BL/6J mice fed a high-fat diet (HFD). Lauric acid (>50% of total BSFL lipids) was the predominant fatty acid. Mice supplemented with BSFL-MCFAs exhibited significantly lower weight gain and food efficiency ratios (FERs) than HFD-fed mice, despite similar food intake. Medium-chain fatty acids derived from BSFL supplementation also attenuated HFD-induced increases in triglycerides, total cholesterol, and low-density lipoprotein cholesterol levels while improving cardiac risk indices. Furthermore, BSFL-MCFAs reduced serum glucose and leptin levels, mitigated hypothalamic endoplasmic reticulum stress marker expression, and decreased serum alanine transaminase levels, indicating protective effects against obesity-related metabolic dysregulation. These findings suggest that BSFL-MCFAs enhance energy expenditure and thermogenesis, thereby contributing to effective weight management and obesity prevention. As a sustainable and functional lipid source, BSFL-MCFAs hold promise as a feed additive for animals and as a dietary ingredient for preventing pet obesity, offering an innovative approach to combat obesity and its associated health risks.
1. Introduction
Obesity is a chronic disease caused by excessive fat accumulation resulting from an imbalance between energy intake and expenditure [1]. The global prevalence of obesity has risen sharply over the past few decades [2]. According to a meta-analysis performed by the Non-Communicable Disease Risk Factor Collaboration, obesity in adults has more than doubled globally, from 13.8% in women and 10.7% in men in 1990 to 28.3% in women and 26.9% in men by 2022, respectively [3]. Obesity is a risk factor for various disorders, including certain cancers, metabolic disorders such as diabetes and hyperlipidemia, cardiovascular diseases (e.g., hypertension and arteriosclerosis), and cerebrovascular events [4,5,6,7]. Furthermore, obesity contributes to musculoskeletal disorders and a reduced quality of life [8]. Also, obesity-related complications pose a risk to animal health [9]. Therefore, the prevention of obesity is important for maintaining health and improving the quality of life for humans and animals.
While obesity can be treated using various approaches, depending on its causes, severity, and associated comorbidities, lifestyle modifications—particularly dietary control and increased physical activity—remain the primary strategy for its prevention and management [10,11]. However, extreme caloric restriction, often used in dietary interventions, may lead to adverse effects, such as a decrease in the basal metabolic rate, bone loss, hormone imbalance, nutrient deficiencies, and hypoglycemia [12,13,14,15]. Therefore, recent efforts have focused on dietary composition rather than reducing total caloric intake, aiming to enhance energy metabolism and reduce fat accumulation without compromising overall health [16,17].
Medium-chain fatty acids (MCFAs) have attracted considerable attention in this context, MCFAs, which are triglycerides found in various plant oils such as olive, palm, and coconut oils, are a fat source better absorbed and metabolized by long-chain triglycerides [18,19,20,21]. Numerous preclinical and clinical studies have shown that MCFAs enhance hepatic thermogenesis in mice [22], increase energy expenditure [23], and improve metabolic profiles by reducing adiposity and insulin resistance [23,24,25,26,27]. Despite these benefits, the supply of traditional plant-based oil has been increasingly affected by climate change-induced agricultural instability, leading to supply shortages and price volatility [28,29,30,31,32].
In response to these challenges, research is being conducted to explore the use of insect oils as alternatives [33,34]. The larvae of the black soldier fly (Hermetia illucens) (BSFL) are known as waste-reducing insects [35]. BSFL are primarily used as animal feed, but recent studies have suggested their potential as a novel nutritional source with reports highlighting antimicrobial activity [34,36,37,38]. Furthermore, studies have shown that obese Zucker rats fed BSFL have reduced hepatic lipid synthesis and fatty acid production and that the lipids in BSFL contribute to lowering the liver fat concentration [34]. It would strengthen the nutritional and industrial relevance of BSFL lipids to compare them directly with conventional MCT sources such as coconut and palm oils. Coconut oil typically contains around 45–53% lauric acid (C12:0), while palm kernel oil contains about 48% [39,40]. In contrast, BSFL lipid profiles are highly modifiable through dietary manipulation, with studies showing that lauric acid content in BSFL oil can be increased to over 70% [41]. Previous studies have demonstrated that BSFL-derived oils, which are rich in MCFA such as lauric acid, can influence metabolic health parameters in animals. For example, BSFL oil supplementation in broiler diets has shown to improve gut microbiota and lipid metabolism [42], while studies in pigs and rodents have suggested potential effects on immune modulation and hepatic lipid reduction [43,44]. This offers a distinct advantage in tailoring fatty acid composition based on application needs. Moreover, BSFL oil production exhibits a significantly lower unit cost compared to plant-based MCT sources, due to shorter production cycles, the use of low-value organic waste as feed, and lower land and water use requirements. However, to date, few investigations have explored the metabolic effects of specific lipid components—particularly MCFAs—derived from BSFL. Therefore, based on these findings, this investigation aimed to quantify the MCFA levels in BSFL-derived lipids and to evaluate the potential of MCFAs extracted from BSFL (BSFL-MCFAs) in preventing high-fat diet-induced obesity in male C57BL/6J mice.
2. Materials and Methods
2.1. Rearing BSFL
The BSFL were reared at the Greenteko Corporation (Hwaseong, Republic of Korea). Briefly, BSFL were reared for 21 days under controlled conditions (26 ± 1 °C and 50% relative humidity). A food waste and waste cooking oil mixture was fermented for 5 days using effective microorganisms, following the method reported by Lee et al., and was used as BSFL feed [45]. The term “effective microorganisms” refers to a mixed culture of beneficial microbes, consisting primarily of lactic acid bacteria, yeasts, and photosynthetic bacteria.
2.2. Extraction of Crude Oil from BSFL and MCFA Preparation
The extraction of crude oil from BSFL and preparation of MCFAs from the crude oil were performed by Greenteko Corporation. The harvested BSFL were dried in a 700 W microwave for 8.5 min. To extract the crude oil, the dried BSFL were pressed at 120 °C (20 MPa), and the liquid extract was collected as crude oil [45]. Subsequently, the crude oil was mixed with water and sulfuric acid in a ratio of 7.0:1.0:0.1 (water/crude; oil/sulfuric acid), and the mixture was reacted at 160 °C for 20 h to prepare the fatty acids. The fatty acid composition was analyzed using gas chromatography (GC)/mass spectrometry at the Korea Quality Testing Institute (Suwon, Republic of Korea). The analysis was performed using an Agilent 7890B GC system equipped with a DB-23 capillary column (60 m × 0.25 mm i.d., 0.25 μm film thickness; Agilent Technologies, Santa Clara, CA, USA). The oven temperature was programmed to start at 130 °C (held for 1 min), increased to 170 °C at 6.5 °C/min, then to 215 °C at 2.75 °C/min, and finally to 230 °C at 40 °C/min (held for 5 min). Helium was used as the carrier gas at a constant flow rate of 1.0 mL/min. Fatty acid methyl esters were prepared according to the standard AOCS Ce 2-66 method [46]. MCFAs were isolated from the prepared fatty acids by vacuum distillation (<7.5 mmHg). Briefly, the fatty acids were heated at 100 °C under vacuum to remove moisture. The temperature was then gradually increased from 200 °C to 245 °C under vacuum, for the evaporation and separation of MCFAs [47].
