Innovations in Cattle Breeding Technology: Prospects in the Era of Gene Editing
Simple Summary
Abstract
1. Introduction
2. From CRISPR/Cas9 to Multi-Gene Editing: New Pathways for Genetic Improvement of Cattle
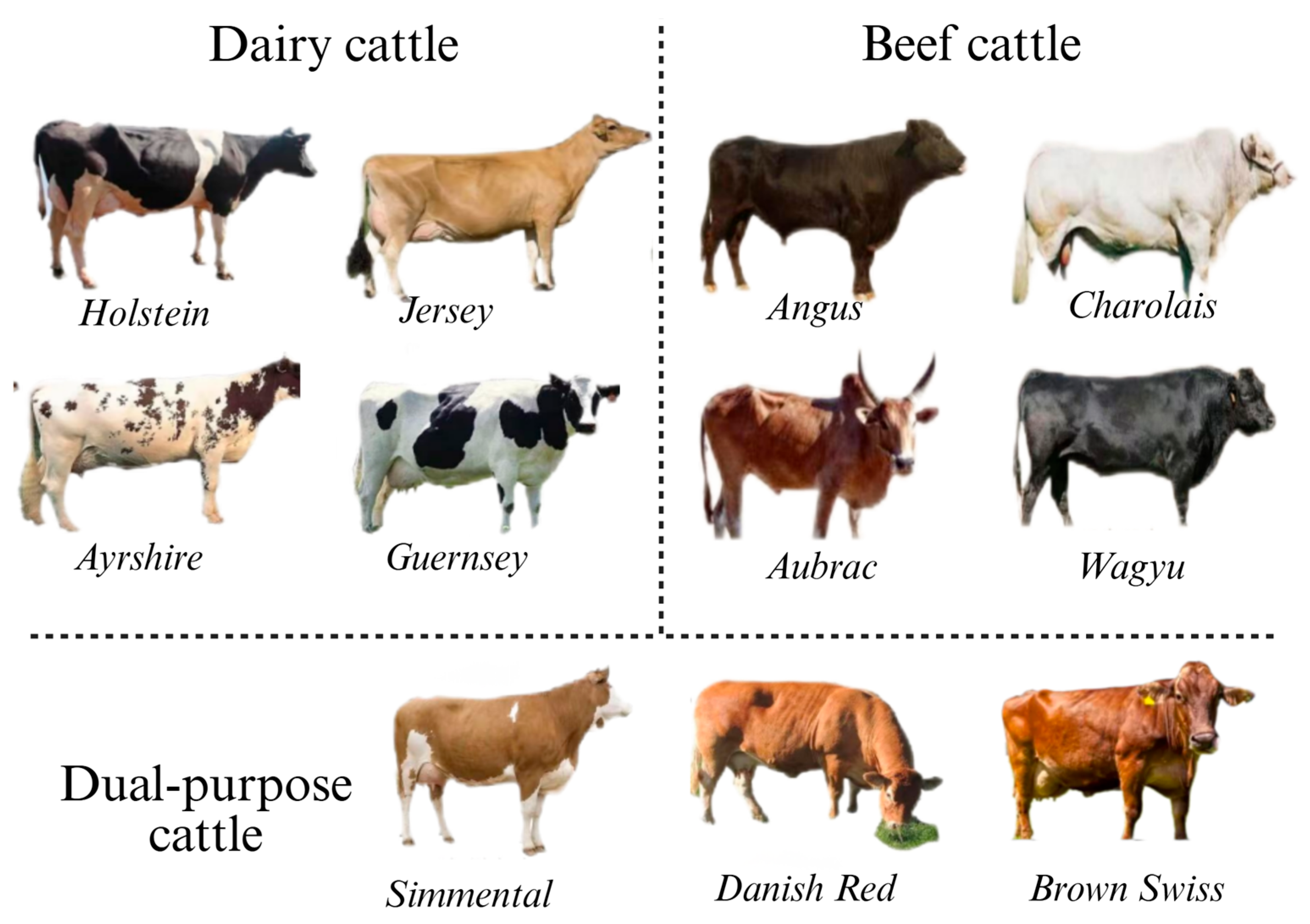
3. Breeding New Lines of Dairy Cattle
3.1. Selection of Key Genes in Dairy Cattle Breeding
3.1.1. DGAT
3.1.2. GHR
3.1.3. PRL
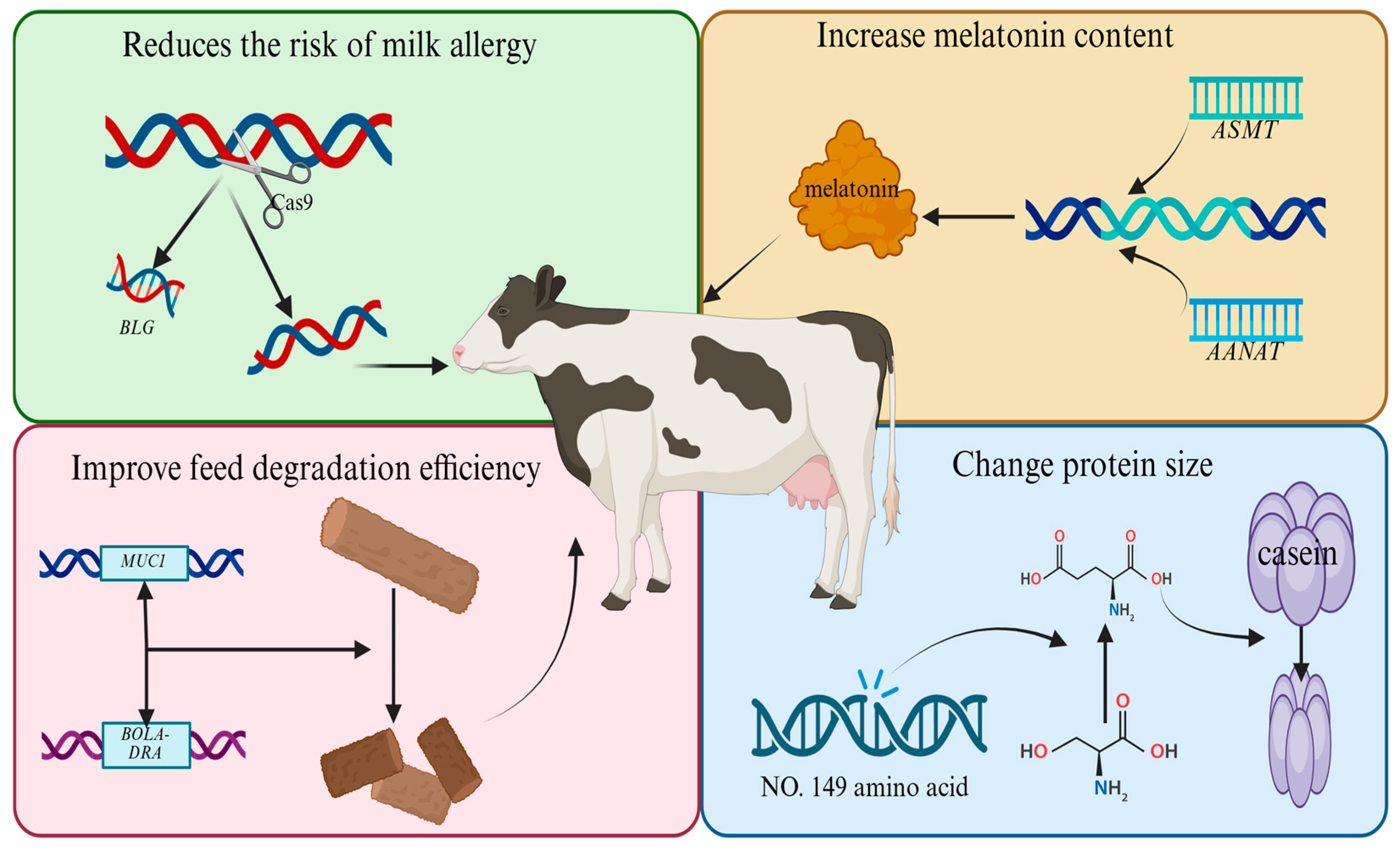
3.2. Outlook on Dairy Breeding
4. Breeding New Lines of Beef Cattle
4.1. Selection of Key Genes in Beef Cattle Breeding
4.1.1. MSTN
4.1.2. CAPN and CAST
4.1.3. LEP
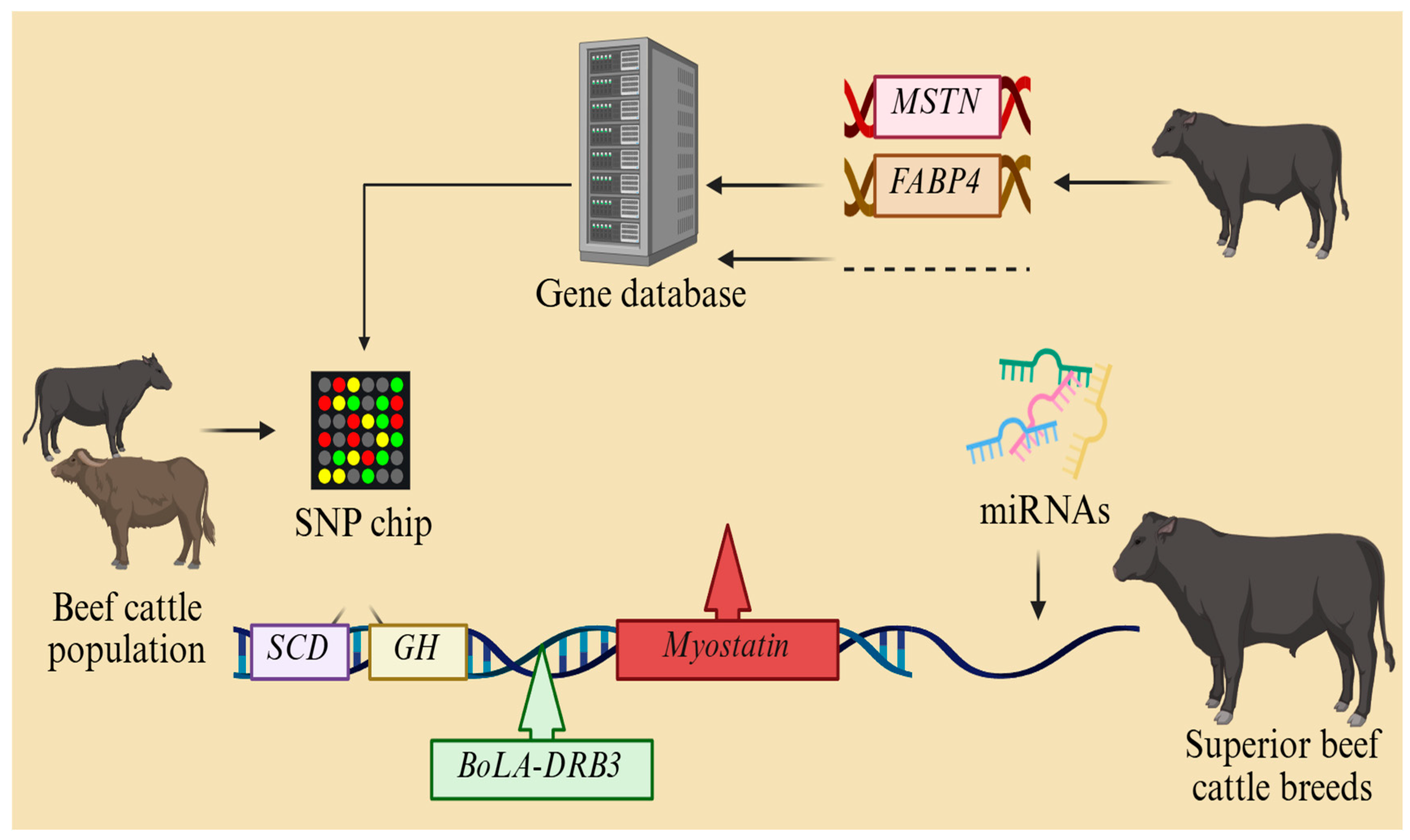
4.2. Beef Cattle Breeding Expectations
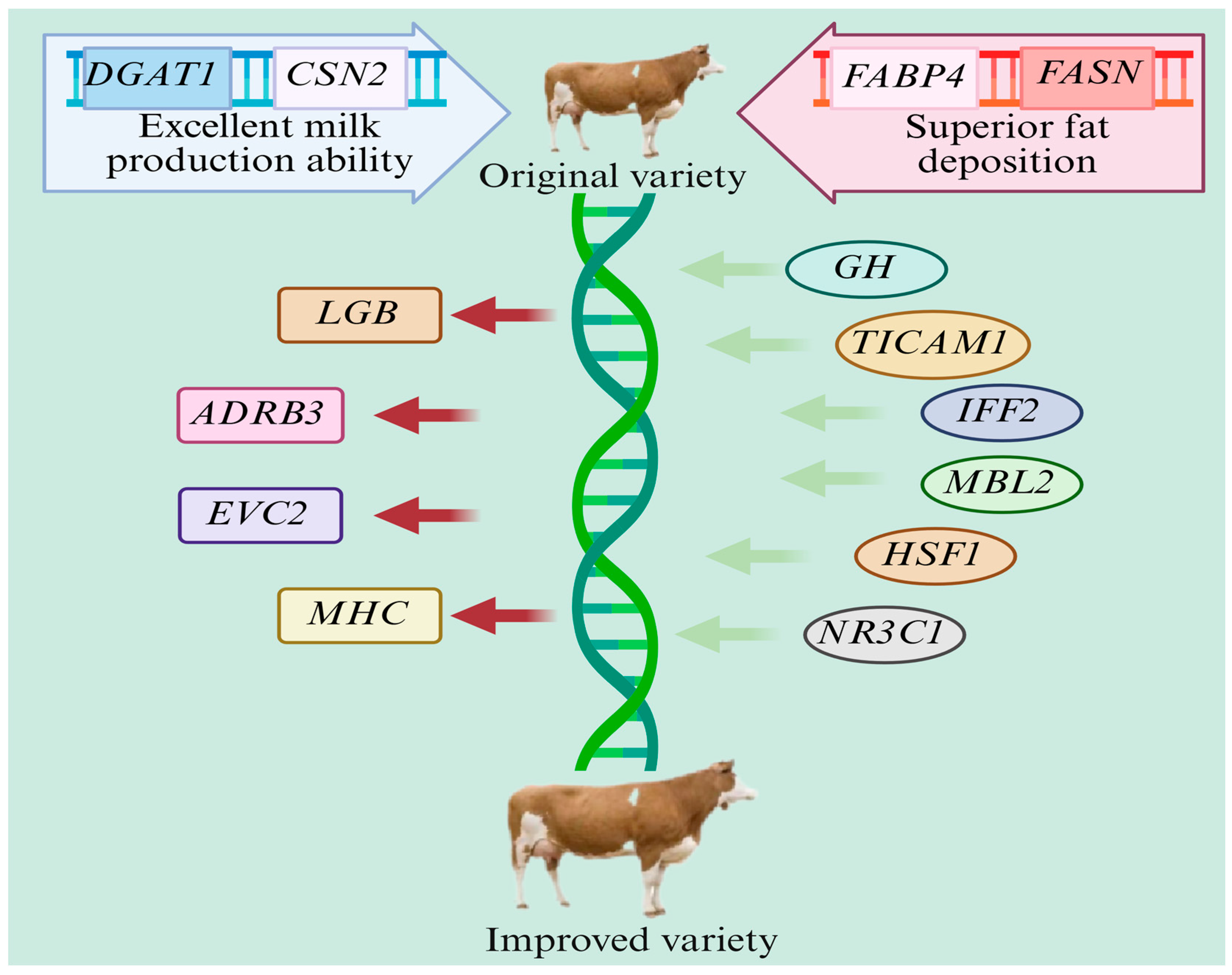
5. Breeding New Lines of Dual-Purpose Cattle
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Oleinik, S.A.; Maltsev, A.E.; Filatov, D.A. Comparative assessment of the productive qualities of Holstein and Jersey dairy cattle. BIO Web. Conf. 2024, 139, 11010. [Google Scholar] [CrossRef]
- Pascottini, O.B.; Crowe, A.D.; Ramil, U.Y.; Hostens, M.; Opsomer, G.; Crowe, M.A. Perspectives in cattle reproduction for the next 20 years—A European context. Theriogenology 2025, 233, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.A.; Mariam, M.H.; Tiezzi, F.; Berg, I.v.d.; Maltecca, C.; Pryce, J.E. Optimizing