Association Analysis of SLC11A1 Polymorphisms with Somatic Cell Score in Chinese Holstein Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Sample Collection and DNA Preparation
2.2. Primer Sequences and PCR Amplification
2.3. Single Strand Conformation Polymorphism (SSCP) Detection
2.4. Cloning and Sequencing
2.5. Restriction Fragment Length Polymorphism (RFLP) Analysis
2.6. Bioinformatics Analysis
2.7. Statistical Analysis
3. Results
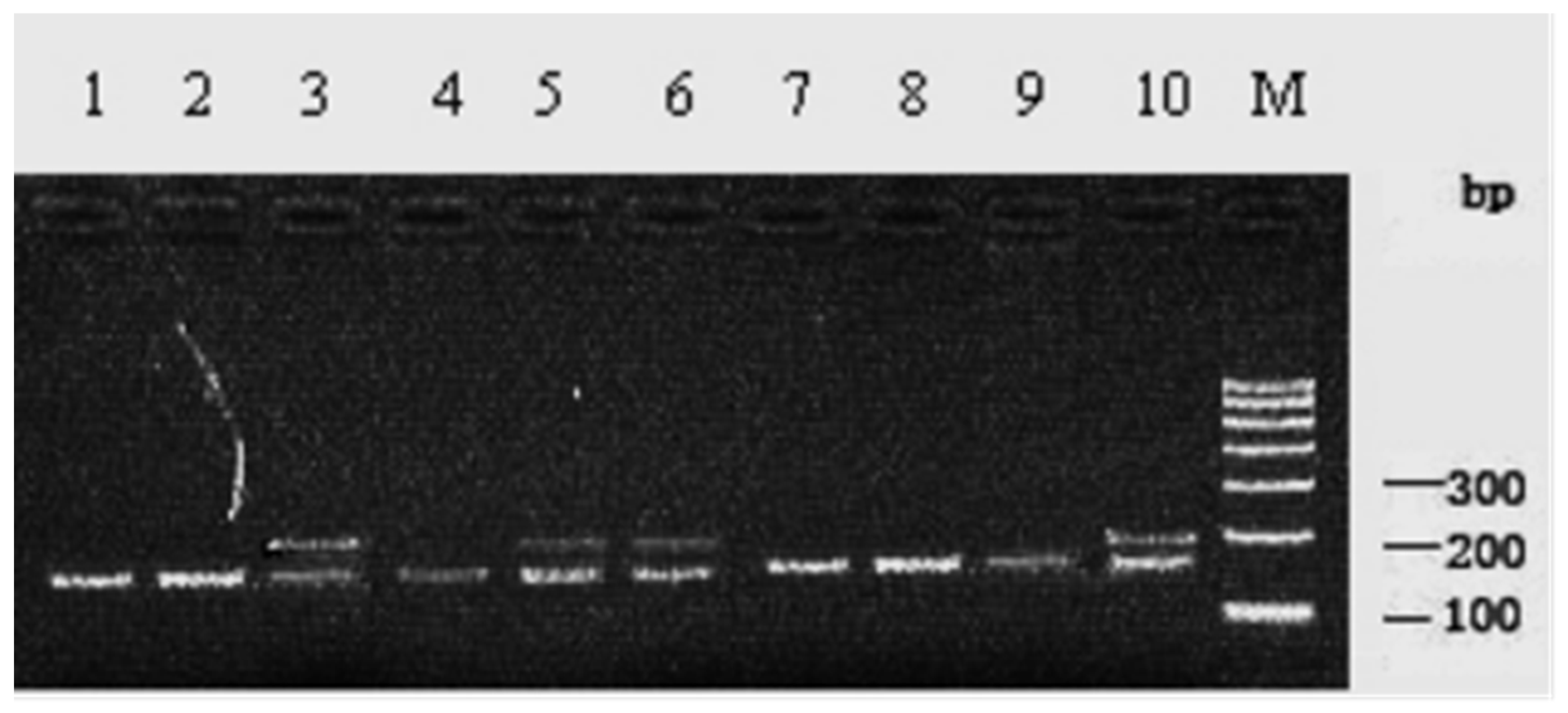
3.1. SSCP Detection and Genotyping
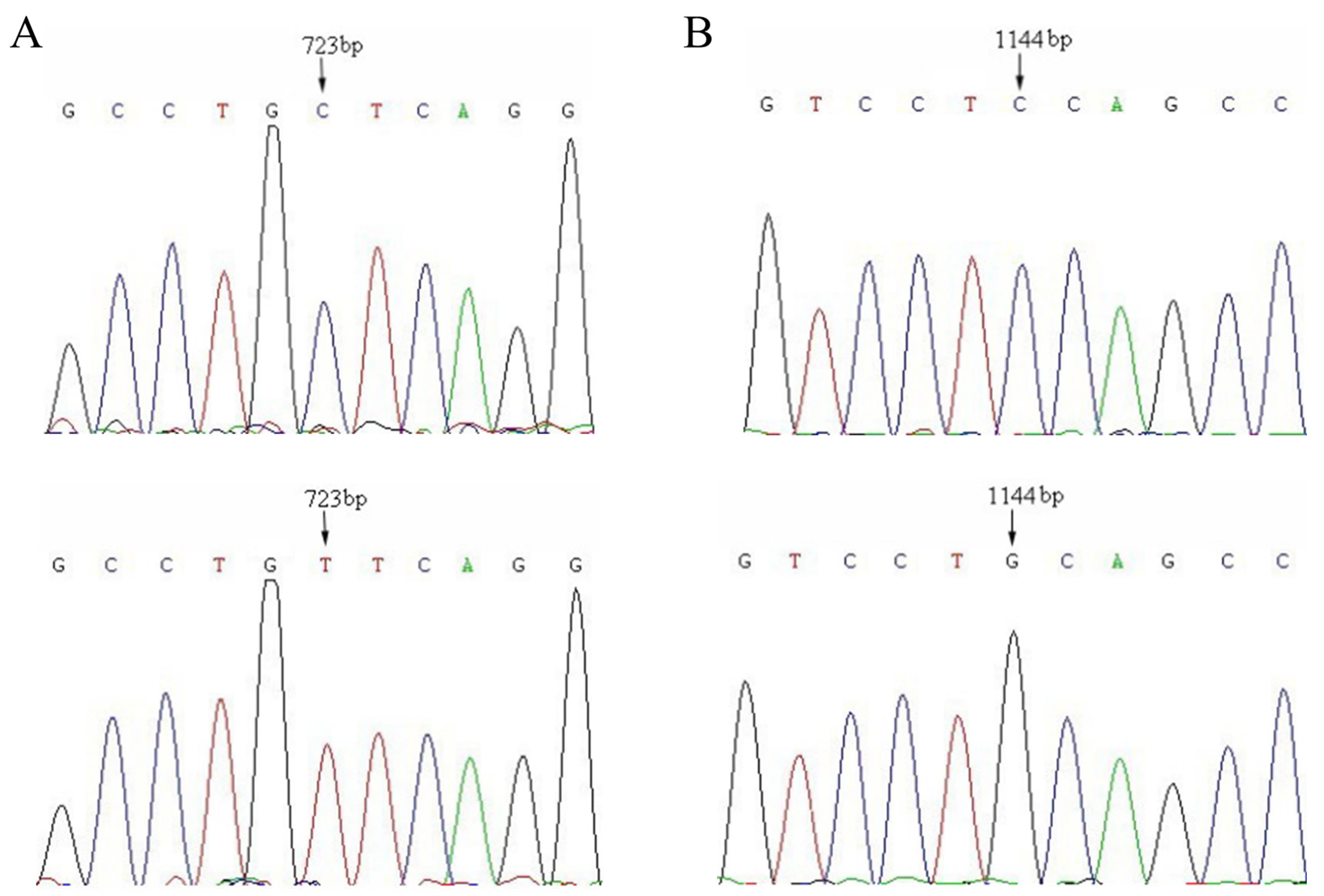
3.2. Bpu10I Analysis of the c.723C>T of SLC11A1
3.3. PstI Analysis of the c.1144C>G of SLC11A1
3.4. Genetic Characteristics of Polymorphic Sites of SLC11A1 Gene in Chinese Holstein Cows
3.5. Association of Two Polymorphic Loci of the SLC11A1 with SCS in Chinese Holstein Cows
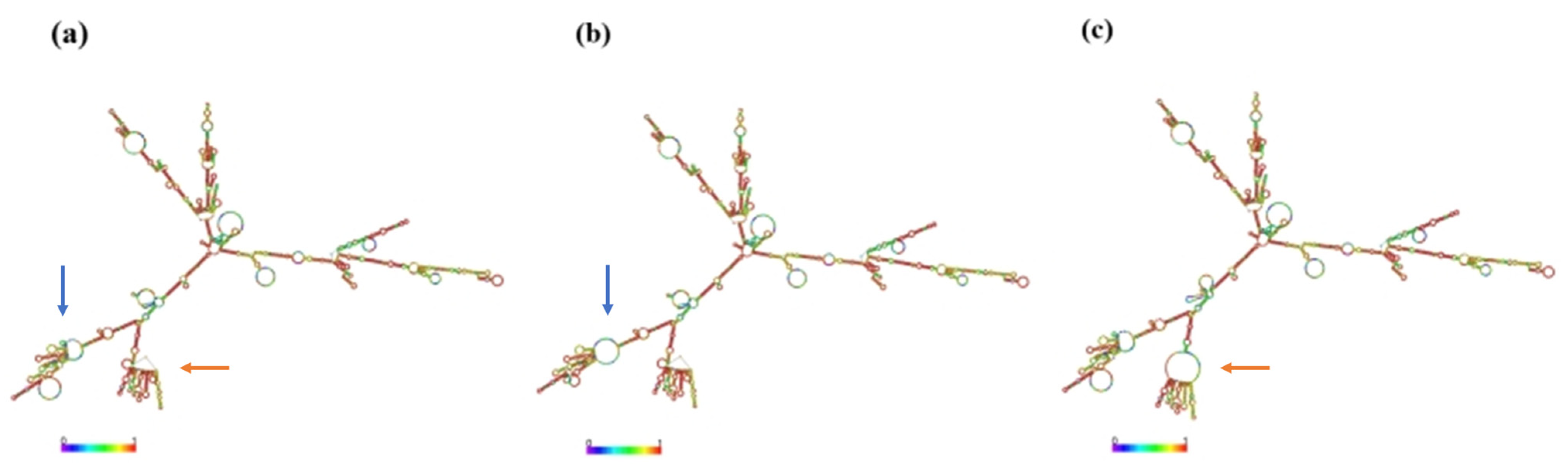
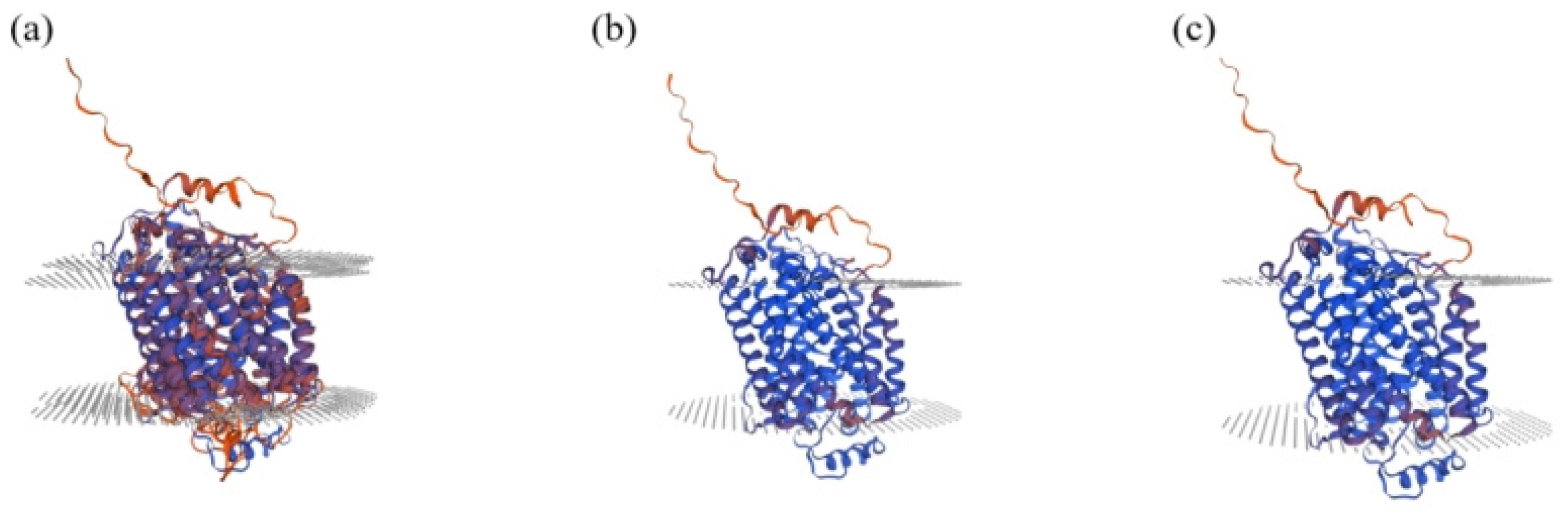
3.6. Bioinformatics Analysis of Chinese Holstein Cows SLC11A1
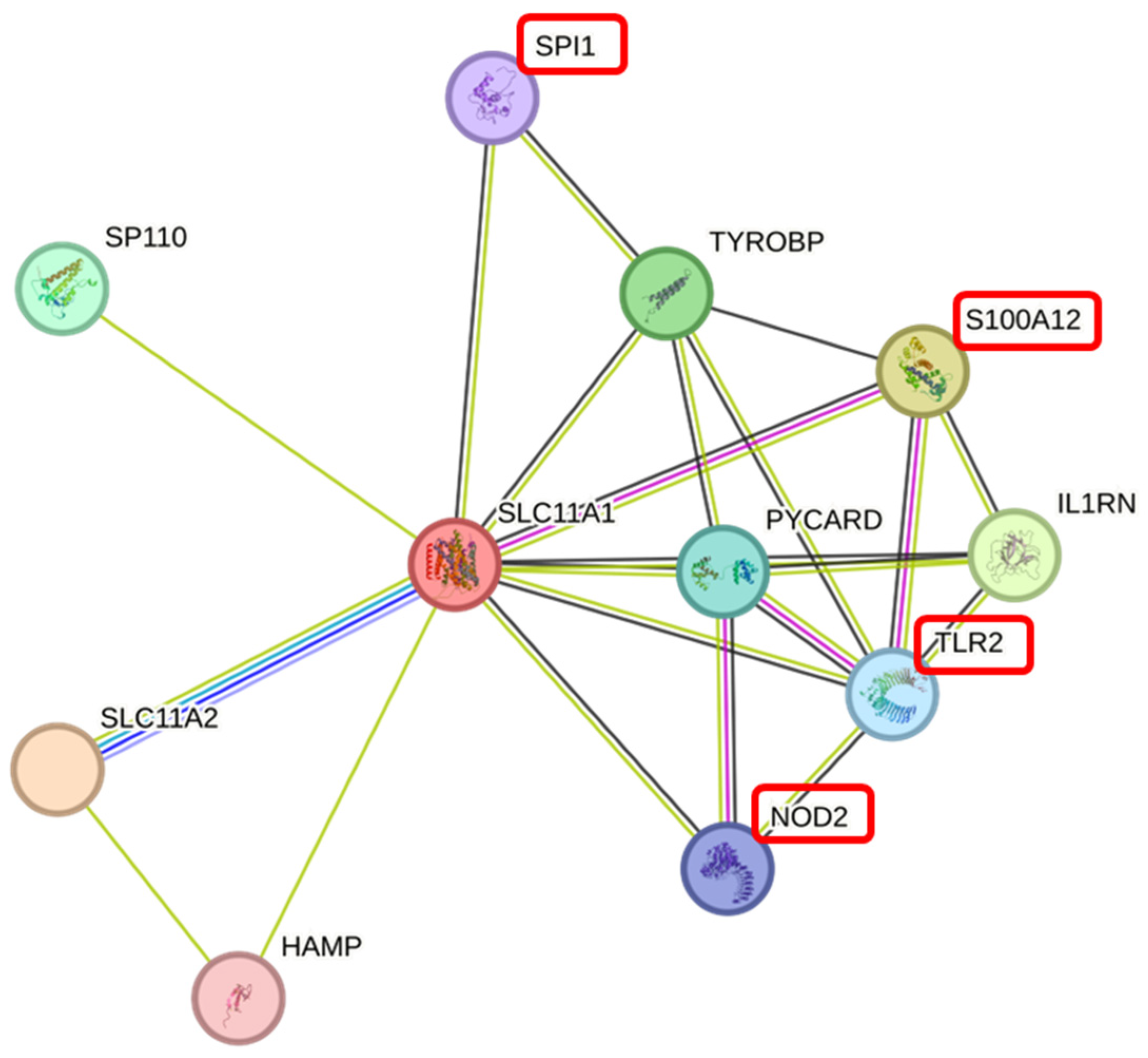
3.7. The PPI Network of the SLC11A1 Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sumon, S.; Parvin, M.S.; Ehsan, M.A.; Islam, M.T. Relationship between somatic cell counts and subclinical mastitis in lactating dairy cows. Vet. World 2020, 13, 1709–1713. [Google Scholar] [CrossRef] [PubMed]
- Touza-Otero, L.; Landin, M.; Diaz-Rodriguez, P. Fighting antibiotic resistance in the local management of bovine mastitis. Biomed. Pharmacother. 2024, 170, 115967. [Google Scholar] [CrossRef] [PubMed]
- Jaglan, K.; Ravikumar, D.; Sukhija, N.; George, L.; Alex, R.; Vohra, V.; Verma, A. Genomic clues of association between clinical mastitis and SNPs identified by ddRAD sequencing in Murrah buffaloes. Anim. Biotechnol. 2023, 34, 4538–4546. [Google Scholar] [CrossRef] [PubMed]