2.3. Animal Experiments
All animal experiments were approved by the Animal Use and Care Committee of Dongguk University (IACUC-2022-09, Gyeongju, Republic of Korea). Forty-five 7-week-old male C57BL/6J mice were purchased from Hana Biotech (Pyeungtaek, Republic of Korea) and stabilized for one week on a normal diet (ND). Mice were then randomly divided into the ND, high-fat diet (HFD), and HFD-containing BSFL-MCFAs (HFD_MCFA) groups (15 mice/group). Each group was further subdivided into three subgroups of five mice each. The mice received ND, HFD, or HFD_MCFA ad libitum for 80 days. For each subgroup, the total body weight and feed intake were measured every 2–3 days during the experiment, and the average body weight and feed intake per mouse were calculated. The mean body weights and feed intake per mouse from each subgroup were aggregated and averaged for each group, and the standard deviations of the average body weight and feed intake per mouse were calculated. The food efficiency ratio (FER) was determined based on the initial and final average body weights, as well as the daily feed intake. During the experiment, mice were housed in polysulfone cages with wood chip bedding and provided with environmental enrichment (e.g., paper rolls and plastic huts) to promote exploratory behavior and reduce stress. Housing conditions were maintained at 22 ± 1 °C, 50 ± 5% relative humidity and a 12 h light/dark cycle. After 80 days, the mice were sacrificed by inhalation of Zoletil50 (Virbac Korea, Seoul, Republic of Korea) after fasting for 12 h. Blood and hypothalami were collected to analyze the biological composition and gene expression of endoplasmic reticulum (ER) stress markers.
2.4. Animal Diet
ND (product #: D12450B) and HFD (product #: D12492) were purchased from Research Diets (New Brunswick, NJ, USA), and HFD_MCFA was prepared by DOOYEOLBIOTECH (Seoul, Republic of Korea). The compositions of the HFD and HFD_MCFA diets are presented in Table 1. All diets were designed to be isocaloric (~4000 kcl/kg) and matched for macronutrient distribution. HFD and HFD_MCT contained ~60% kcal from fat, 20% from protein, and 20% from carbohydrates, with lard replaced by MCFAs in HFD_MCFA. ND provided ~10% kcal from fat, 20% from protein, and 70% from carbohydrates. A summary of the proximate macronutrient composition is shown in Supplementary Materials.

Table 1.
HFD and HFD_MCFA formulas.
2.5. Measurement of Blood Lipid Levels
Triglyceride (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL) levels in the blood were analyzed by the Korea Non-Clinical Technology Solution Center (Seongnam, Republic of Korea) using standard enzymatic colorimetric analysis based on the Trinder reaction [48,49]. Low-density lipoprotein cholesterol (LDL) levels were determined using the Friedewald formula. The formula is as follows: [LDL (mg/dL) = TC (mg/dL) − HDL (mg/dL) − TG (mg/dL)]/5 which is valid when TG < 400 mg/dL [50]. Based on the lipid levels, the atherogenic index (AI) and cardiac risk factor (CRF) were calculated using the Lauer and Rosenfeld formulae, respectively. The formulae are as follows: AI = ([TC] − [HDL-c])/[HDL-c] and CRF = [TC]/[HDL-c] [51].
2.6. Analysis of Liver Injury Indicators in the Blood
We evaluated the activities of aspartic acid transaminase (AST) and alanine transaminase (ALT) in the blood as liver damage indicators. The activities were assessed by the Korea Non-Clinical Technology Solution Center using the Reitman–Frankel method.
2.7. Analysis of Kidney Function Indicators in the Blood
Blood urea nitrogen (BUN) and creatinine levels were measured as indicators of kidney function. BUN and creatinine levels were assessed by the Korea Non-Clinical Technology Solution Center using the enzymatic methods of Talke and Schubert and Jeff reaction, respectively [52,53]. BUN was quantified by the enzymatic conversion of urea to ammonia using urease, followed by a colorimetric reaction. Creatinine was measured via the Jaffe reaction, in which creatinine reacts with alkaline picrate to form a colored complex, which is quantified spectrophotometrically.
2.8. Evaluation of Glucose and Leptin Concentrations in the Blood
We analyzed the blood glucose and leptin levels, because circulating glucose and leptin concentrations are closely associated with obesity. The analyses were performed using a Glucose Colorimetric Detection Kit and mouse leptin ELISA kit (Invitrogen, Thermo Fisher Scientific; Waltham, MA, USA), respectively.
2.9. Quantitative Real-Time Polymerase Chain Reaction (PCR)
We analyzed the expression of obesity-associated ER stress markers (Grp7, Chop, Xbp-1s, Atf4, and ErdJ4) in the hypothalamus. The mouse hypothalamus was homogenized, and the total RNA was extracted using TRIzol reagent (Invitrogen, Thermo Fischer Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. Single-strand cDNA was then synthesized using a high-capacity reverse transcription kit (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA), and the relative gene expression of ER stress markers was determined using the cDNA and real-time PCR. GAPDH was used as an internal control. The primer sequences for ER stress markers and GAPDH are presented in Table 2. The primers used in this investigation were identical in sequence to those previously reported [54].

Table 2.
Primer sequences for the analysis of obesity-associated ER stress markers.
2.10. Statistical Analysis
Statistical analysis of the data was performed using one-way analysis of variance (ANOVA), followed by Tukey’s honestly significant difference test. SPSS software (version 20.0; SPSS, Inc., Chicago, IL, USA) was used for the analysis, and p-values < 0.05 were considered significant.
3. Results
3.1. Fatty Acid Compositon of BSFL Crude Lipids
To prepare the BSFL-MCFAs, the fatty acid composition of the crude lipids extracted from BSFL was analyzed. Lauric acid was the most prevalent among the crude lipids (51.1%), followed by stearic, palmitic, and oleic acids (12.8%, 12.1%, and 11.1%, respectively) (Table 3). Additionally, fatty acids with a carbon chain length of ≤12 comprised 53.2% of the crude lipids. Furthermore, 85.6% of crude lipids were saturated fatty acids. Consequently, these findings suggest that the crude lipids extracted from BSFL contain >50% MCFAs, implying an association between high MCFA levels in BSFL crude lipids and their weight reduction effects.

Table 3.
The composition of fatty acid in curd oil extracted from BSFL.
3.2. Effect of MCFAs on Weight Gain of Mice Fed HFD
We evaluated the effects of BSFL-MCFAs on weight gain in mice. Mice in the HFD group showed the greatest increase in body weight, whereas the weight gain was significantly attenuated in the HFD_MCFA group (Figure 1). Notably, the weight gain in mice in the HFD_MCFA group was lower than that in the ND group. The highest daily food intake was observed in the ND group, with no significant difference in food intake between the HFD and HFD_MCFA groups (Figure 1b). On the basis of these findings, we calculated the FER for each group. The FER in the HFD_MCFA group was lower than that in the ND and HFD groups (Figure 1b). These results demonstrate that BSFL-MCFAs may help suppress weight gain in HFD-fed mice.
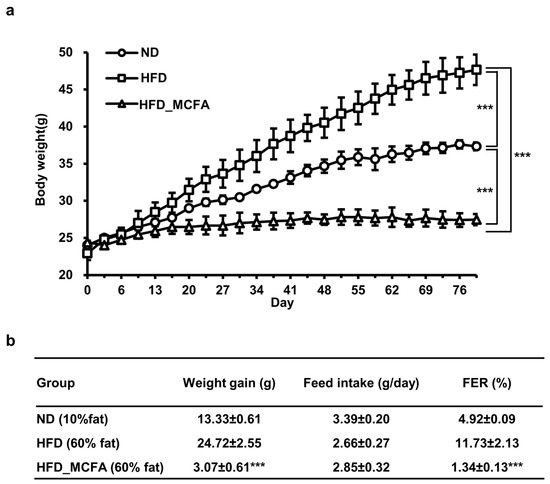
Figure 1.