genetic diversity in Australian Holsteins and Jerseys: A comparative analysis of whole-genome and regional inbreeding depression effects. J. Dairy Sci. 2024, 108, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.P.; Wall, E.; Pryce, J.E. Genetics and genomics of reproductive performance in dairy and beef cattle. Animal 2014, 8, 105–121. [Google Scholar] [CrossRef]
- Ahmad, S.; Agung, I.; Hendra, H.; Jasmadi; Ainsyar, H.M.; Angger, S.A.; Erma, S.A.; Hilda, N.; Taufik, K.; Guna, D.I.N.; et al. Effects of various macroalgae species on methane production, rumen fermentation, and ruminant production: A meta-analysis from in vitro and in vivo experiments. Anim. Feed Sci. Technol. 2022, 294, 115503. [Google Scholar]
- Mikkola, M.; Desmet, K.L.J.; Kommisrud, E.; Riegler, M.A. Recent advancements to increase success in assisted reproductive technologies in cattle. Anim. Reprod. 2024, 21, e20240031. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.L.; Van Eenennaam, A.L. Synergistic power of genomic selection, assisted reproductive technologies, and gene editing to drive genetic improvement of cattle. CABI Agric. Biosci. 2022, 3, 13. [Google Scholar] [CrossRef]
- Xu, L.; Bickhart, D.M.; Cole, J.B.; Schroeder, S.G.; Song, J.; Tassell, C.P.V.; Sonstegard, T.S.; Liu, G.E. Genomic signatures reveal new evidences for selection of important traits in domestic cattle. Mol. Biol. Evol. 2015, 32, 711–725. [Google Scholar] [CrossRef]
- Alves, F.J.G.; Alves, S.D.; Macedo, M.L.F.; Pinto, d.M.T.; Simielli, F.L.F.; Santos, S.D.B.d.; Roberto, C.; Galvão, A.L. Sustainable Intensification of Beef Production in the Tropics: The Role of Genetically Improving Sexual Precocity of Heifers. Animals 2022, 12, 174. [Google Scholar] [CrossRef]
- Alves, F.J.G.; Elisa, P.; Iana, S.P.; Soares, C.G.; Macedo, M.L.F.; Zerlotti, M.M.E.; Fernando, B.; Roberto, C.; Galvão, d.A.L. Current applications and perspectives of genomic selection in Bos indicus (Nellore) cattle. Livest. Sci. 2022, 263, 105001. [Google Scholar]
- Zhang, Z.; Erbe, M.; He, J.; Ober, U.; Gao, N.; Zhang, H.; Simianer, H.; Li, J. Accuracy of whole-genome prediction using a genetic architecture-enhanced variance-covariance matrix. G3 2015, 5, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Brøndum, R.F.; Su, G.; Janss, L.; Sahana, G.; Guldbrandtsen, B.; Boichard, D.; Lund, M.S. Quantitative trait loci markers derived from whole genome sequence data increases the reliability of genomic prediction. J. Dairy Sci. 2015, 98, 4107–4116. [Google Scholar] [CrossRef] [PubMed]