- Rupp, R.; Boichard, D. Genetic parameters for clinical mastitis, somatic cell score, production, udder type traits, and milking ease in first lactation Holsteins. J. Dairy Sci. 1999, 82, 2198–2204. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Z.; Yang, Z.; Zhu, X.; Ji, D.; Mao, Y. Association of IL8 -105G/A with mastitis somatic cell score in Chinese Holstein dairy cows. Anim. Biotechnol. 2015, 26, 143–147. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, H.; Li, R.; Zhang, Y.; Liu, Y.; Wang, J.; Wang, X.; Ju, Z.; Liu, W.; Hou, M.; et al. In silico genome-wide miRNA-QTL-SNPs analyses identify a functional SNP associated with mastitis in Holsteins. BMC Genet. 2019, 20, 46. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Hui, T.; Sun, J.; Cai, W.; Lin, G.; Wang, L.; Dou, X.; Wang, Z.; Han, D.; et al. Association analysis for SNPs of KRT26 and TCHH genes with cashmere production performance, body measurement traits and milk production traits in Liaoning cashmere goats. Anim. Biotechnol. 2023, 34, 698–708. [Google Scholar] [CrossRef]
- An, D.; Chen, X.; Li, Z.; Dai, L.; Huang, J.; Xiao, M.; Liu, H.; Xu, J.; Ruan, Y. Genetic variation in the BLM gene and its expression in the ovaries is closely related to kidding number in goats. Theriogenology 2024, 218, 254–266. [Google Scholar] [CrossRef]
- Clancey, E.; Kiser, J.N.; Moraes, J.G.N.; Dalton, J.C.; Spencer, T.E.; Neibergs, H.L. Genome-wide association analysis and gene set enrichment analysis with SNP data identify genes associated with 305-day milk yield in Holstein dairy cows. Anim. Genet. 2019, 50, 254–258. [Google Scholar] [CrossRef]
- Chu, M.X.; Ye, S.C.; Qiao, L.; Wang, J.X.; Feng, T.; Huang, D.W.; Cao, G.L.; Di, R.; Fang, L.; Chen, G.H. Polymorphism of exon 2 of BoLA-DRB3 gene and its relationship with somatic cell score in Beijing Holstein cows. Mol. Biol. Rep. 2012, 39, 2909–2914. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, Q.; Zhang, Q.; Arbab, A.A.I.; Li, M.; Yang, Z.; Karrow, N.A.; Mao, Y. Polymorphisms of the ACSL1 Gene Influence Milk Production Traits and Somatic Cell Score in Chinese Holstein Cows. Animals 2020, 10, 2282. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sadee, W.; Schlesinger, L.S. Innate immune gene polymorphisms in tuberculosis. Infect. Immun. 