MCFAs attenuate the HFD-induced increase in body weight in mice with a decrease in FER. (a) The total body weight of each subgroup was measured every 2–3 days. Subsequently, the mean body weight per mouse was calculated for each subgroup. The subgroup averages were summed, and an overall mean was calculated to determine the average body weight per mouse of each group. Statistical differences between groups were determined through pairwise comparisons. *** p < 0.001. (b) Initial and final body weights of each subgroup were measured, and the mean initial and final body weights per mouse was calculated for each subgroup. The average initial body weight was subtracted from the average final body weight to determine the weight gain per mouse in each subgroup. The subgroup weight gains were averaged to calculate the overall weight gain per mouse for each group. The total feed intake for each subgroup was measured every 2–3 days, and the mean feed intake per mouse was calculated for each subgroup. The subgroup averages were then summed to calculate the overall feed intake per mouse for each group. The FER was calculated as a percentage by dividing the daily weight gain by the daily feed intake. *** indicates that there is a significant difference between the HFD_MCFA group and the HFD group at p < 0.001.
3.3. Effect of MCFAs on Blood Lipid Parameters
To evaluate the effects of MCFAs on blood lipid parameters, the TG, TC, LDL, and HDL concentrations were measured. The TG, TC, and LDL levels were significantly elevated in the HFD group; however, the increases were attenuated in the HFD_MCFA group. In contrast, the HDL levels in both the HFD and HFD_MCFA groups were higher than that in the ND group (Figure 2a). Based on these results, we calculated the AI and CRF. The AI and CRF were significantly increased in the HDF group, whereas these increases were suppressed in the HFD_MCFA group (Figure 2b). Moreover, the AI and CRF in the HFD_MCFA group were lower than those in the ND group. These findings suggest that BSFL-MCFAs may help prevent atherogenesis and cardiac disease development.
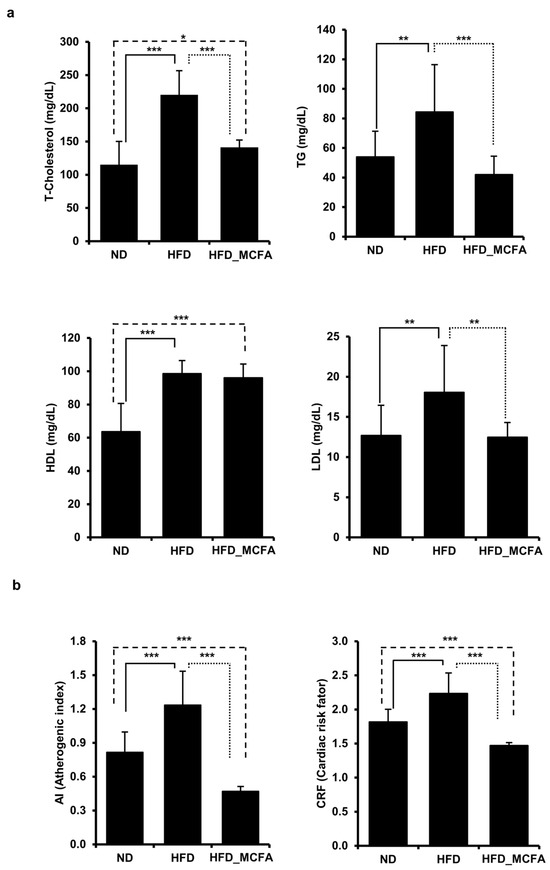
Figure 2.
MCFAs prevent the HFD-induced increase in TC, TG, and LDL levels in mice, with decreases in the AI and CRF. (a) Blood samples were collected from each mouse after anesthesia, followed by euthanasia. TC, TG, HDL, and LDL levels of individual mice were measured and summed, and group averages were determined. * p < 0.05, ** p < 0.01, and *** p < 0.001. (b) The average AI and CRF for each group are presented. The AI and CRF were calculated based on the lipid levels of individual mice, and the group averages were then determined.
3.4. Effect of MCFAs on Liver Injury and Kidney Function
An HFD can induce metabolic changes that lead to liver and kidney injury, as evidenced by alterations in serum biomarkers such as AST, ALT, BUN, and creatinine [55,56,57,58,59,60]. Therefore, we analyzed these markers to investigate the effects of MCFAs on the liver and kidney functions in HFD-fed mice. The results showed no significant increase in AST, ALT, BUN, or creatinine levels in the HFD group compared to those in the ND group (Figure 3). Interestingly, ALT levels in the HFD_MCFA group were significantly lower than those in the ND and HFD groups, suggesting a potential protective effect of MCFAs on liver function (Figure 3a). Additionally, although the HFD_MCFA group exhibited a significant increase in BUN levels compared with the HFD group, no significant difference was observed between the ND and HFD_MCFA groups (Figure 3b). These findings demonstrate that BSFL-MCFAs may exert differential effects on serum biomarkers associated with liver and kidney functions in HFD-fed mice, particularly by reducing ALT levels.
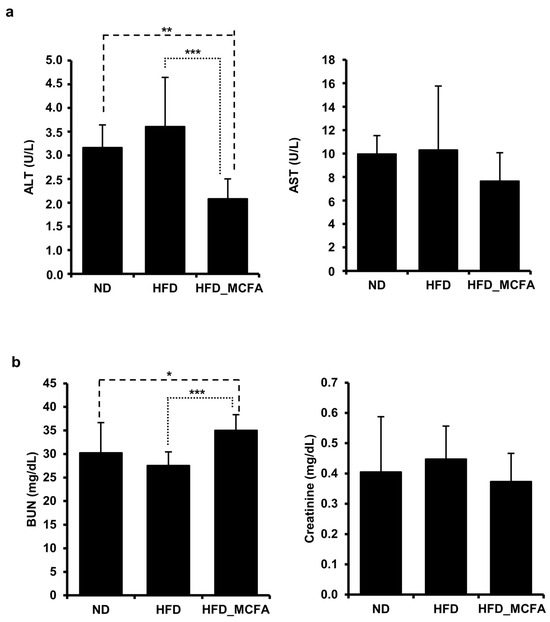
Figure 3.
MCFAs decrease levels of the liver injury marker ALT. (a,b) Blood samples were collected from each mouse after anesthesia, followed by euthanasia. (a) The average ALT and AST levels for each group are presented. The ALT and AST levels of individual mice were measured and summed, and group averages were determined. ** p < 0.01 and *** p < 0.001. (b) Average BUN and creatinine levels for each group are presented. BUN and creatinine levels of individual mice were measured and summed, and group averages were calculated. * p < 0.05 and *** p < 0.001.
3.5. Effect of MCFAs on Blood Glucose and Leptin Levels
Animal experiments have elucidated the relationship between an HFD and increased serum glucose, and the relationship between obesity and increased serum glucose levels has been reported [61,62,63,64,65]. Therefore, we measured blood glucose and leptin levels to examine the effect of MCFAs on obesity. Significant increases in the blood glucose and leptin levels were observed in the HFD group. In contrast, the blood glucose and leptin levels in the HFD_MCFA group were significantly lower than those in the HFD group (Figure 4). Furthermore, lower leptin levels were observed in the HFD_MCFA group than in the ND group. These results suggest that BSFL-MCFAs may help prevent HFD-induced obesity.
