Dietary Supplementation with Fermented Milk Improves Growth Performance and Intestinal Functions in Intrauterine Growth-Restricted Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Fermented Milk
2.3. Animals and Dietary Treatments
2.4. Sample Collection
2.5. Analysis of Amino Acid Profiles in Milk and Plasma
2.6. Examination of Intestinal Morphology
2.7. Determination of Digestive Enzyme Activities
2.8. Quantification of Glutathione (GSH), Glutathione Disulfide (GSSG) and MDA
2.9. Quantitative Real-Time Polymerase Chain Reaction (qPCR) Analysis
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Composition of the Fermented Milk
3.2. Dietary Supplementation with Fermented Milk Improved the Growth Performance of Weaned IUGR Piglets
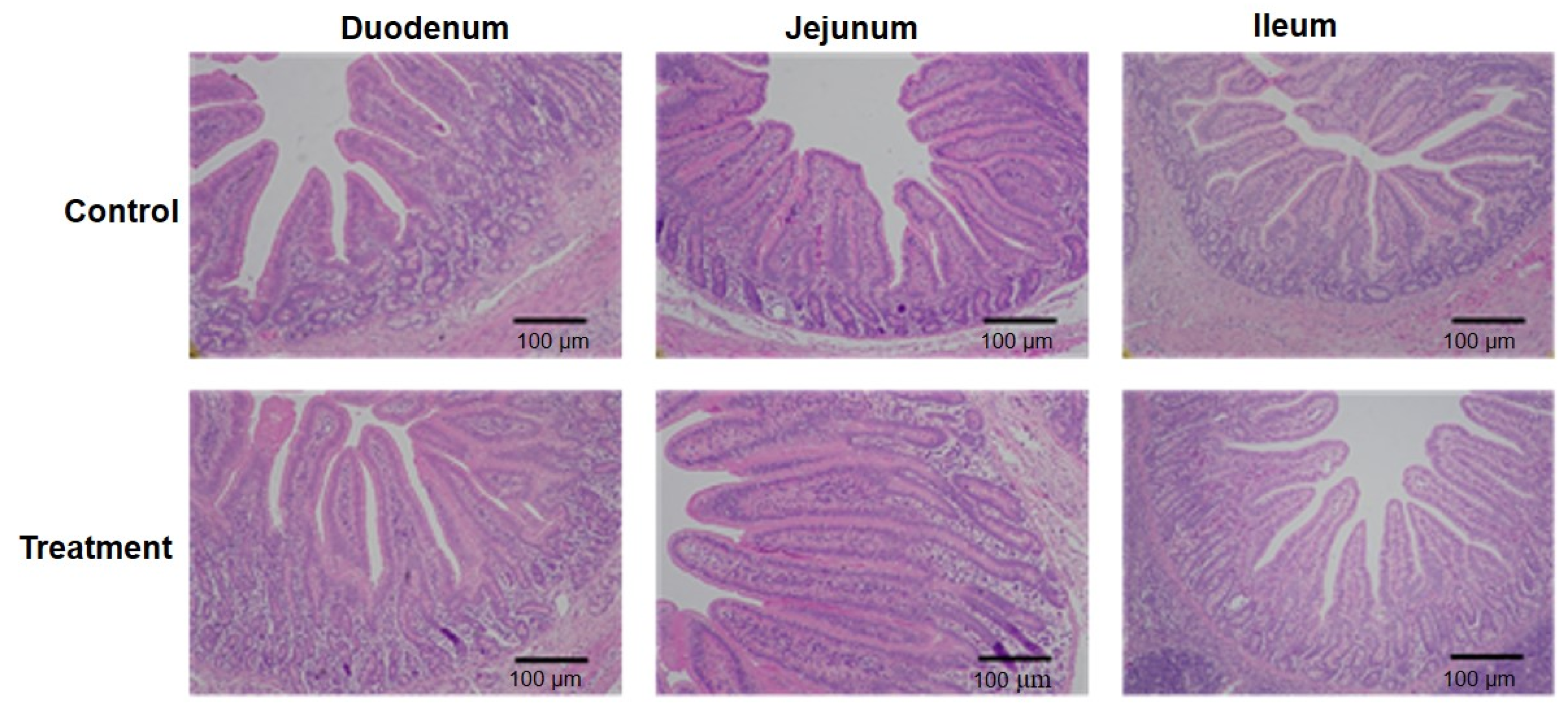
3.3. Fermented Milk Improved Intestinal Morphology in IUGR Piglets
3.4. Fermented Milk Increased Digestive Enzyme Activity in the Intestine of Weaned IUGR Pigs
3.5. Fermented Milk Enhanced Antioxidant Capacity in Weaned IUGR Piglets
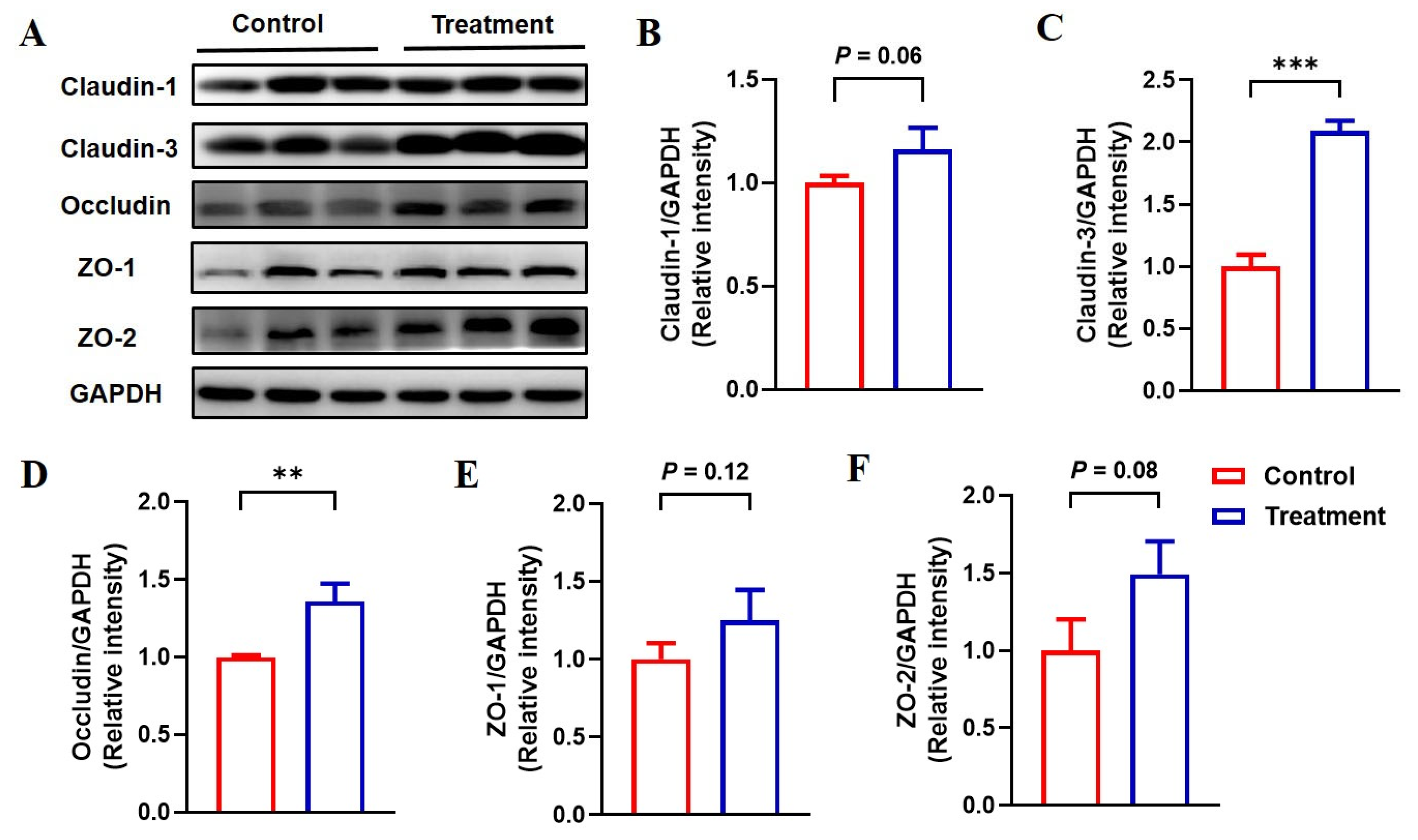
3.6. Feeding Fermented Milk Improved the Intestinal Barrier Function of IUGR Pigs
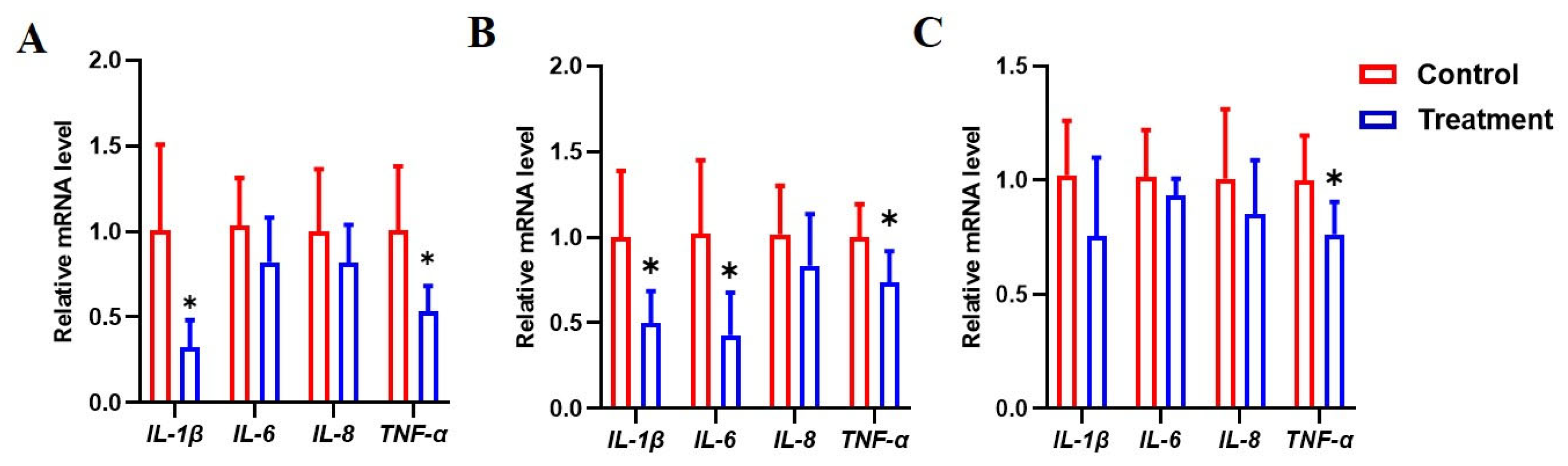
3.7. Dietary Fermented Milk Suppressed Intestinal Inflammation in IUGR Piglets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Datta, S.; Johnson, G.A.; Li, P.; Satterfield, M.C.; Spencer, T.E. Proline metabolism in the conceptus: Implications for fetal growth and development. Amino Acids 2008, 35, 691–702. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal nutrition and fetal development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef]
- Cui, C.; Wu, C.; Wang, J.; Ma, Z.; Zheng, X.; Zhu, P.; Wang, N.; Zhu, Y.; Guan, W.; Chen, F. Restored intestinal integrity, nutrients transporters, energy metabolism, antioxidative capacity and decreased harmful microbiota were associated with IUGR piglet's catch-up growth before weanling. J. Anim. Sci. Biotechnol. 2022, 13, 129. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Zou, Y.; Zhang, X.; Wang, Z.; Hu, J.; Han, D.; Zhao, J.; Dai, Z.; Wang, J. Lactobacillus amylovorus promotes lactose utilization in small intestine and enhances intestinal barrier function in intrauterine growth restricted piglets. J. Nutr. 2024, 154, 535–542. [Google Scholar] [CrossRef]