2012, 80, 3343–3359. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.; Teng, Z.; Wang, Y.; Dong, G.; Xu, C.; Qin, B.; Song, C.; Chai, J.; Li, Y.; et al. Association between SLC11A1 (NRAMP1) polymorphisms and susceptibility to tuberculosis in Chinese Holstein cattle. Tuberculosis 2017, 103, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Lokken-Toyli, K.L.; Diaz-Ochoa, V.E.; Camacho, L.; Stull-Lane, A.R.; Van Hecke, A.E.R.; Mooney, J.P.; Muñoz, A.D.; Walker, G.T.; Hampel, D.; Jiang, X.; et al. Vitamin A deficiency impairs neutrophil-mediated control of Salmonella via SLC11A1 in mice. Nat. Microbiol. 2024, 9, 727–736. [Google Scholar] [CrossRef]
- Vidal, S.M.; Malo, D.; Vogan, K.; Skamene, E.; Gros, P. Natural resistance to infection with intracellular parasites: Isolation of a candidate for Bcg. Cell 1993, 73, 469–485. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Gao, Z.; Lu, Y.; Deng, W.; Xi, D.; Chong, Y. An Exhaustive Structural Scrutiny and Initial Functional Appraisal of the Yak SLC11A1 Gene. Russ. J. Genet. 2024, 60, 493–502. [Google Scholar] [CrossRef]
- Vidal, S.; Tremblay, M.L.; Govoni, G.; Gauthier, S.; Sebastiani, G.; Malo, D.; Skamene, E.; Olivier, M.; Jothy, S.; Gros, P. The Ity/Lsh/Bcg locus: Natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 1995, 182, 655–666. [Google Scholar] [CrossRef]
- Govoni, G.; Vidal, S.; Gauthier, S.; Skamene, E.; Malo, D.; Gros, P. The Bcg/Ity/Lsh locus: Genetic transfer of resistance to infections in C57BL/6J mice transgenic for the Nramp1 Gly169 allele. Infect. Immun. 1996, 64, 2923–2929. [Google Scholar] [CrossRef]
- Perry, I.D.; Krishnan, L.; Murphy, S.P. SLC11A1 is expressed in the human placenta across multiple gestational ages. Placenta 2019, 75, 23–26. [Google Scholar] [CrossRef]
- Holder, A.; Garty, R.; Elder, C.; Mesnard, P.; Laquerbe, C.; Bartens, M.C.; Salavati, M.; Shabbir, M.Z.; Tzelos, T.; Connelly, T.; et al. Analysis of Genetic Variation in the Bovine SLC11A1 Gene, Its Influence on the Expression of NRAMP1 and Potential Association with Resistance to Bovine Tuberculosis. Front. Microbiol. 2020, 11, 1420. [Google Scholar] [CrossRef]