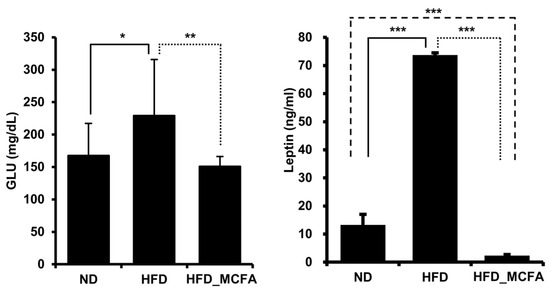
Figure 4.
MCFAs significantly suppress the increases in blood glucose and leptin levels. Blood samples were collected from each mouse after anesthesia, followed by euthanasia. The average ALT and AST levels for each group are presented. ALT and AST levels of individual mice were measured and summed, and group averages were determined. * p < 0.05, ** p < 0.01, and *** p < 0.001.
3.6. Effect of MCFAs on the Expression of ER Stress Markers
Several studies have reported that hypothalamic ER stress is closely associated with obesity and leptin resistance [66,67,68,69,70,71,72]. Therefore, we investigated changes in the expression of ER stress markers, such as Grp78, Erdj4, Xbp-1s, Atf4, and Chop, in the hypothalamus. The mRNA levels of ER stress markers were significantly increased in the HFD group, implying that the increase in body weight in HFD-fed mice was associated with obesity (Figure 5). In contrast, we observed decreased mRNA levels of ER stress markers in the HFD_MCFA group, suggesting the prevention of obesity by MCFAs (Figure 5). These findings indicate that BSFL-MCFAs may exhibit anti-obesity effects in HFD-fed C57BL/6J mice.
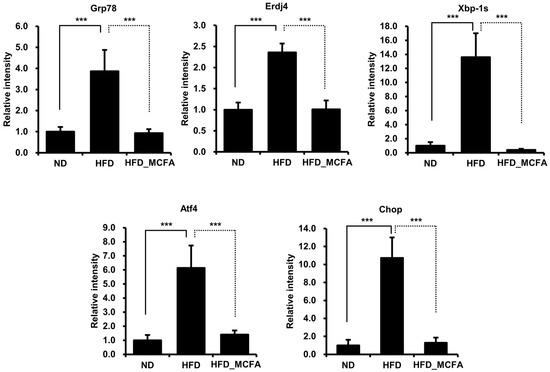
Figure 5.
MCFAs significantly suppress the increases in the expression of ER stress markers. The mice were sacrificed under anesthesia, and the hypothalami were collected. Total RNA was extracted from the hypothalami, and equal amounts of total RNA from individual samples were pooled for each group. Gene expression levels of ER stress markers were then analyzed. *** p < 0.001.
4. Discussion
BSFL have been utilized in agricultural and animal feed applications due to their high protein and lipid levels of approximately 42% and 35%, respectively [73]. Many studies have shown that lauric acid is the major fatty acid component in BSFL lipids [74,75,76,77,78]. We also found that the most prevalent fatty acid component of BSFL lipids was lauric acid [45]. Many studies have demonstrated the beneficial effects of lauric acid and triglycerides on metabolic disorders, including obesity, fatty liver, and diabetes [79,80,81,82,83]. In this investigation, mice supplemented with BSFL_MCFAs exhibited significantly reduced weight gain (Cohen’s d value: 11.68, effect-size r: 0.99) and FER (Cohen’s d value: 6.89, effect-size r: 0.96), without no significant change in food intake (Figure 1). Notably, both the weight gain and FER in the HFD_MCFA group were also lower than those observed in the ND group (Figure 1b). These findings are consistent with previous reports indicating that MCFA intake may increase thermogenesis and enhance energy expenditure, thereby contributing to the reduction in body weight in both animals and humans [22,23,84,85]. Taken together, these results suggest that the anti-obesity potential of BSFL-MCFAs may be associated with thermogenic activation and metabolic rate enhancement.
In this investigation, only male C57BL/6J mice were used to minimize potential variability associated with hormonal fluctuations in females, particularly those related to the estrous cycle, which can influence metabolic outcomes. This sex-specific selection is consistent with the design of many preclinical metabolic studies that aim to establish proof-of-concept efficacy. However, we acknowledge this as a limitation of the current study, and we have emphasized the importance of including female animals in future experiments to improve the generalizability and translational relevance of the findings.
Obesity is known to be associated with increased TC, TG, and LDL levels in the blood, which are established risk factors for metabolic disorders and cardiovascular diseases [86]. Accordingly, preventing obesity is a well-established strategy to improve lipid profiles and to reduce cardiovascular risk. In this study, significantly lower TC (Cohen’s d value: 2.97, effect-size r: 0.83), TG (Cohen’s d value: 1.26, effect-size r: 0.53), and LDL (Cohen’s d value: 1.30, effect-size r: 0.0.55) levels were observed in the HFD_MCFA group than in the HFD group (Figure 2). Additionally, the AI and CRF in the HFD_MCFA group were also lower than those in both the ND and HFD groups. Interestingly, HDL levels were elevated in both the HFD and HFD_MCFAs groups. This may reflect a compensatory response to high-fat intake, as increased HDL is often observed in rodent models fed high-fat diets [87]. In particular, MCFAs have been reported to promote HDL biosynthesis and improve reverse cholesterol transport by enhancing hepatic expression of apolipoproteins and lipid transporters [88,89]. These observations are consistent with previous reports indicating the lipid-lowering effects of MCFAs in animal models [90,91,92], and are further supported by recent meta-analysis report showing that MCFA-enriched diets contribute to reduced body weight, improved lipid profiles in blood, and decreased insulin resistance in overweight or obese individuals [93]. Therefore, these results imply that the significantly lower levels of TC, TG, and LDL in the HFD_MCFA group may be closely associated with the attenuated weight gain caused by the inhibition of body fat accumulation.
Although several investigations have reported increased blood levels of liver and kidney injury markers following HFD consumption, such increases were not observed in this investigation (Figure 3). Previous investigations have shown increases in liver and kidney injury markers such as ALT, AST, BUN, and creatinine after ≥14 weeks of HFD administration in C57B/6J mice [59,94,95]. In contrast, the present study was limited to 80 days (approximately 12 weeks), which may not have been sufficient to induce the damage markers. Therefore, the unobserved increase in these markers in our investigation may be attributed to the relatively short duration of HDF administration.
Interestingly, the HFD_MCFA group exhibited the lowest ALT levels and highest BUN levels, whereas no significant changes were observed in AST and creatinine levels. Reductions in ALT levels may be partially influenced by decreases in muscle mass, and increases in BUN levels are often associated with enhanced protein catabolism. As shown in Figure 1, the HFD_MCFA group exhibited less weight gain than the ND group, although no actual weight loss was observed. Collectively, these findings suggest that the changes in ALT and BUN levels in the HFD_MCFA group are unlikely to be the result of liver or kidney damage. Instead, the reduced weight gain in the HFD_MCFA group likely results from the inhibition of fat accumulation rather than from a loss of muscle mass. Considering that increased BUN levels are a common outcome of high-protein diets, it is plausible that MCFAs enhance the metabolic breakdown of dietary proteins, thereby contributing to the observed increase in BUN levels.