- Fang, T.; Tian, G.; Chen, D.; He, J.; Zheng, P.; Mao, X.; Yan, H.; Yu, B. Endoplasmic reticulum stress contributes to intestinal injury in intrauterine growth restriction newborn piglets. Animals 2024, 14, 2677. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K. Intrauterine growth retardation affects intestinal health of suckling piglets via altering intestinal antioxidant capacity, glucose uptake, tight junction, and immune responses. Oxidative Med. Cell. Longev. 2022, 2022, 2644205. [Google Scholar] [CrossRef]
- Wan, J.; Yu, Q.; Luo, J.; Zhang, L.; Ruan, Z. Effects of ferulic acid on the growth performance, antioxidant capacity, and intestinal development of piglets with intrauterine growth retardation. J. Anim. Sci. 2022, 100, skac144. [Google Scholar] [CrossRef]
- Xiong, L.; Azad, M.A.K.; Liu, Y.; Zhang, W.; Zhu, Q.; Hu, C.; You, J.; Kong, X. Intrauterine growth restriction affects colonic barrier function via regulating the Nrf2/Keap1 and TLR4-NF-κb/ERK pathways and altering colonic microbiome and metabolome homeostasis in growing-finishing pigs. Antioxidants 2024, 13, 283. [Google Scholar] [CrossRef]
- He, W.; Posey, E.A.; Steele, C.C.; Savell, J.W.; Bazer, F.W.; Wu, G. Dietary glycine supplementation enhances glutathione availability in tissues of pigs with intrauterine growth restriction. J. Anim. Sci. 2024, 102, skae025. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, W.; Azad, M.A.K.; Ma, C.; Zhu, Q.; Kong, X. Metabolome, microbiome, and gene expression alterations in the colon of newborn piglets with intrauterine growth restriction. Front. Microbiol. 2022, 13, 989060. [Google Scholar] [CrossRef]
- Zhang, X.; Yun, Y.; Lai, Z.; Ji, S.; Yu, G.; Xie, Z.; Zhang, H.; Zhong, X.; Wang, T.; Zhang, L. Supplemental Clostridium butyricum modulates lipid metabolism by reshaping the gut microbiota composition and bile acid profile in IUGR suckling piglets. J. Anim. Sci. Biotechnol. 2023, 14, 36. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Sigala-Robles, R.; Santiago-López, L.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Mata-Haro, V.; Wall-Medrano, A.; González-Córdova, A.F. Peptides, exopolysaccharides, and short-chain fatty acids from fermented milk and perspectives on inflammatory bowel diseases. Dig. Dis. Sci. 2022, 67, 4654–4665. [Google Scholar] [CrossRef]
- Samtiya, M.; Samtiya, S.; Badgujar, P.C.; Puniya, A.K.; Dhewa, T.; Aluko, R.E. Health-promoting and therapeutic attributes of milk-derived bioactive peptides. Nutrients 2022, 14, 3001. [Google Scholar] [CrossRef]
- Lin, A.; Yan, X.; Xu, R.; Wang, H.; Su, Y.; Zhu, W. Effects of lactic acid bacteria-fermented formula milk supplementation on colonic microbiota and mucosal transcriptome profile of weaned piglets. Animal 2023, 17, 100959. [Google Scholar] [CrossRef]
