Innovative Approaches to Avoid Antibiotic Use in Equine Semen Cryopreservation: Advancing Sustainable Reproductive Technologies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Semen Collection and Hygiene Conditions
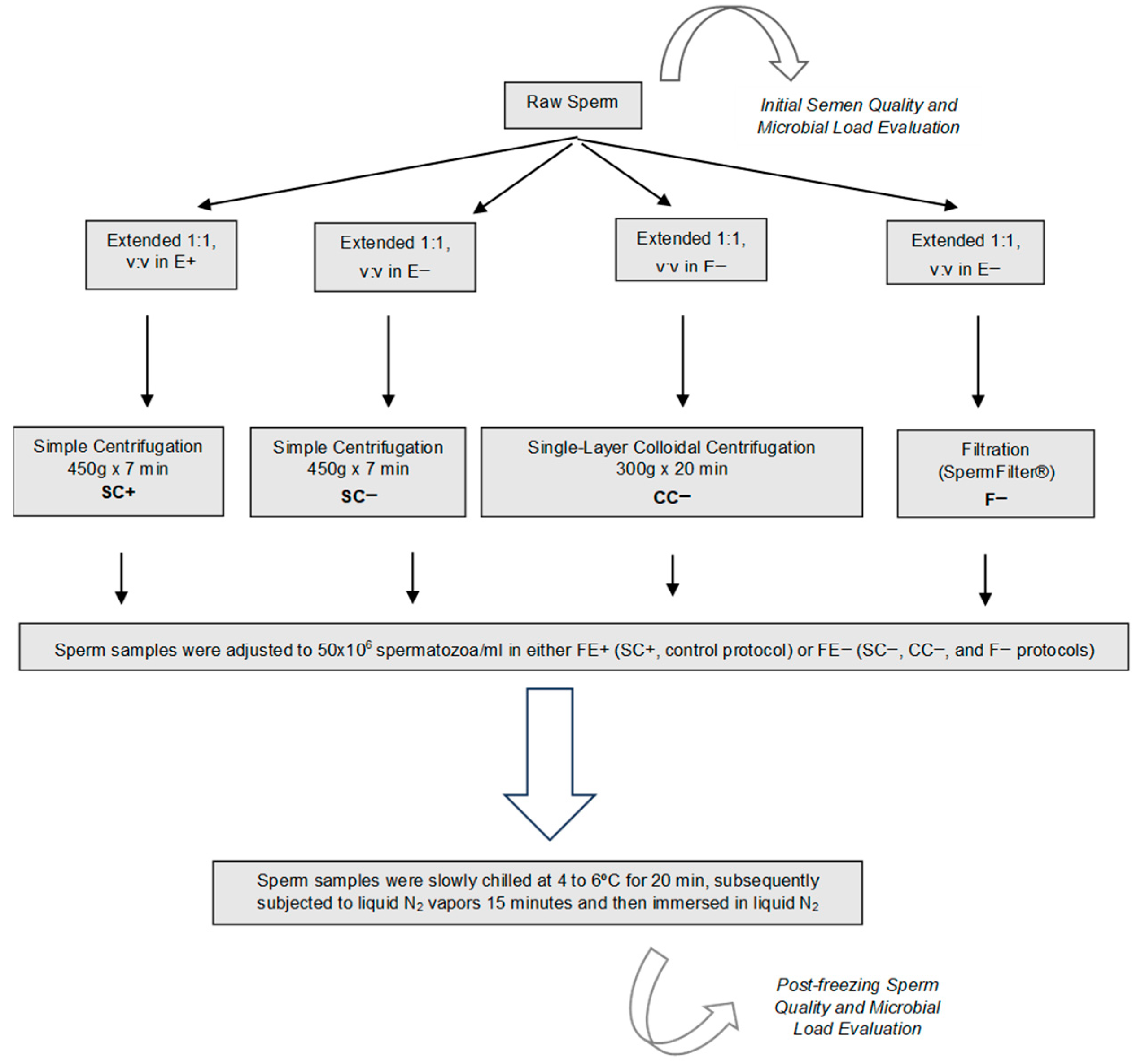
2.3. Experimental Design
2.4. Semen Processing Techniques
2.5. Cooling and Freezing–Thawing Processing
2.6. Semen Microbial Load Evaluation
2.7. Semen Quality Evaluation
2.8. Statistical Analysis
3. Results
3.1. Evaluation of Microbial Load
3.2. Evaluation of Semen Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| S+ | Simple Centrifugation with antibiotics |
| S− | Simple Centrifugation without antibiotics |
| F− | Filtration without antibiotics |
| C− | Single-Layer Colloidal Centrifugation without antibiotics |
| EU | European Union |
| AMR | Antimicrobial resistance |
| CFUs | Colony-forming units |
| E+ | Equiplus® extender with gentamicin |
| E− | Equiplus® extender without antibiotics |
| FE+ | Freezing extender with antibiotics |
| FE− | Freezing extender without antibiotics |
| COS | Columbia 5% Sheep Blood Agar |
| SDA | Sabouraud Dextrose Agar |
| SCH | Schaedler vitamin K1 5% Sheep Blood Agar |
| SD | Standard deviation |
| LQ | Lower Quartile |
| UQ | Upper Quartile |
| TMOT | Total motility |
| PMOT | Progressive motility |
| VCL | Curvilinear velocity |
| VAP | Average path velocity |
| VSL | Straight-line velocity |
| STR | Straightness |
| LIN | Linearity |
| WOB | Wobble |
| AHL | Amplitude of lateral displacement |
| BCF | Beat cross frequency |
References
- Loomis, P.R.; Graham, J.K. Commercial Semen Freezing: Individual Male Variation in Cryosurvival and the Response of Stallion Sperm to Customized Freezing Protocols. Anim. Reprod. Sci. 2008, 105, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Loomis, P.R.; Squires, E.L. Frozen Semen Management in Equine Breeding Programs. Theriogenology 2005, 64, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Samper, J.C.; Morris, C.A. Current Methods for Stallion Semen Cryopreservation: A Survey. Theriogenology 1998, 49, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Althouse, G. Sanitary Procedures for the Production of Extended Semen. Reprod. Domest. Anim. 2008, 43, 374–378. [Google Scholar] [CrossRef]
- Hernández-Avilés, C.; Love, C.C.; Serafini, R.; Teague, S.R.; Varner, D.D. Supplemental Antibiotics in a Commercial Extender for Stallion Semen. J. Equine Vet. Sci. 2019, 80, 33–35. [Google Scholar] [CrossRef]
- Pickett, B.W.; Voss, J.L.; Jones, R.L. Control of Bacteria in Stallions and Their Semen. J. Equine Vet. Sci. 1999, 19, 424–469. [Google Scholar] [CrossRef]