In this study, we found that supplementation with BSFL-MCFAs was associated with improvement in several obesity-related metabolic parameters in mice fed HFD. Lauric acid (C12;0), the predominant MCFA in BSFL crud lipids, may have contributed to these effects through its known metabolic properties. The HFD_MCFA group showed reduced serum glucose (Cohen’s d value: 1.28, effect-size r: 0.54) and leptin (Cohen’s d value: 112.89, effect-size r: 0.99) levels compared to the HFD group, which may reflect improvements in glucose regulation and metabolic hormone balance (Figure 4). In addition, gene expression analysis revealed that BSFL_MCFAs intake was accompanied by decreased expression of several ER stress markers, including Grp78, Erdj4, Xbp-1s, Atf4, and Chop, suggesting a potential reduction in HFD-induced ER stress (Figure 5). MCFAs, including lauric acid, have been reported to attenuate ER stress via modulation of the PERK-eIF2α-ATF4 pathway [96,97]. ER stress has been implicated in metabolic dysfunctions associated with obesity, including insulin resistance, leptin resistance, and chronic inflammation in adipose and hepatic tissues [66,69,98,99,100,101,102]. Specifically, ER stress can impair leptin signaling by activating the SOCS3 pathway and suppressing JAK2-STAT3 signaling, leading to leptin resistance [103,104]. The lower expression of Chop and Atf4, in particular, may demonstrate a downregulation of stress pathways related to the PERK-eIF2α axis, which is known to mediate cellular stress and inflammatory signaling [105,106]. Interestingly, the leptin levels in the HFD-MCFA group were not only lower than those in the HFD group but also the significantly reduced compared to the ND group. While this finding may suggest that BSFL-MCFAs enhance leptin sensitivity or energy expenditure, further investigation is needed to clarify the underlying mechanisms, in potential effects on thermogenesis or metabolic rates. Furthermore, the reduced weight gain observed in the HFD_MCFA group likely reflects fat mass loss rather than muscle catabolism, as indicators of muscle damage (AST) and renal function (creatinine) remained stable across groups (Figure 3). Overall, the results suggest that BSFL-MCFAs supplementation may contribute to metabolic improvements in the context of diet-induced obesity, possibly through modulating ER stress and related endocrine responses. However, further studies are necessary to reveal these findings and to explore the mechanistic pathways involved, particularly those linking ER stress with systemic metabolic regulation (Figure 6).
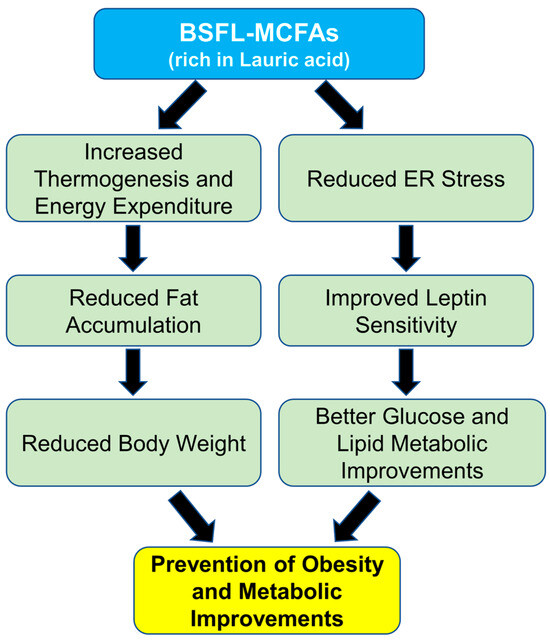
Figure 6.
Schematic representation of the proposed mechanisms underlying the effects of MCFAs on HFD-induced obesity.
5. Conclusions
In conclusion, the findings of this study suggest that BSFL-MCFAs may contribute to the prevention of obesity by inhibiting fat accumulation and may serve as an effective nutritional supplement that promotes weight management by increasing the basal metabolic rate. These results demonstrate that BSFL-MCFAs should be considered as a feed additive for mitigating obesity risk in companion animals, with potential applicability as a functional dietary ingredient.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani15101384/s1, Table S1. Proximate macronutrient composition of diets.
Author Contributions
K.-S.L. and M.-G.L. performed the experiments and analyzed the data. K.-S.L. prepared the original manuscript and K.-S.L. and K.J. organized the data into tables and figures. T.-W.G. and E.-Y.Y. directed the study and was involved in all aspects of the experimental design, data analysis, and manuscript preparation. All the authors critically reviewed the text, tables, and figures. All authors have read and agreed to the published version of the manuscript.
Funding
This was supported by the Korea Institute of Planning and Evaluation in Food., Agriculture, and Forestry (IPET) via the Technology Commercialization Program funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (122041-03).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Use and Care Committee at Dongguk University in accordance with the guidelines established by the Korean Animal Protection Act and the Laboratory Animal Act (IACUC-2022-09).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We thank the Greenteko Corporation for providing the breeding site for BSFL.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MCFAs | Medium-chain fatty acids |
| BSFL | Black soldier fly larvae |
| HFD | High-fat diet |
| ER | Endoplasmic reticulum |
| ND | Normal diet |
| FER | Food efficiency |
| AI | Atherogenic index |
| CRF | Cardiac risk factor |
References
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; Paciorek, C.J.; Lhoste, V.P.; Carrillo-Larco, R.M.; Stevens, G.A. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Obesity: The disease. J. Med. Chem. 2006, 49, 4001–4007. [Google Scholar] [CrossRef]
- Sullivan, P.W.; Ghushchyan, V.H.; Ben-Joseph, R. The impact of obesity on diabetes, hyperlipidemia and hypertension in the United States. Qual. Life Res. 2008, 17, 1063–1071. [Google Scholar] [CrossRef]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and cardiovascular disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef]
- Fortunato, L.M.; Kruk, T.; Júnior, E.L. Relationship between obesity and musculoskeletal disorders: Systematic review and meta-analysis. Res. Soc. Dev. 2021, 10, e119101320212. [Google Scholar] [CrossRef]
- Xing, W.; Li, S. Fat Metabolism-related lncRNA and Target Regulation and Application Studies in Chickens. Pak. Vet. J. 2023, 43, 579–584. [Google Scholar]
- Baker, J.; Supriya, R.; Dutheil, F.; Gao, Y. Obesity: Treatments, conceptualizations, and future directions for a growing problem. Biology 2022, 11, 160. [Google Scholar] [CrossRef]
- Tucker, S.; Bramante, C.; Conroy, M.; Fitch, A.; Gilden, A.; Wittleder, S.; Jay, M. The most undertreated chronic disease: Addressing obesity in primary care settings. Curr. Obes. Rep. 2021, 10, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; Heilbronn, L.K.; De Jonge, L.; DeLany, J.P.; Volaufova, J.; Anton, S.D.; Redman, L.M.; Smith, S.R.; Ravussin, E. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity 2007, 15, 2964–2973. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rosen, C.J. New insights into calorie restriction induced bone loss. Endocrinol. Metab. 2023, 38, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Abedelmalek, S.; Chtourou, H.; Souissi, N.; Tabka, Z. Caloric restriction effect on proinflammatory cytokines, growth hormone, and steroid hormone concentrations during exercise in judokas. Oxid. Med. Cell. Longev. 2015, 2015, 809492. [Google Scholar] [CrossRef]