- Lin, A.; Yan, X.; Wang, H.; Su, Y.; Zhu, W. Effects of lactic acid bacteria-fermented formula milk supplementation on ileal microbiota, transcriptomic profile, and mucosal immunity in weaned piglets. J. Anim. Sci. Biotechnol. 2022, 13, 113. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of the Official Analytical Chemists: Gaithersburg, MD, USA, 2007. [Google Scholar]
- NRC. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Pedersen, K.S.; Toft, N. Intra- and inter-observer agreement when using a descriptive classification scale for clinical assessment of faecal consistency in growing pigs. Prev. Vet. Med. 2011, 98, 288–291. [Google Scholar] [CrossRef]
- Engelsmann, M.N.; Jensen, L.D.; van der Heide, M.E.; Hedemann, M.S.; Nielsen, T.S.; Nørgaard, J.V. Age-dependent development in protein digestibility and intestinal morphology in weaned pigs fed different protein sources. Animal 2022, 16, 100439. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Ji, Y.; Sun, Y.; Liu, N.; Jia, H.; Dai, Z.; Yang, Y.; Wu, Z. L-leucine supplementation reduces growth performance accompanied by changed profiles of plasma amino acids and expression of jejunal amino acid transporters in breast-fed intra-uterine growth-retarded piglets. Br. J. Nutr. 2023, 129, 2025–2035. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Z.; Jia, S.; Wu, G. Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 964, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, X.; Dai, Z.; Wu, Z.; Bazer, F.W.; Wu, G. Analysis of glutathione in biological samples by HPLC involving pre-column derivatization with o-phthalaldehyde. Methods Mol. Biol. 2018, 1694, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ruggeri, R.; Bee, G.; Trevisi, P.; Ollagnier, C. Intrauterine growth restriction defined by increased brain-to-liver weight ratio affects postnatal growth and protein efficiency in pigs. Animal 2024, 18, 101044. [Google Scholar] [CrossRef]
- Santos, T.G.; Fernandes, S.D.; de Oliveira Araújo, S.B.; Felicioni, F.; de Mérici Domingues, E.P.T.; Caldeira-Brant, A.L.; Ferreira, S.V.; de Paula Naves, L.; de Souza, S.P.; Campos, P.; et al. Intrauterine growth restriction and its impact on intestinal morphophysiology throughout postnatal development in pigs. Sci. Rep. 2022, 12, 11810. [Google Scholar] [CrossRef]