- Pasing, S.S.; Aurich, C.; Von Lewinski, M.; Wulf, M.; Krüger, M.; Aurich, J.E. Development of the Genital Microflora in Stallions Used for Artificial Insemination throughout the Breeding Season. Anim. Reprod. Sci. 2013, 139, 53–61. [Google Scholar] [CrossRef]
- Samper, J.C.; Tibary, A. Disease Transmission in Horses. Theriogenology 2006, 66, 551–559. [Google Scholar] [CrossRef]
- Herlong, J.L.; Reubish, K.; Higdon, H.L.; Boone, W.R. Quantitative and Qualitative Analysis of Microorganisms in an Assisted Reproductive Technology Facility. Fertil. Steril. 2008, 89, 847–853. [Google Scholar] [CrossRef]
- Dever, L.A. Mechanisms of Bacterial Resistance to Antibiotics. Arch. Intern. Med. 1991, 151, 886. [Google Scholar] [CrossRef]
- Ortega-Ferrusola, C.; González-Fernández, L.; Muriel, A.; Macías-García, B.; Rodríguez-Martínez, H.; Tapia, J.; Alonso, J.; Peña, F. Does the Microbial Flora in the Ejaculate Affect the Freezeability of Stallion Sperm? Reprod. Domest. Anim. 2009, 44, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, T.; Lopes, G.; Pinto, M.; Silva, E.; Miranda, C.; Correia, M.J.; Damásio, L.; Thompson, G.; Rocha, A. Colloid Centrifugation of Fresh Stallion Semen before Cryopreservation Decreased Microorganism Load of Frozen-Thawed Semen without Affecting Seminal Kinetics. Theriogenology 2015, 83, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, C.; Rooks, D.; McCarthy, A.; Murray, R. Antimicrobial Resistance in Some Gram-Negative Bacteria Isolated from the Bovine Ejaculate. Reprod. Domest. Anim. 2013, 48, 525–528. [Google Scholar] [CrossRef]
- Zampieri, D.; Santos, V.G.; Braga, P.A.C.; Ferreira, C.R.; Ballottin, D.; Tasic, L.; Basso, A.C.; Sanches, B.V.; Pontes, J.H.F.; Da Silva, B.P.; et al. Microorganisms in Cryopreserved Semen and Culture Media Used in the in vitro Production (IVP) of Bovine Embryos Identified by Matrix-Assisted Laser Desorption Ionization Mass Spectrometry (MALDI-MS). Theriogenology 2013, 80, 337–345. [Google Scholar] [CrossRef]
- Goularte, K.L.; Voloski, F.L.S.; Redú, J.F.M.; Ferreira, C.E.R.; Vieira, A.D.; Duval, E.H.; Mondadori, R.G.; Lucia, T. Antibiotic Resistance in Microorganisms Isolated in a Bull Semen Stud. Reprod. Domest. Anim. 2020, 55, 318–324. [Google Scholar] [CrossRef]
- Bresciani, C.; Cabassi, C.S.; Morini, G.; Taddei, S.; Bettini, R.; Bigliardi, E.; Ianni, F.D.; Sabbioni, A.; Parmigiani, E. Boar Semen Bacterial Contamination in Italy and Antibiotic Efficacy in a Modified Extender. Ital. J. Anim. Sci. 2014, 13, 3082. [Google Scholar] [CrossRef]
- Gòdia, M.; Ramayo-Caldas, Y.; Zingaretti, L.M.; Darwich, L.; López, S.; Rodríguez-Gil, J.E.; Yeste, M.; Sánchez, A.; Clop, A. A Pilot RNA-Seq Study in 40 Pietrain Ejaculates to Characterize the Porcine Sperm Microbiome. Theriogenology 2020, 157, 525–533. [Google Scholar] [CrossRef]