- DuVall, M.A.; Coulter, C.E.; Gosey, J.L.; Herrera, M.J.; Hill, C.M.; Jariwala, R.R.; Maisano, L.E.; Moldovan, L.A.; Morrison, C.D.; Nwabueze, N.V. Leptin treatment prevents impaired hypoglycemic counterregulation induced by exposure to severe caloric restriction or exposure to recurrent hypoglycemia. Auton. Neurosci. 2021, 235, 102853. [Google Scholar] [CrossRef]
- Blundell, J.E.; Stubbs, J. Diet composition and the control of food intake in humans. In Handbook of Obesity; CRC Press: Boca Raton, FL, USA, 2003; pp. 443–476. [Google Scholar]
- Koliaki, C.; Spinos, T.; Spinou, Μ.; Brinia, Μ.-E.; Mitsopoulou, D.; Katsilambros, N. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare 2018, 6, 73. [Google Scholar] [CrossRef]
- Nimbkar, S.; Leena, M.M.; Moses, J.; Anandharamakrishnan, C. Medium chain triglycerides (MCT): State-of-the-art on chemistry, synthesis, health benefits and applications in food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 843–867. [Google Scholar] [CrossRef]
- Watanabe, S.; Tsujino, S. Applications of medium-chain triglycerides in foods. Front. Nutr. 2022, 9, 802805. [Google Scholar] [CrossRef]
- Rasheed, M.; Zaman, M.A.; Zafar, A.; Khan, M.A.; Anjum, S.; Ali, H.M.; Hussain, S.; Zafar, M.; Yasin, J.; Hussain, R. Prophylactic Effects of Methylene Blue, Coconut and Olive Oils Supplements on Hemato-Biochemical and Histo-pathological Parameters against p-Phenylenediamine Toxicity in Male Albino Rats. Pak. Vet. J. 2024, 44, 840–846. [Google Scholar]
- Ndiaye, E.M.; El Idrissi, Y.; Sow, A.; Ayessou, N.C.; El Moudden, H.; Harhar, H.; Cisse, M.; Tabyaoui, M. Influence of the extraction process on the chemical composition and oxidation state of baobab (Adansonia digitata L.) seed oil. J. Glob. Innov. Agric. Sci. 2024, 12, 45–52. [Google Scholar] [CrossRef]
- Rial, S.A.; Jutras-Carignan, A.; Bergeron, K.-F.; Mounier, C. A high-fat diet enriched in medium chain triglycerides triggers hepatic thermogenesis and improves metabolic health in lean and obese mice. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2020, 1865, 158582. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Ross, R.; Parsons, W.D.; Jones, P.J. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes. Res. 2003, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Wein, S.; Wolffram, S.; Schrezenmeir, J.; Gašperiková, D.; Klimeš, I.; Šeböková, E. Medium-chain fatty acids ameliorate insulin resistance caused by high-fat diets in rats. Diabetes Metab. Res. Rev. 2009, 25, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Alexandrou, E.; Herzberg, G.R.; White, M.D. High-level medium-chain triglyceride feeding and energy expenditure in normal-weight women. Can. J. Physiol. Pharmacol. 2007, 85, 507–513. [Google Scholar] [CrossRef]
- Takeuchi, H.; Noguchi, O.; Sekine, S.; Kobayashi, A.; Aoyama, T. Lower weight gain and higher expression and blood levels of adiponectin in rats fed medium-chain TAG compared with long-chain TAG. Lipids 2006, 41, 207–212. [Google Scholar] [CrossRef]
- Jia, M.; Yue, H.; Wang, X.; Zong, A.; Xu, T.; Xu, Y.-J.; Liu, Y. Medium-chain triglyceride attenuates obesity by activating brown adipose tissue via upregulating the AMPK signaling pathway. J. Nutr. Biochem. 2025, 141, 109914. [Google Scholar] [CrossRef]
- Gupta, R.; Pierdzioch, C. Climate risk and the volatility of agricultural commodity price fluctuations: A prediction experiment. In Behavioral Finance and Asset Prices: The Influence of Investor’s Emotions; Springer: Berlin/Heidelberg, Germany, 2022; pp. 23–44. [Google Scholar]
- Umar, Z.; Gubareva, M.; Naeem, M.; Akhter, A. Return and volatility transmission between oil price shocks and agricultural commodities. PLoS ONE 2021, 16, e0246886. [Google Scholar] [CrossRef]
- Nguyen, H.; Randall, M.; Lewis, A. Factors affecting crop prices in the context of climate change—A review. Agriculture 2024, 14, 135. [Google Scholar] [CrossRef]
- Dossa, L.I.K.-T.; Bashir, M.K.; Hassan, S.; Mushtaq, K. Impact of climate change on agricultural production in Burkina Faso, West Africa. J. Glob. Innov. Agric. Sci. 2023, 11, 319–332. [Google Scholar] [CrossRef]
- Kioko, M.; Ndirangu, S.; Nyarindo, W. Evaluating effect of climate smart agricultural practices adoption on productivity of drought-tolerant pulses: Insights from dryland areas of Makueni County, Kenya. J. Glob. Innov. Agric. Sci. 2024, 12, 803–813. [Google Scholar] [CrossRef]
- Mun, S.-K.; Jang, C.J.; Jo, S.; Park, S.-H.; Sim, H.B.; Ramos, S.C.; Kim, H.; Choi, Y.-J.; Park, D.-H.; Park, K.-W. Anti-obesity and immunomodulatory effects of oil and fermented extract dried from Tenebrio molitor larvae on aged obese mice. Anim. Cells Syst. 2024, 28, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Marschall, M.J.; Grundmann, S.M.; Seel, W.; Simon, M.-C.; Schuchardt, S.; Most, E.; Gessner, D.K.; Wen, G.; Ringseis, R.; Eder, K. Fat from Hermetia illucens alters the cecal gut microbiome and lowers hepatic triglyceride concentration in comparison to palm oil in obese zucker rats. J. Nutr. 2024, 154, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Boakye-Yiadom, K.A.; Ilari, A.; Duca, D. Greenhouse gas emissions and life cycle assessment on the black soldier fly (Hermetia illucens L.). Sustainability 2022, 14, 10456. [Google Scholar] [CrossRef]
- Lee, K.-S.; Yun, E.-Y.; Goo, T.-W. Antimicrobial activity of an extract of Hermetia illucens larvae immunized with Lactobacillus casei against Salmonella species. Insects 2020, 11, 704. [Google Scholar] [CrossRef]
- Xia, J.; Ge, C.; Yao, H. Antimicrobial peptides from black soldier fly (Hermetia illucens) as potential antimicrobial factors representing an alternative to antibiotics in livestock farming. Animals 2021, 11, 1937. [Google Scholar] [CrossRef]
- Franco, A.; Scieuzo, C.; Salvia, R.; Pucciarelli, V.; Borrelli, L.; Addeo, N.F.; Bovera, F.; Laginestra, A.; Schmitt, E.; Falabella, P. Antimicrobial activity of lipids extracted from Hermetia illucens reared on different substrates. Appl. Microbiol. Biotechnol. 2024, 108, 167. [Google Scholar] [CrossRef]
- Dayrit, F.M. The properties of lauric acid and their significance in coconut oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Ibrahim, N.A. Characteristics of Malaysian palm kernel and its products. J. Oil Palm Res. 2013, 25, 245–252. [Google Scholar]
- Suryati, T.; Julaeha, E.; Farabi, K.; Ambarsari, H.; Hidayat, A.T. Lauric acid from the black soldier fly (Hermetia illucens) and its potential applications. Sustainability 2023, 15, 10383. [Google Scholar] [CrossRef]
- Yoon, J.S. Effects of Black Soldier Fly (Hermetia illucens) Larvae Oil and Meal on Growth Performance, Cecal Microflora, and Meat Quality in Broiler. Ph.D. Thesis, Seoul National University Graduate School, Seoul, Republic of Korea, 2021. [Google Scholar]