- Lynegaard, J.C.; Hansen, C.F.; Kristensen, A.R.; Amdi, C. Body composition and organ development of intra-uterine growth restricted pigs at weaning. Animal 2020, 14, 322–329. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Gao, L.; Chen, L.; Zhang, H. Nutrient-intake-level-dependent regulation of intestinal development in newborn intrauterine growth-restricted piglets via glucagon-like peptide-2. Animal 2016, 10, 1645–1654. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Yan, C.; Peng, X.; Xu, Q.; Xuan, Y.; Han, F.; Tian, G.; Fang, Z.; Lin, Y.; et al. Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction. Br. J. Nutr. 2015, 114, 53–62. [Google Scholar] [CrossRef]
- Van Ginneken, C.; Ayuso, M.; Van Bockstal, L.; Van Cruchten, S. Preweaning performance in intrauterine growth-restricted piglets: Characteristics and interventions. Mol. Reprod. Dev. 2023, 90, 697–707. [Google Scholar] [CrossRef]
- Hu, L.; Peng, X.; Chen, H.; Yan, C.; Liu, Y.; Xu, Q.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; et al. Effects of intrauterine growth retardation and Bacillus subtilis PB6 supplementation on growth performance, intestinal development and immune function of piglets during the suckling period. Eur. J. Nutr. 2017, 56, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Jiang, L.; Li, Q.; Zhang, J.; Zhang, L.; Wang, T. Dietary dimethylglycine sodium salt supplementation alleviates redox status imbalance and intestinal dysfunction in weaned piglets with intrauterine growth restriction. Anim. Nutr. 2022, 10, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Li, M. Effects of dietary epidermal growth factor supplementation on liver antioxidant capacity of piglets with intrauterine growth retardation. J. Anim. Sci. 2023, 101, skad323. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Jiang, L.; Wang, T. Dimethylglycine sodium salt alleviates intrauterine growth restriction-induced low growth performance, redox status imbalance, and hepatic mitochondrial dysfunction in suckling piglets. Front. Vet. Sci. 2022, 9, 905488. [Google Scholar] [CrossRef]
- Gao, H.; Chen, X.; Zhao, J.; Xue, Z.; Zhang, L.; Zhao, F.; Wang, B.; Wang, L. Integrative analysis of liver metabolomics and transcriptomics reveals oxidative stress in piglets with intrauterine growth restriction. Biology 2022, 11, 1430. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Li, X.L.; Satterfield, M.C.; Spencer, T.E. Impacts of amino acid nutrition on pregnancy outcome in pigs: Mechanisms and implications for swine production. J. Anim. Sci. 2010, 88, E195–E204. [Google Scholar] [CrossRef]