- Feng, J.; Li, Y.; Hashad, M.; Schurr, E.; Gros, P.; Adams, L.G.; Templeton, J.W. Bovine natural resistance associated macrophage protein 1 (Nramp1) gene. Genome Res. 1996, 6, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Barthel, R.; Feng, J.; Piedrahita, J.A.; McMurray, D.N.; Templeton, J.W.; Adams, L.G. Stable transfection of the bovine NRAMP1 gene into murine RAW264.7 cells: Effect on Brucella abortus survival. Infect. Immun. 2001, 69, 3110–3119. [Google Scholar] [CrossRef]
- Borriello, G.; Capparelli, R.; Bianco, M.; Fenizia, D.; Alfano, F.; Capuano, F.; Ercolini, D.; Parisi, A.; Roperto, S.; Iannelli, D. Genetic resistance to Brucella abortus in the water buffalo (Bubalus bubalis). Infect. Immun. 2006, 74, 2115–2120. [Google Scholar] [CrossRef]
- Capparelli, R.; Alfano, F.; Amoroso, M.G.; Borriello, G.; Fenizia, D.; Bianco, A.; Roperto, S.; Roperto, F.; Iannelli, D. Protective effect of the Nramp1 BB genotype against Brucella abortus in the water buffalo (Bubalus bubalis). Infect. Immun. 2007, 75, 988–996. [Google Scholar] [CrossRef]
- Ateya, A.I.; Ibrahim, S.S.; Al-Sharif, M.M. Single Nucleotide Polymorphisms, Gene Expression and Economic Evaluation of Parameters Associated with Mastitis Susceptibility in European Cattle Breeds. Vet. Sci. 2022, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Moradi-Sharhrbabak, M.; Miraie-Ashtiani, R.; Safdari-Shahroudi, M.; Abdollahi-Arpanahi, R. Case–control approach application for finding a relationship between candidate genes and clinical mastitis in Holstein dairy cattle. J. Appl. Genet. 2016, 57, 107–112. [Google Scholar] [CrossRef]
- Cheng, Z.; Buggiotti, L.; Salavati, M.; Marchitelli, C.; Palma-Vera, S.; Wylie, A.; Takeda, H.; Tang, L.; Crowe, M.A.; Wathes, D.C.; et al. Global transcriptomic profiles of circulating leucocytes in early lactation cows with clinical or subclinical mastitis. Mol. Biol. Rep. 2021, 48, 4611–4623. [Google Scholar] [CrossRef]