- Koutsos, E.; Modica, B.; Freel, T. Immunomodulatory potential of black soldier fly larvae: Applications beyond nutrition in animal feeding programs. Transl. Anim. Sci. 2022, 6, txac084. [Google Scholar] [CrossRef]
- Eickleberry, C. Impacts of Black Soldier Fly (Hermetia Illucens) Larvae Oil on Sow Reproductive Efficiency, Nursery Pig Performance, and Hematological Criteria; North Carolina State University: Raleigh, NC, USA, 2023. [Google Scholar]
- Lee, K.-S.; Yun, E.-Y.; Goo, T.-W. Optimization of feed components to improve Hermetia illucens growth and development of oil extractor to produce biodiesel. Animals 2021, 11, 2573. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, Y.; Kim, J.; Park, S.Y.; Lee, K.; Hwang, K.T. Physicochemical characteristics and anti-oxidant activities of farm-cultivated and mountain-cultivated ginseng seeds. Food Sci. Biotechnol. 2018, 27, 1257–1264. [Google Scholar] [CrossRef]
- Chen, Y.; She, Y.; Lei, J.; Wang, D.; Wu, S.; Men, K. Medium chain fatty acids: Extraction, isolation, purification, bioactive properties and application. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 705, p. 012013. [Google Scholar]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Olamoyegun, M.A.; Oluyombo, R.; Asaolu, S.O. Evaluation of dyslipidemia, lipid ratios, and atherogenic index as cardiovascular risk factors among semi-urban dwellers in Nigeria. Ann. Afr. Med. 2016, 15, 194–199. [Google Scholar] [CrossRef]
- Talke, H.; Schubert, G. Enzymatic urea determination in the blood and serum in the Warburg optical test. Klin. Wochenschr. 1965, 43, 174–175. [Google Scholar] [CrossRef]
- Toora, B.; Rajagopal, G. Measurement of creatinine by Jaffe’s reaction-determination of concentration of sodium hydroxide required for maximum color development in standard, urine and protein free filtrate of serum. Indian J. Exp. Biol. 2002, 40, 352–354. [Google Scholar]
- Kim, J.; Yun, E.-Y.; Park, S.-W.; Goo, T.-W.; Seo, M. Allomyrina dichotoma larvae regulate food intake and body weight in high fat diet-induced obese mice through mTOR and Mapk signaling pathways. Nutrients 2016, 8, 100. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef]
- Kume, S.; Uzu, T.; Araki, S.-i.; Sugimoto, T.; Isshiki, K.; Chin-Kanasaki, M.; Sakaguchi, M.; Kubota, N.; Terauchi, Y.; Kadowaki, T. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J. Am. Soc. Nephrol. 2007, 18, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Declèves, A.-E.; Zolkipli, Z.; Satriano, J.; Wang, L.; Nakayama, T.; Rogac, M.; Le, T.P.; Nortier, J.L.; Farquhar, M.G.; Naviaux, R.K. Regulation of lipid accumulation by AMK-activated kinase in high fat diet–induced kidney injury. Kidney Int. 2014, 85, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, J.; Li, S.; Guo, F.; Li, A.; Wu, H.; Chen, J.; Pan, Q.; Liao, S.; Liu, H.-f. High-fat diet-induced renal proximal tubular inflammatory injury: Emerging risk factor of chronic kidney disease. Front. Physiol. 2021, 12, 786599. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, Y.-L.; Zhang, J.-L.; Zhang, P.; Wang, J.-Q.; Li, Z.-H. Alpinetin improved high fat diet-induced non-alcoholic fatty liver disease (NAFLD) through improving oxidative stress, inflammatory response and lipid metabolism. Biomed. Pharmacother. 2018, 97, 1397–1408. [Google Scholar] [CrossRef]
- Ha, S.-K.; Chae, C. Inducible nitric oxide distribution in the fatty liver of a mouse with high fat diet-induced obesity. Exp. Anim. 2010, 59, 595–604. [Google Scholar] [CrossRef][Green Version]
- Lichtenstein, A.H.; Schwab, U.S. Relationship of dietary fat to glucose metabolism. Atherosclerosis 2000, 150, 227–243. [Google Scholar] [CrossRef]
- McAllan, L.; Skuse, P.; Cotter, P.D.; Connor, P.O.; Cryan, J.F.; Ross, R.P.; Fitzgerald, G.; Roche, H.M.; Nilaweera, K.N. Protein quality and the protein to carbohydrate ratio within a high fat diet influences energy balance and the gut microbiota in C57BL/6J mice. PLoS ONE 2014, 9, e88904. [Google Scholar] [CrossRef]
- Li, W.-C.; Hsiao, K.-Y.; Chen, I.-C.; Chang, Y.-C.; Wang, S.-H.; Wu, K.-H. Serum leptin is associated with cardiometabolic risk and predicts metabolic syndrome in Taiwanese adults. Cardiovasc. Diabetol. 2011, 10, 36. [Google Scholar] [CrossRef]
- Phipps, P.; Starritt, E.; Caterson, I.; Grunstein, R. Association of serum leptin with hypoventilation in human obesity. Thorax 2002, 57, 75–76. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.; Gojobori, T.; Isenovic, E. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Liu, G.; Guo, J.; Su, Z. Hypothalamic endoplasmic reticulum stress as a key mediator of obesity-induced leptin resistance. Obes. Rev. 2018, 19, 770–785. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; González-García, I.; Seoane-Collazo, P.; Martínez-Sánchez, N.; Liñares-Pose, L.; Rial-Pensado, E.; Fernø, J.; Tena-Sempere, M.; Casals, N.; Diéguez, C. Reduction of hypothalamic endoplasmic reticulum stress activates browning of white fat and ameliorates obesity. Diabetes 2017, 66, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Çakir, I.; Cyr, N.E.; Perello, M.; Litvinov, B.P.; Romero, A.; Stuart, R.C.; Nillni, E.A. Obesity induces hypothalamic endoplasmic reticulum stress and impairs proopiomelanocortin (POMC) post-translational processing. J. Biol. Chem. 2013, 288, 17675–17688. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Liu, S.; Klionsky, D.J.; Lip, G.Y.; Tuomilehto, J.; Kavalakatt, S.; Pereira, D.M.; Samali, A.; Ren, J. ER stress in obesity pathogenesis and management. Trends Pharmacol. Sci. 2022, 43, 97–109. [Google Scholar] [CrossRef]
- Williams, L.M. Hypothalamic dysfunction in obesity. Proc. Nutr. Soc. 2012, 71, 521–533. [Google Scholar] [CrossRef]
- Ramírez, S.; Claret, M. Hypothalamic ER stress: A bridge between leptin resistance and obesity. FEBS Lett. 2015, 589, 1678–1687. [Google Scholar] [CrossRef]
- Ozcan, L.; Ergin, A.S.; Lu, A.; Chung, J.; Sarkar, S.; Nie, D.; Myers, M.G.; Ozcan, U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009, 9, 35–51. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Tian, J.; Zhou, J.; Yu, H. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2016, 465, 43–52. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)–Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Kim, C.-H.; Ryu, J.; Lee, J.; Ko, K.; Lee, J.-y.; Park, K.Y.; Chung, H. Use of black soldier fly larvae for food waste treatment and energy production in Asian countries: A review. Processes 2021, 9, 161. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Bueno, R.P.; González-Fernández, M.J.; Sánchez-Muros-Lozano, M.J.; García-Barroso, F.; Guil-Guerrero, J.L. Fatty acid profiles and cholesterol content of seven insect species assessed by several extraction systems. Eur. Food Res. Technol. 2016, 242, 1471–1477. [Google Scholar] [CrossRef]