- European Union. Regulation (Eu) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/Ec. Off. J. Eur. Union 2016, 119, 1–88. [Google Scholar] [CrossRef]
- Malaluang, P.; Wilén, E.; Frosth, S.; Lindahl, J.; Hansson, I.; Morrell, J.M. Vaginal Bacteria in Mares and the Occurrence of Antimicrobial Resistance. Microorganisms 2022, 10, 2204. [Google Scholar] [CrossRef]
- Malaluang, P.; Wilén, E.; Frosth, S.; Lindahl, J.F.; Hansson, I.; Morrell, J.M. Antimicrobial Resistance in Vaginal Bacteria in Inseminated Mares. Pathogens 2023, 12, 375. [Google Scholar] [CrossRef]
- Morrell, J.M.; Cojkic, A.; Malaluang, P.; Ntallaris, T.; Lindahl, J.; Hansson, I. Antibiotics in Semen Extenders—A Multiplicity of Paradoxes. Reprod. Fertil. Dev. 2024, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zabala, S.M.; Serres, C.; Montero, N.; Crespo, F.; Lorenzo, P.L.; Pérez-Aguilera, V.; Galán, C.; Domínguez-Gimbernat, M.; Oliet, A.; Moreno, S.; et al. Strategies to Reduce the Use of Antibiotics in Fresh and Chilled Equine Semen. Animals 2024, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Ďuračka, M.; Benko, F.; Chňapek, M.; Tvrdá, E. Strategies for Bacterial Eradication from Human and Animal Semen Samples: Current Options and Future Alternatives. Sensors 2023, 23, 6978. [Google Scholar] [CrossRef]
- Schulze, M.; Nitsche-Melkus, E.; Hensel, B.; Jung, M.; Jakop, U. Antibiotics and Their Alternatives in Artificial Breeding in Livestock. Anim. Reprod. Sci. 2020, 220, 106284. [Google Scholar] [CrossRef]
- Alvarenga, M.A.; Papa, F.O.; Carmo, M.T.; Kievitsbosch, T.; Castro Chaves, M.M.B.; Ramires Neto, C. Methods of Concentrating Stallion Semen. J. Equine Vet. Sci. 2012, 32, 424–429. [Google Scholar] [CrossRef]
- Pérez, V.; Crespo, F.; López, A.I.; Cárdenas, S.; Bautista, M.J.; Hidalgo, M.; Dorado, J.; Ortiz, I. Effect of Silver Nanoparticles on Donkey Sperm Parameters and Ultrastructure. Reprod. Domest. Anim. 2024, 59, e14662. [Google Scholar] [CrossRef]
- Sancho, S.; Briz, M.; Yeste, M.; Bonet, S.; Bussalleu, E. Effects of the Antimicrobial Peptide Protegrine 1 on Sperm Viability and Bacterial Load of Boar Seminal Doses. Reprod. Domest. Anim. 2017, 52, 69–71. [Google Scholar] [CrossRef]
- Reckinger, F. Fertility with Cervical Insemination and Boar Effects Using Hypothermic Stored Semen. Theriogenology 2025, 236, 45–51. [Google Scholar] [CrossRef]
- Morrell, J.M.; Klein, C.; Lundeheim, N.; Erol, E.; Troedsson, M.H.T. Removal of Bacteria from Stallion Semen by Colloid Centrifugation. Anim. Reprod. Sci. 2014, 145, 47–53. [Google Scholar] [CrossRef]
- Cochran, J.D.; Amann, R.P.; Froman, D.P.; Pickett, B.W. Effects of Centrifugation, Glycerol Level, Cooling to 5 °C, Freezing Rate and Thawing Rate on the Post-Thaw Motility of Equine Sperm. Theriogenology 1984, 22, 25–38. [Google Scholar] [CrossRef]