- Ali, A.K.A.; Shook, G. An Optimum Transformation for Somatic Cell Concentration in Milk. J. Dairy Sci. 1980, 63, 487–490. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de-Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Dalanezi, F.M.; Joaquim, S.F.; Guimarães, F.F.; Guerra, S.T.; Lopes, B.C.; Schmidt, E.M.S.; Cerri, R.L.A.; Langoni, H. Influence of pathogens causing clinical mastitis on reproductive variables of dairy cows. J. Dairy Sci. 2020, 103, 3648–3655. [Google Scholar] [CrossRef]
- Oviedo-Boyso, J.; Valdez-Alarcón, J.J.; Cajero-Juárez, M.; Ochoa-Zarzosa, A.; López-Meza, J.E.; Bravo-Patiño, A.; Baizabal-Aguirre, V.M. Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J. Infect. 2007, 54, 399–409. [Google Scholar] [CrossRef]
- Hogeveen, H.; Huijps, K.; Lam, T. Economic aspects of mastitis: New developments. N. Z. Vet. J. 2011, 59, 16–23. [Google Scholar] [CrossRef]
- Cunrath, O.; Bumann, D. Host resistance factor SLC11A1 restricts Salmonella growth through magnesium deprivation. Science 2019, 366, 995–999. [Google Scholar] [CrossRef]
- Brochado, M.J.; Gatti, M.F.; Zago, M.A.; Roselino, A.M. Association of the solute carrier family 11 member 1 gene polymorphisms with susceptibility to leprosy in a Brazilian sample. Mem. Do Inst. Oswaldo Cruz 2016, 111, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Delaby, C.; Rondeau, C.; Pouzet, C.; Willemetz, A.; Pilard, N.; Desjardins, M.; Canonne-Hergaux, F. Subcellular localization of iron and heme metabolism related proteins at early stages of erythrophagocytosis. PLoS ONE 2012, 7, e42199. [Google Scholar] [CrossRef]
- Cellier, M.F.M. Developmental Control of NRAMP1 (SLC11A1) Expression in Professional Phagocytes. Biology 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Dunner, S.; Toro, R.; Tobón, J.; Gallego, J.; Cañón, J. Effect of polymorphisms in the Slc11a1 coding region on resistance to brucellosis by macrophages in vitro and after challenge in two Bos breeds (Blanco Orejinegro and Zebu). Genet. Mol. Biol. 2010, 33, 463–470. [Google Scholar] [CrossRef]