- Surendra, K.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef]
- Saraswathi, V.; Kumar, N.; Gopal, T.; Bhatt, S.; Ai, W.; Ma, C.; Talmon, G.A.; Desouza, C. Lauric acid versus palmitic acid: Effects on adipose tissue inflammation, insulin resistance, and non-alcoholic fatty liver disease in obesity. Biology 2020, 9, 346. [Google Scholar] [CrossRef]
- Xia, J.; Yu, P.; Zeng, Z.; Ma, M.; Zhang, G.; Wan, D.; Gong, D.; Deng, S.; Wang, J. Lauric triglyceride ameliorates high-fat-diet-induced obesity in rats by reducing lipogenesis and increasing lipolysis and β-oxidation. J. Agric. Food Chem. 2021, 69, 9157–9166. [Google Scholar] [CrossRef]
- Sedik, A.A.; Elgohary, R.; Khalifa, E.; Khalil, W.K.; Shafey, H.I.; Shalaby, M.B.; Gouida, M.S.O.; Tag, Y.M. Lauric acid attenuates hepato-metabolic complications and molecular alterations in high-fat diet-induced nonalcoholic fatty liver disease in rats. Toxicol. Mech. Methods 2024, 34, 454–467. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Liu, T.; Wang, J.; Cai, H.; Zhang, X.; Xia, D.Q.H.; Feng, F.; Tang, J. Differential modulations of lauric acid and its glycerides on high fat diet-induced metabolic disorders and gut microbiota dysbiosis. Food Res. Int. 2022, 157, 111437. [Google Scholar] [CrossRef]
- Tham, Y.Y.; Choo, Q.C.; Muhammad, T.S.T.; Chew, C.H. Lauric acid alleviates insulin resistance by improving mitochondrial biogenesis in THP-1 macrophages. Mol. Biol. Rep. 2020, 47, 9595–9607. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Han, X.; Zhou, F.; Guo, J.; Huang, W.; Zhan, J.; You, Y. Coconut oil and medium-chain fatty acids attenuate high-fat diet-induced obesity in mice through increased thermogenesis by activating brown adipose tissue. Front. Nutr. 2022, 9, 896021. [Google Scholar] [CrossRef]
- Shinohara, H.; Ogawa, A.; Kasai, M.; Aoyama, T. Effect of randomly interesterified triacylglycerols containing medium-and long-chain fatty acids on energy expenditure and hepatic fatty acid metabolism in rats. Biosci. Biotechnol. Biochem. 2005, 69, 1811–1818. [Google Scholar] [CrossRef]
- Han, T.S.; Lean, M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016, 5, 2048004016633371. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Yanagita, T. Medium-chain fatty acids: Functional lipids for the prevention and treatment of the metabolic syndrome. Pharmacol. Res. 2010, 61, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Swift, L.L.; Hill, J.O.; Peters, J.C.; Greene, H.L. Plasma lipids and lipoproteins during 6 d of maintenance feeding with long-chain, medium-chain, and mixed-chain triglycerides. Am. J. Clin. Nutr. 1992, 56, 881–886. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhang, X.; Xu, Q.; Yang, X.; Xue, C. Medium-chain fatty acids reduce serum cholesterol by regulating the metabolism of bile acid in C57BL/6J mice. Food Funct. 2017, 8, 291–298. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhang, X.; Xu, Q.; Zhang, Y.; Xue, C.; Guo, C. Medium-chain fatty acids decrease serum cholesterol via reduction of intestinal bile acid reabsorption in C57BL/6J mice. Nutr. Metab. 2018, 15, 37. [Google Scholar] [CrossRef]
- Xu, Q.; Xue, C.; Zhang, Y.; Liu, Y.; Wang, J.; Yu, X.; Zhang, X.; Zhang, R.; Yang, X.; Guo, C. Medium-chain fatty acids enhanced the excretion of fecal cholesterol and cholic acid in C57BL/6J mice fed a cholesterol-rich diet. Biosci. Biotechnol. Biochem. 2013, 77, 1390–1396. [Google Scholar] [CrossRef]
- He, H.; Liu, K.; Liu, M.; Yang, A.-J.; Cheng, K.-W.; Lu, L.W.; Liu, B.; Chen, J.-H. The Impact of Medium-Chain Triglycerides on Weight Loss and Metabolic Health in Individuals with Overweight or Obesity: A Systematic Review and Meta-Analysis. Clin. Nutr. 2024, 43, 1755–1768. [Google Scholar] [CrossRef]
- Sánchez-Navarro, A.; Martínez-Rojas, M.Á.; Caldiño-Bohn, R.I.; Pérez-Villalva, R.; Zambrano, E.; Castro-Rodríguez, D.C.; Bobadilla, N.A. Early triggers of moderately high-fat diet-induced kidney damage. Physiol. Rep. 2021, 9, e14937. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, H.; Liu, H.; Jia, W.; Yan, J.; Ding, W.; Zhang, Y.; Xiao, Z.; Zhu, Z. Protective effects of ferroptosis inhibition on high fat diet-induced liver and renal injury in mice. Int. J. Clin. Exp. Pathol. 2020, 13, 2041. [Google Scholar]
- Nghiem, T.-H.T.; Nguyen, K.A.; Kusuma, F.; Park, S.; Park, J.; Joe, Y.; Han, J.; Chung, H.T. The PERK-eIF2α-ATF4 Axis Is Involved in Mediating ER-Stress-Induced Ferroptosis via DDIT4-mTORC1 Inhibition and Acetaminophen-Induced Hepatotoxicity. Antioxidants 2025, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Walter, N.S.; Gorki, V.; Bhardwaj, R.; Punnakkal, P. Endoplasmic Reticulum Stress: Implications in Diseases. Protein J. 2025, 44, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Lebeaupin, C.; Wu, N.N.; Kaufman, R.J.; Ren, J. ER stress and inflammation crosstalk in obesity. Med. Res. Rev. 2023, 43, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.-H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.r.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.k.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef]
- Hosoi, T.; Sasaki, M.; Miyahara, T.; Hashimoto, C.; Matsuo, S.; Yoshii, M.; Ozawa, K. Endoplasmic reticulum stress induces leptin resistance. Mol. Pharmacol. 2008, 74, 1610–1619. [Google Scholar] [CrossRef]
- Kim, O.-K.; Jun, W.; Lee, J. Mechanism of ER stress and inflammation for hepatic insulin resistance in obesity. Ann. Nutr. Metab. 2015, 67, 218–227. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Li, Z.; Guo, B. ER stress-induced inflammasome activation contributes to hepatic inflammation and steatosis. J. Clin. Cell. Immunol. 2016, 7, 457. [Google Scholar] [CrossRef]
- Liu, H.; Du, T.; Li, C.; Yang, G. STAT3 phosphorylation in central leptin resistance. Nutr. Metab. 2021, 18, 39. [Google Scholar] [CrossRef]
- Engin, A. The mechanism of leptin resistance in obesity and therapeutic perspective. In Obesity and Lipotoxicity; Advances in Experimental Medicine and Biology; Engin, A.B., Engin, A., Eds.; Springer: Cham, Switzerland, 2024; Volume 1460, pp. 463–487. [Google Scholar]
- Yuan, Z.; Xiao-Wei, L.; Juan, W.; Xiu-Juan, L.; Nian-Yun, Z.; Lei, S. HIIT and MICT attenuate high-fat diet-induced hepatic lipid accumulation and ER stress via the PERK-ATF4-CHOP signaling pathway. J. Physiol. Biochem. 2022, 78, 641–652. [Google Scholar] [CrossRef]
- Amen, O.M.; Sarker, S.D.; Ghildyal, R.; Arya, A. Endoplasmic reticulum stress activates unfolded protein response signaling and mediates inflammation, obesity, and cardiac dysfunction: Therapeutic and molecular approach. Front. Pharmacol. 2019, 10, 977. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).