- Wang, J.; Feng, C.; Liu, T.; Shi, M.; Wu, G.; Bazer, F.W. Physiological alterations associated with intrauterine growth restriction in fetal pigs: Causes and insights for nutritional optimization. Mol. Reprod. Dev. 2017, 84, 897–904. [Google Scholar] [CrossRef]
- Ashworth, C.J.; Nwagwu, M.O.; McArdle, H.J. Genotype and fetal size affect maternal-fetal amino acid status and fetal endocrinology in Large White × Landrace and Meishan pigs. Reprod. Fertil. Dev. 2013, 25, 439–445. [Google Scholar] [CrossRef]
- Lin, G.; Liu, C.; Feng, C.; Fan, Z.; Dai, Z.; Lai, C.; Li, Z.; Wu, G.; Wang, J. Metabolomic analysis reveals differences in umbilical vein plasma metabolites between normal and growth-restricted fetal pigs during late gestation. J. Nutr. 2012, 142, 990–998. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Huang, S.; Wang, W.; Dai, Z.; Feng, C.; Wu, G.; Wang, J. Maternal L-glutamine supplementation during late gestation alleviates intrauterine growth restriction-induced intestinal dysfunction in piglets. Amino Acids 2018, 50, 1289–1299. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Satterfield, M.C.; Li, X.; Wang, X.; Johnson, G.A.; Burghardt, R.C.; Dai, Z.; Wang, J.; Wu, Z. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 2013, 45, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Knabe, D.A.; Kim, S.W. Arginine nutrition in neonatal pigs. J. Nutr. 2004, 134, 2783S–2790S; discussion 2796S–2797S. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Wu, G.; Sun, Y.; Wang, B.; He, B.; Dai, Z.; Wu, Z. Glutamine enhances tight junction protein expression and modulates corticotropin-releasing factor signaling in the jejunum of weanling piglets. J. Nutr. 2015, 145, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Ji, S.; Yu, G.; Jia, P.; Niu, Y.; Zhang, H.; Zhang, X.; Wang, T.; Zhang, L. Effects of Bacillus subtilis on jejunal integrity, redox status, and microbial composition of intrauterine growth restriction suckling piglets. J. Anim. Sci. 2021, 99, skab255. [Google Scholar] [CrossRef]
- Xie, Z.; Yun, Y.; Yu, G.; Zhang, X.; Zhang, H.; Wang, T.; Zhang, L. Bacillus subtilis alleviates excessive apoptosis of intestinal epithelial cells in intrauterine growth restriction suckling piglets via the members of Bcl-2 and caspase families. J. Sci. Food Agric. 2024, 104, 6924–6932. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Fang, J. Progress on the mechanisms of Lactobacillus plantarum to improve intestinal barrier function in ulcerative colitis. J. Nutr. Biochem. 