- Gutiérrez-Cepeda, L.; Fernández, A.; Crespo, F.; Gosálvez, J.; Serres, C. Simple and Economic Colloidal Centrifugation Protocols May Be Incorporated into the Clinical Equine Sperm Processing Procedure. Anim. Reprod. Sci. 2011, 124, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Loomis, P.R. Advanced Methods for Handling and Preparation of Stallion Semen. Vet. Clin. N. Am. Equine Pract. 2006, 22, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Kenney, R.M. Society for Theriogenology. Manual for Clinical Fertility Evaluation of the Stallion; Society for Theriogenology: Hastings, NE, USA, 1983. [Google Scholar]
- Al-Kass, Z.; Eriksson, E.; Bagge, E.; Wallgren, M.; Morrell, J.M. Bacteria Detected in the Genital Tract, Semen or Pre-Ejaculatory Fluid of Swedish Stallions from 2007 to 2017. Acta Vet. Scand. 2019, 61, 25. [Google Scholar] [CrossRef] [PubMed]
- Webb, E.M.; Holman, D.B.; Schmidt, K.N.; Crouse, M.S.; Dahlen, C.R.; Cushman, R.A.; Snider, A.P.; McCarthy, K.L.; Amat, S. A Longitudinal Characterization of the Seminal Microbiota and Antibiotic Resistance in Yearling Beef Bulls Subjected to Different Rates of Gain. Microbiol. Spectr. 2023, 11, e05180-22. [Google Scholar] [CrossRef]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence That the Endometrial Microbiota Has an Effect on Implantation Success or Failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Gholiof, M.; Adamson-De Luca, E.; Wessels, J.M. The Female Reproductive Tract Microbiotas, Inflammation, and Gynecological Conditions. Front. Reprod. Health 2022, 4, 963752. [Google Scholar] [CrossRef]
- Varela, E.; Rey, J.; Plaza, E.; Muñoz De Propios, P.; Ortiz-Rodríguez, J.M.; Álvarez, M.; Anel-López, L.; Anel, L.; De Paz, P.; Gil, M.C.; et al. How Does the Microbial Load Affect the Quality of Equine Cool-Stored Semen? Theriogenology 2018, 114, 212–220. [Google Scholar] [CrossRef]
- Nicholson, C.M.; Abramsson, L.; Holm, S.E.; Bjurulf, E. Bacterial Contamination and Sperm Recovery after Semen Preparation by Density Gradient Centrifugation Using Silane-Coated Silica Particles at Different g Forces. Hum. Reprod. 2000, 15, 662–666. [Google Scholar] [CrossRef]
- Morrell, J.M.; Wallgren, M. Removal of Bacteria from Boar Ejaculates by Single Layer Centrifugation Can Reduce the Use of Antibiotics in Semen Extenders. Anim. Reprod. Sci. 2011, 123, 64–69. [Google Scholar] [CrossRef]
- Malaluang, P.; Wagner, L.H.; Cojkic, A.; Spergser, J.; Aurich, C.; Morrell, J.M. Reduced Bacterial Load in Stallion Semen by Modified Single Layer Centrifugation or Sperm Washing. Theriogenology 2024, 216, 111–117. [Google Scholar] [CrossRef]