- John, v.d.O.; Constantinos, P. The genome editing revolution. Trends Biotechnol. 2023, 41, 396–409. [Google Scholar]
- Christian, M.; Cermak, T.; Doyle, E.L.; Schmidt, C.; Zhang, F.; Hummel, A.; Bogdanove, A.J.; Voytas, D.F. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 2010, 186, 757–761. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Magdalena, H.; Daniel, L.; Joanna, Z.; Ryszard, S. CRISPR/Cas9 Immune System as a Tool for Genome Engineering. Arch. Immunol. Ther. Exp. 2017, 65, 233–240. [Google Scholar]
- Kuznetsov, V.; Revina, G.; Astashenkova, L. Additive-Polygenic Inheritance of Reproductive System Diseases in Holstein Cows in Subpopulations. In International Scientific Conference the Fifth Technological Order: Prospects for the Development and Modernization of the Russian Agro-Industrial Sector (TFTS 2019); Atlantis Press: Dordrecht, The Netherlands, 2020. [Google Scholar]
- Dong, F.; Xie, K.; Chen, Y.; Yang, Y.; Mao, Y. Polycistronic tRNA and CRISPR guide-RNA enables highly efficient multiplexed genome engineering in human cells. Biochem. Biophys. Res. Commun. 2017, 482, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhao, D.; Zhang, X.; Ding, X.; Bi, C. CRISPR/Cas9 assisted Multiplex Genome Editing Technique in Escherichia coli. Biotechnol. J. 2018, 13, e1700604. [Google Scholar] [CrossRef]