2024, 124, 109505. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, L.; Lv, L.; Li, L. Pediococcus pentosaceus, a future additive or probiotic candidate. Microb. Cell Fact. 2021, 20, 45. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Tan, Z.L.; Abu Hafsa, S.H.; Adegbeye, M.J.; Greiner, R.; Ugbogu, E.A.; Cedillo Monroy, J.; Salem, A.Z.M. Saccharomyces cerevisiae as a probiotic feed additive to non and pseudo-ruminant feeding: A review. J. Appl. Microbiol. 2020, 128, 658–674. [Google Scholar] [CrossRef]
- Sharma, H.; Ozogul, F.; Bartkiene, E.; Rocha, J.M. Impact of lactic acid bacteria and their metabolites on the techno-functional properties and health benefits of fermented dairy products. Crit. Rev. Food Sci. Nutr. 2023, 63, 4819–4841. [Google Scholar] [CrossRef]
| Item | Before Fermentation | After Fermentation | p-Value |
|---|---|---|---|
| Pediococcus pentosaceus, CFU/L | 4.0 × 107 | 1.4 × 1010 | — |
| Lactiplantibacillus plantarum, CFU/L | 2.0 × 107 | 1.3 × 1010 | — |
| Bacillus subtilis, CFU/L | 2.7 × 106 | 6.0 × 107 | — |
| Saccharomyces cerevisae, CFU/L | 1.1 × 107 | 1.1 × 109 | — |
| Dry matter, % | 18.72 ± 0.05 | 19.04 ± 0.21 | 0.13 |
| Crude protein, g/L | 38.51 ± 1.30 | 40.12 ± 1.28 | 0.25 |
| Glucose, mmol/L | 207.90 ± 12.64 a | 184.30 ± 5.72 b | <0.01 |
| Lactic acid, mmol/L | 10.28 ± 1.09 a | 33.60 ± 5.54 b | <0.01 |
| Ammonia, mmol/L | 0.14 ± 0.02 | 0.18 ± 0.05 | 0.46 |
| pH | 6.51 ± 0.02 a | 5.72 ± 0.10 b | <0.01 |
| Item | Control | Treatment | p-Value |
|---|---|---|---|
| BW, kg | |||
| Day 0 | 3.73 ± 0.28 | 3.75 ± 0.42 | 0.91 |
| Day 7 | 4.60 ± 0.35 | 4.78 ± 0.59 | 0.40 |
| Day 14 | 6.25 ± 0.48 | 6.75 ± 0.87 | 0.08 |
| Day 19 | 7.59 ± 0.73 | 8.00 ± 0.87 | 0.23 |
| ADG, g/d | |||
| Days 0 to 7 | 125.43 ± 38.11 | 146.29 ± 41.57 | 0.20 |
| Days 8 to 14 | 235.14 ± 38.11 a | 276.43 ± 58.89 b | 0.05 |
| Days 0 to 14 | 180.10 ± 27.02 a | 216.14 ± 41.57 b | <0.05 |
| Days 0 to 19 | 209.29 ± 32.22 | 235.57 ± 41.57 | 0.11 |
| ADFI, g/d | |||
| Days 0 to 7 | 186.47 ± 19.11 | 185.61 ± 26.94 | 0.93 |
| Days 8 to 14 | 344.29 ± 36.74 | 378.20 ± 13.47 | 0.06 |
| Days 0 to 14 | 260.03 ± 22.05 | 275.22 ± 16.66 | 0.21 |
| Days 0 to 19 | 311.35 ± 29.39 | 326.24 ± 16.66 | 0.32 |
| F:G, g/g | |||
| Days 0 to 7 | 1.50 ± 0.29 | 1.28 ± 0.20 | 0.15 |
| Days 8 to 14 | 1.53 ± 0.20 | 1.38 ± 0.12 | 0.16 |
| Days 0 to 14 | 1.46 ± 0.10 a | 1.29 ± 0.05 b | <0.01 |
| Days 0 to 19 | 1.46 ± 0.15 | 1.38 ± 0.22 | 0.53 |
| Incidence of diarrhea, % | |||