- Kadarmideen, H.N.; Ali, A.A.; Thomson, P.C.; Müller, B.; Zinsstag, J. Polymorphisms of the SLC11A1 gene and resistance to bovine tuberculosis in African Zebu cattle. Anim. Genet. 2011, 42, 656–658. [Google Scholar] [CrossRef]
- Taka, S.; Liandris, E.; Gazouli, M.; Sotirakoglou, K.; Theodoropoulos, G.; Bountouri, M.; Andreadou, M.; Ikonomopoulos, J. In vitro expression of the SLC11A1 gene in goat monocyte-derived macrophages challenged with Mycobacterium avium subsp paratuberculosis. Infect. Genet. Evol. 2013, 17, 8–15. [Google Scholar] [CrossRef]
- Cosenza, G.; Martin, P.; Garro, G.; Gallo, D.; Auzino, B.; Ciampolini, R.; Pauciullo, A. A novel allelic donkey β-lactoglobulin I protein isoform generated by a non-AUG translation initiation codon is associated with a nonsynonymous SNP. J. Dairy Sci. 2023, 106, 4158–4170. [Google Scholar] [CrossRef]
- Wang, P.; Li, K.; Fan, Y.; Zhang, H.; Zhang, Y.; Liu, Z.; Li, W.; Han, H.; Gao, Y.; Liu, J.; et al. Association analysis and expression level of ACE polymorphisms with egg-laying trait in Taihang chicken. Poult. Sci. 2022, 101, 102163. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, A.; Mishra, S.K.; Dubey, P.K.; Goyal, S.; Sehgal, M.; Niranjan, S.K.; Sodhi, M.; Mishra, B.P.; Kataria, R.S. Identification of genetic variation in NOD-like receptor 2 gene and influence of polymorphism on gene structure and function in buffalo (Bubalus bubalis). Res. Vet. Sci. 2017, 115, 43–50. [Google Scholar] [CrossRef]
- Moretti, R.; Soglia, D.; Chessa, S.; Sartore, S.; Finocchiaro, R.; Rasero, R.; Sacchi, P. Identification of SNPs Associated with Somatic Cell Score in Candidate Genes in Italian Holstein Friesian Bulls. Animals 2021, 11, 366. [Google Scholar] [CrossRef]