- Tetsushi, S.; Ayami, N.; Satoshi, K.; Kazuaki, C.; Takashi, Y. Multiplex genome engineering in human cells using all-in-one CRISPR/Cas9 vector system. Sci. Rep. 2014, 4, 5400. [Google Scholar]
- Kor, S.D.; Chowdhury, N.; Keot, A.K.; Yogendra, K.; Chikkaputtaiah, C.; Sudhakar Reddy, P. RNA Pol III promoters-key players in precisely targeted plant genome editing. Front. Genet. 2022, 13, 989199. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Thakur, M.D.; Wang, Y.; Su, Q.; Zhao, Y.; Feng, Y. Regulation of U6 Promoter Activity by Transcriptional Interference in Viral Vector-Based RNAi. Genom. Proteom. Bioinform. 2010, 8, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Muntazir, M.; Aejaz, A.D.; Milan, S.; Anshika, T.; Nancy, B.; Umer, B.; Ahmad, B.B.; Abbu, Z.; Sajad, A.; TanvirUlHassan, D.; et al. CRISPR-Based Genome Editing Tools: Insights into Technological Breakthroughs and Future Challenges. Genes 2021, 12, 797. [Google Scholar] [CrossRef]
- Kishimoto, T.; Nishimura, K.; Morishita, K.; Fukuda, A.; Miyamae, Y.; Kumagai, Y.; Sumaru, K.; Nakanishi, M.; Hisatake, K.; Sano, M. An engineered ligand-responsive Csy4 endoribonuclease controls transgene expression from Sendai virus vectors. J. Biol. Eng. 2024, 18, 9. [Google Scholar] [CrossRef]