| Days 0 to 7 | 10.32 ± 11.44 | 11.11 ± 7.17 | 0.89 |
| Days 8 to 14 | 0 | 0.79 ± 1.94 | 0.34 |
| Days 0 to 14 | 3.97 ± 3.92 | 6.35 ± 2.45 | 0.23 |
| Days 0 to 19 | 3.80 ± 4.16 | 5.26 ± 1.91 | 0.44 |
| Item | Control | Treatment | p-Value |
|---|---|---|---|
| Alanine | 435.03 ± 44.11 | 447.56 ± 90.63 | 0.81 |
| Arginine | 148.60 ± 9.55 a | 169.31 ± 6.12 b | <0.05 |
| Asparagine | 51.73 ± 22.54 | 53.18 ± 13.96 | 0.92 |
| Aspartate | 17.68 ± 6.61 | 17.03 ± 4.90 | 0.82 |
| Glutamine | 340.60 ± 71.04 a | 428.88 ± 46.54 b | <0.05 |
| Glutamate | 159.73 ± 17.64 | 178.08 ± 39.19 | 0.31 |
| Glycine | 661.82 ± 18.62 | 676.22 ± 90.63 | 0.71 |
| Histidine | 88.11 ± 21.31 | 91.50 ± 23.03 | 0.80 |
| Isoleucine | 74.12 ± 13.23 | 67.45 ± 19.60 | 0.51 |
| Leucine | 208.93 ± 46.54 | 229.40 ± 66.14 | 0.56 |
| Lysine | 152.98 ± 58.79 | 149.05 ± 56.34 | 0.91 |
| Methionine | 26.65 ± 4.41 | 23.98 ± 5.39 | 0.42 |
| Phenylalanine | 48.20 ± 11.02 | 48.88 ± 16.90 | 0.94 |
| Serine | 188.88 ± 41.64 | 160.72 ± 8.57 | 0.17 |
| Threonine | 87.42 ± 31.84 | 64.63 ± 17.64 | 0.17 |
| Tryptophan | 25.43 ± 10.04 | 23.56 ± 5.39 | 0.57 |
| Tyrosine | 132.39 ± 41.64 | 115.32 ± 26.94 | 0.42 |
| Valine | 103.44 ± 26.94 | 117.67 ± 41.64 | 0.49 |
| β-Alanine | 74.13 ± 14.45 | 83.87 ± 10.78 | 0.31 |
| Citrulline | 104.45 ± 26.94 | 102.67 ± 31.84 | 0.90 |
| Ornithine | 126.94 ± 41.64 | 115.83 ± 41.64 | 0.65 |
| Taurine | 92.32 ± 34.29 | 81.35 ± 36.74 | 0.59 |
| Item | Control | Treatment | p-Value |
|---|---|---|---|
| Duodenum | |||
| Villous height, μm | 371.23 ± 31.84 a | 420.84 ± 46.54 b | <0.05 |
| Crypt depth, μm | 276.41 ± 24.25 a | 253.78 ± 14.70 b | 0.05 |
| Villous height/crypt depth | 1.38 ± 0.15 a | 1.68 ± 0.22 b | <0.01 |
| Jejunum | |||
| Villous height, μm | 420.87 ± 29.39 | 422.61 ± 29.39 | 0.92 |
| Crypt depth, μm | 227.42 ± 20.58 a | 195.88 ± 17.88 b | <0.01 |
| Villous height/crypt depth | 1.94 ± 0.24 a | 2.25 ± 0.27 b | <0.05 |
| Ileum | |||
| Villous height, μm | 402.11 ± 29.39 | 424.45 ± 15.43 | 0.10 |
| Crypt depth, μm | 163.09 ± 13.47 | 153.84 ± 9.55 | 0.24 |
| Villous height/crypt depth | 2.52 ± 0.22 a | 3.09 ± 0.17 b | <0.01 |
| Item | Control | Treatment | p-Value |
|---|---|---|---|
| Duodenum | |||
| Lipase, U/g protein | 75.31 ± 34.29 a | 120.33 ± 24.49 b | <0.05 |
| α-amylase, U/mg protein | 98.09 ± 26.94 a | 152.35 ± 36.74 b | <0.05 |
| Sucrase, U/mg protein | 44.64 ± 6.37 a | 87.41 ± 44.09 b | <0.05 |
| Maltase, U/mg protein | 131.03 ± 58.79 | 198.58 ± 51.44 | 0.08 |