- Li, B.; Wan, Z.; Wang, Z.; Zuo, J.; Xu, Y.; Han, X.; Phouthapane, V.; Miao, J. TLR2 Signaling Pathway Combats Streptococcus uberis Infection by Inducing Mitochondrial Reactive Oxygen Species Production. Cells 2020, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, X.; Lin, T.; Dong, W.; Gao, Y.; Ji, P.; Zhang, Y.; Zhao, X.; Zhang, Q. Toll-like Receptor 2 Is Associated with the Immune Response, Apoptosis, and Angiogenesis in the Mammary Glands of Dairy Cows with Clinical Mastitis. Int. J. Mol. Sci. 2022, 23, 10717. [Google Scholar] [CrossRef] [PubMed]
- Lutzow, Y.C.; Donaldson, L.; Gray, C.P.; Vuocolo, T.; Pearson, R.D.; Reverter, A.; Byrne, K.A.; Sheehy, P.A.; Windon, R.; Tellam, R.L. Identification of immune genes and proteins involved in the response of bovine mammary tissue to Staphylococcus aureus infection. BMC Vet. Res. 2008, 4, 18. [Google Scholar] [CrossRef]
- Zhong, K.; Zhang, C.Y.; Zha, G.M.; Wang, X.J.; Jiao, X.Q.; Zhu, H.S.; Wang, Y.Y. S100 calcium-binding protein A12 as a diagnostic index for subclinical mastitis in cows. Reprod. Domest. Anim. 2018, 53, 1442–1447. [Google Scholar] [CrossRef]

| Gene | Primer | Primer Sequence (5′→3′) | Amplified Region | Size (bp) | Tm (°C) |
|---|---|---|---|---|---|
| SLC11A1 | P1 | F: TGTGAGGACAGCTGAGGAT R: CTGGAGAAGGGAATGGCTAT | Exons 1,2 | 1318 | 66 |
| P2 | F: ACCGGGCTCCTCTGTCCATG R: GTGGCAAAGCTGGGGGTCTC | Exons 3 | 798 | 63 | |
| P3 | F: TATATGGCTCCCCTCTCCCT R: GTTACCGCAATGAATCCTCC | Intron 6 | 400 | 63 | |
| P4 | F: GGCTGACGGCCTCTCCCTAA R: TTCCTGTCCAGGGGCACCA | Exons 4 | 176 | 66 | |
| P5 | F: AGCCTTGCTGACGGGACTAC R: AAGAACTAGGGCCCCTCTG | Exons 5 | 165 | 58 | |
| P6 | F: CTGACCCAGGCCACTCTGGT R: TGAGAGGCACACACCCACG | Exons 6 | 114 | 65 | |
| P7 | F: TGCTCTCACCCTAGGGTTGC R: GGCCCCTGTTATGGCTCTGG | Exons 7 | 110 | 62 | |
| P8 | F: GGGACTTTCACTCACCCCAC R: CCTCCCCTCCAGTCTGCTCA | Exons 8 | 199 | 61 | |
| P9 | F: TCGATGCTTCCGTCCTTTTA R: CTTTGGAGTCCCCTGCAGAA | Exons 9 | 242 | 57 | |
| P10 | F: CGGTGCCCGCAGTTCAACAT R: GGAAGGAGGGGTGTGGTCG | Exons 10 | 241 | 62 | |
| P11 | F: TACAGGGGCGGAGCCTGCT R: CACATCCGAGTCCTGAGTGG | Exons 11 | 243 | 64 | |
| P12 | F: CGTCCCCTGGAACCCCAGTC R: GTTCCTGTTTGGCCCCACAG | Exons 12 | 259 | 65 | |
| P13 | F: TAACGGCAGGGTCCTTCAGC R: AGTGCGATAAGCCCAGTGCT | Exons 13 | 156 | 65 | |