- Al-Kass, Z.; Spergser, J.; Aurich, C.; Kuhl, J.; Schmidt, K.; Morrell, J.M. Effect of Presence or Absence of Antibiotics and Use of Modified Single Layer Centrifugation on Bacteria in Pony Stallion Semen. Reprod. Domest. Anim. 2019, 54, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Vidament, M. French Field Results (1985–2005) on Factors Affecting Fertility of Frozen Stallion Semen. Anim. Reprod. Sci. 2005, 89, 115–136. [Google Scholar] [CrossRef]
- Morris, G.J.; Faszer, K.; Green, J.E.; Draper, D.; Grout, B.W.W.; Fonseca, F. Rapidly Cooled Horse Spermatozoa: Loss of Viability Is Due to Osmotic Imbalance during Thawing, Not Intracellular Ice Formation. Theriogenology 2007, 68, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Hoogewijs, M.; Morrell, J.; Van Soom, A.; Govaere, J.; Johannisson, A.; Piepers, S.; De Schauwer, C.; De Kruif, A.; De Vliegher, S. Sperm Selection Using Single Layer Centrifugation Prior to Cryopreservation Can Increase Thawed Sperm Quality in Stallions. Equine Vet. J. 2011, 43, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Mancill, S.S.; Love, C.; Brinsko, S.; Edmond, A.J.; Foster, M.L.; Teague, S.; Waite, J.A.; Varner, D. Effect of Density Gradient Centrifugation on Cryopreservation of Equine Spermatozoa. Anim. Reprod. Sci. 2010, 121, 208–209. [Google Scholar]
- Gutiérrez-Cepeda, L.; Crespo, F.; Blazquez, J.C.; Serres, C. Optimization of the Equine-Sperm Freeze Test in Purebred Spanish Horses by Incorporating Colloidal Centrifugation. Animals 2023, 13, 382. [Google Scholar] [CrossRef]
- Neto, C.R.; Monteiro, G.A.; Soares, R.F.; Pedrazzi, C.; Dell’aqua, J.A.; Papa, F.O.; Alvarenga, M.A. Effect of Removing Seminal Plasma Using a Sperm Filter on the Viability of Refrigerated Stallion Semen. J. Equine Vet. Sci. 2013, 33, 40–43. [Google Scholar] [CrossRef]
- Malaluang, P.; Wilén, E.; Lindahl, J.; Hansson, I.; Morrell, J.M. Antimicrobial Resistance in Equine Reproduction. Animals 2021, 11, 3035. [Google Scholar] [CrossRef]
- Morrell, J.; Wallgren, M. Alternatives to Antibiotics in Semen Extenders: A Review. Pathogens 2014, 3, 934–946. [Google Scholar] [CrossRef]
| Culture Media | Mean ± Std. Deviation | Minimum | Median | Maximum |
|---|---|---|---|---|
| COS | 2436 ± 3371.24 | 160 | 700 | 10,400 |
| SDA | 287 ± 674.65 | 0 | 15 | 2170 |
| SCH | 461.11 ± 563.50 | 0 | 180 | 1640 |
| Culture Media | Descriptive Statistics | S+ | S− | F− | C− |
|---|---|---|---|---|---|
| COS (CFU/mL) | Mean ± SD | 78 ± 203.84 | 602 ± 1268.38 | 42 ± 65.96 | 256 ± 478.27 |
| LQ | 0 | 20 | 0 | 0 | |
| Median | 0 a | 135 b | 10 a,b | 35 a,b | |
| UQ | 10 | 290 | 60 | 160 | |
| SDA (CFU/mL) | Mean ± SD | 1 ± 3.16 | 11 ± 14.49 | 19 ± 47.71 | 5 ± 10.80 |
| LQ | 0 | 0 | 0 | 0 | |
| Median | 0 a | 5 a | 0 a | 0 a | |
| UQ | 0 | 20 | 0 | 0 | |
| SCH (CFU/mL) | Mean ± SD | 6 ± 9.66 | 6.3 ± 12.53 | 8.89 ± 16.92 | 11 ± 28.07 |