| Jejunum | |||
| Lipase, U/g protein | 64.57 ± 17.64 | 77.29 ± 23.03 | 0.31 |
| α-amylase, U/mg protein | 88.40 ± 26.94 | 101.38 ± 51.44 | 0.59 |
| Sucrase, U/mg protein | 71.81 ± 13.23 a | 98.08 ± 18.62 b | <0.05 |
| Maltase, U/mg protein | 192.92 ± 39.19 | 261.79 ± 93.08 | 0.14 |
| Ileum | |||
| Lipase, U/g protein | 78.78 ± 13.96 | 82.84 ± 5.88 | 0.57 |
| α-amylase, U/mg protein | 68.60 ± 16.90 | 88.06 ± 19.35 | 0.10 |
| Sucrase, U/mg protein | 70.23 ± 26.94 | 95.25 ± 18.62 | 0.13 |
| Maltase, U/mg protein | 202.06 ± 53.89 | 302.23 ± 90.63 | 0.06 |
| Item | Control | Treatment | p-Value |
|---|---|---|---|
| Jejunum | |||
| GSH, nmol/mg tissue | 1.42 ± 0.34 | 1.62 ± 0.24 | 0.29 |
| GSSG, nmol/mg tissue | 0.11 ± 0.02 | 0.12 ± 0.02 | 0.46 |
| GSH:GSSG | 13.13 ± 0.44 a | 14.46 ± 1.05 b | <0.05 |
| MDA, nmol/mg protein | 0.36 ± 0.12 | 0.34 ± 0.10 | 0.88 |
| Ileum | |||
| GSH, nmol/mg tissue | 1.18 ± 0.12 a | 1.73 ± 0.20 b | <0.01 |
| GSSG, nmol/mg tissue | 0.11 ± 0.02 | 0.12 ± 0.02 | 0.67 |
| GSH:GSSG | 10.69 ± 0.49 a | 15.14 ± 2.42 b | <0.01 |
| MDA, nmol/mg protein | 0.30 ± 0.05 | 0.27 ± 0.05 | 0.45 |
| Liver | |||
| GSH, nmol/mg tissue | 13.27 ± 0.42 | 15.47 ± 0.29 | 0.15 |
| GSSG, nmol/mg tissue | 1.06 ± 0.02 | 1.05 ± 0.02 | 0.47 |
| GSH:GSSG | 12.15 ± 1.64 a | 15.21 ± 1.52 b | <0.01 |
| MDA, nmol/mg protein | 1.50 ± 0.39 a | 0.86 ± 0.07 b | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Cui, L.; Yang, Y.; Yang, Y.; Dai, Z.; Wu, Z. Dietary Supplementation with Fermented Milk Improves Growth Performance and Intestinal Functions in Intrauterine Growth-Restricted Piglets. Animals 2025, 15, 1367. https://doi.org/10.3390/ani15101367
Yang Q, Cui L, Yang Y, Yang Y, Dai Z, Wu Z. Dietary Supplementation with Fermented Milk Improves Growth Performance and Intestinal Functions in Intrauterine Growth-Restricted Piglets. Animals. 2025; 15(10):1367. https://doi.org/10.3390/ani15101367
Chicago/Turabian StyleYang, Qing, Lu Cui, Yang Yang, Ying Yang, Zhaolai Dai, and Zhenlong Wu. 2025. "Dietary Supplementation with Fermented Milk Improves Growth Performance and Intestinal Functions in Intrauterine Growth-Restricted Piglets" Animals 15, no. 10: 1367. https://doi.org/10.3390/ani15101367
APA StyleYang, Q., Cui, L., Yang, Y., Yang, Y., Dai, Z., & Wu, Z. (2025). Dietary Supplementation with Fermented Milk Improves Growth Performance and Intestinal Functions in Intrauterine Growth-Restricted Piglets. Animals, 15(10), 1367. https://doi.org/10.3390/ani15101367