| P14 | F: GCAGCCGATCCTGAATCTCC R: TGGTCTCCTCTCCTTGCCTC | Exons 14 | 243 | 60 | |
| P15 | F: CGGGGTTCCTCGTAGGTCT R: CCCTTGTCTGGCAGGCCAGT | Exons 15 | 353 | 65 | |
| P16 | F: ACGTGAGTTCCAGAGGGAC R: GGGGAAGCAAAGAATCTGC | 3′ untranslated region | 432 | 64 |
| Polymorphic Site | Genotype | Genotype Frequency | Allele | Allele Frequency | Heterozy- Gosity | Effective Number of Alleles | PIC | χ2(P) |
|---|---|---|---|---|---|---|---|---|
| c.723C>T | AA (166) | 0.790 | A | 0.895 | 0.188 | 1.231 | 0.170 | 2.88 (p = 0.09) |
| AB (44) | 0.210 | B | 0.105 | |||||
| c. 1144C>G | CC (132) | 0.629 | C | 0.805 | 0.314 | 1.458 | 0.265 | 3.09 (p = 0.08) |
| CD (74) | 0.352 | D | 0.195 | |||||
| DD (4) | 0.019 |
| Gene | Polymorphic Site | Genotype | Number of Samples | Somatic Cell Score |
|---|---|---|---|---|
| SLC11A1 | c. 723C>T | AA | 166 | 4.12 b ± 0.15 |
| AB | 44 | 4.75 a ± 0.24 | ||
| c. 1144C>G | CC | 132 | 4.19 a ± 0.17 | |
| CD | 74 | 4.34 a ± 0.20 | ||
| DD | 4 | 4.65 a ± 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Liu, Y.; Li, T.; Li, Q.; Wang, J.; An, Y.; Yang, Y.; Li, K.; Chu, M. Association Analysis of SLC11A1 Polymorphisms with Somatic Cell Score in Chinese Holstein Cows. Animals 2025, 15, 1370. https://doi.org/10.3390/ani15101370
Liu K, Liu Y, Li T, Li Q, Wang J, An Y, Yang Y, Li K, Chu M. Association Analysis of SLC11A1 Polymorphisms with Somatic Cell Score in Chinese Holstein Cows. Animals. 2025; 15(10):1370. https://doi.org/10.3390/ani15101370
Chicago/Turabian StyleLiu, Kai, Yufang Liu, Tuo Li, Qiuling Li, Jinyu Wang, Yongfu An, Yuze Yang, Kaiyang Li, and Mingxing Chu. 2025. "Association Analysis of SLC11A1 Polymorphisms with Somatic Cell Score in Chinese Holstein Cows" Animals 15, no. 10: 1370. https://doi.org/10.3390/ani15101370
APA StyleLiu, K., Liu, Y., Li, T., Li, Q., Wang, J., An, Y., Yang, Y., Li, K., & Chu, M. (2025). Association Analysis of SLC11A1 Polymorphisms with Somatic Cell Score in Chinese Holstein Cows. Animals, 15(10), 1370. https://doi.org/10.3390/ani15101370