| LQ | 0 | 0 | 0 | 0 | |
| Median | 0 a | 0 a | 0 a | 0 a | |
| UQ | 10 | 10 | 10 | 10 |
| Variable | Mean ± Std. Deviation |
|---|---|
| Volume (mL) | 45.52 ± 30.84 |
| Concentration (×106 sperm/mL) | 212.20 ± 76.29 |
| TMOT (%) | 86.01 ± 9.86 |
| PMOT (%) | 51.94 ± 14.80 |
| Viable (%) | 70.39 ± 13.45 |
| Protocol | TMOT (%) | PMOT (%) | VCL (μm/s) | VAP (μm/s) | VSL (μm/s) | STR (%) | LIN (%) | WOB (%) | ALH (μm) | BCF (Hz) | Viable (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S+ | 71.37 a ± 9.52 | 44.71 a ± 9.32 | 84.62 a,b ± 11.53 | 48.89 a ± 7.76 | 35.21 a ± 7.37 | 63.22 c ± 6.2 | 37.02 b ± 5.17 | 55.31 a ± 3.43 | 2.05 a ± 0.25 | 14.88 b ± 3.09 | 58.8 a ± 9.17 |
| S− | 64.88 a,b ± 8.96 | 43.63 a ± 9.06 | 87.92 a ± 16.25 | 48.79 a ± 11.92 | 36.85 a ± 10.51 | 65.97 b,c ± 5.88 | 37.17 b ± 5.67 | 53.14 a ± 4.44 | 2.11 a ± 0.3 | 16.87 a,b ± 3.59 | 52.29 b ± 9.41 |
| F− | 56.96 c ± 12.07 | 38.77 a ± 9.36 | 87.03 a ± 10.25 | 49.86 a ± 6.63 | 39.52 a ± 6.37 | 70.7 a ± 3.6 | 40.91 a ± 4.75 | 55.41 a ± 5.13 | 1.99 a ± 0.31 | 18.17 a ± 1.77 | 48.86 b ± 11.09 |
| C− | 62.41 b,c ± 8.28 | 40.2 a ± 6.12 | 75.96 b ± 11.72 | 43.24 a ± 7.52 | 35.44 a ± 9.14 | 68.25 a,b ± 3.81 | 39.53 a,b ± 3.3 | 55.24 a ± 2.84 | 1.81 b ± 0.23 | 15.54 b ± 2.26 | 42.88 c ± 9.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabala, S.M.; Serres, C.; Montero, N.; Crespo, F.; Lorenzo, P.L.; Pérez-Aguilera, V.; Oliet, A.; Hijón, V.; Moreno, S.; González-Zorn, B.; et al. Innovative Approaches to Avoid Antibiotic Use in Equine Semen Cryopreservation: Advancing Sustainable Reproductive Technologies. Animals 2025, 15, 1368. https://doi.org/10.3390/ani15101368
Zabala SM, Serres C, Montero N, Crespo F, Lorenzo PL, Pérez-Aguilera V, Oliet A, Hijón V, Moreno S, González-Zorn B, et al. Innovative Approaches to Avoid Antibiotic Use in Equine Semen Cryopreservation: Advancing Sustainable Reproductive Technologies. Animals. 2025; 15(10):1368. https://doi.org/10.3390/ani15101368
Chicago/Turabian StyleZabala, Sonsoles Mercedes, Consuelo Serres, Natalia Montero, Francisco Crespo, Pedro Luis Lorenzo, Verónica Pérez-Aguilera, Agustín Oliet, Virginia Hijón, Santiago Moreno, Bruno González-Zorn, and et al. 2025. "Innovative Approaches to Avoid Antibiotic Use in Equine Semen Cryopreservation: Advancing Sustainable Reproductive Technologies" Animals 15, no. 10: 1368. https://doi.org/10.3390/ani15101368
APA StyleZabala, S. M., Serres, C., Montero, N., Crespo, F., Lorenzo, P. L., Pérez-Aguilera, V., Oliet, A., Hijón, V., Moreno, S., González-Zorn, B., & Gutiérrez-Cepeda, L. (2025). Innovative Approaches to Avoid Antibiotic Use in Equine Semen Cryopreservation: Advancing Sustainable Reproductive Technologies. Animals, 15(10), 1368. https://doi.org/10.3390/ani15101368