- Haurwitz, R.E.; Sternberg, S.H.; Doudna, J.A. Csy4 relies on an unusual catalytic dyad to position and cleave CRISPR RNA. EMBO J. 2012, 31, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- Zalatan, J.G.; Lee, M.E.; Almeida, R.; Gilbert, L.A.; Whitehead, E.H.; Russa, M.L.; Tsai, J.C.; Weissman, J.S.; Dueber, J.E.; Qi, L.S.; et al. Engineering Complex Synthetic Transcriptional Programs with CRISPR RNA Scaffolds. Cell 2015, 160, 339–350. [Google Scholar] [CrossRef]
- Cuellar, C.J.; Amaral, T.F.; Rodriguez-Villamil, P.; Ongaratto, F.; Martinez, D.O.; Labrecque, R.; Losano, J.D.D.A.; Estrada-Cortés, E.; Bostrom, J.R.; Martins, K.; et al. Consequences of gene editing of PRLR on thermotolerance, growth, and male reproduction in cattle. FASEB BioAdvances 2024, 6, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, L.; Su, G.; Wei, Z.; Liu, X.; Song, L.; Hai, C.; Wu, D.; Hao, Z.; Wu, Y.; et al. Growth Traits and Sperm Proteomics Analyses of Myostatin Gene-Edited Chinese Yellow Cattle. Life 2022, 12, 627. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, D.; Qin, G.; Song, R.; Yao, J.; Cao, C.; Zheng, Q.; Hou, N.; Wang, Y.; Zhao, J. Cytosine Base Editor (hA3A-BE3-NG)-Mediated Multiple Gene Editing for Pyramid Breeding in Pigs. Front. Genet. 2020, 11, 592623. [Google Scholar] [CrossRef]
- Kumar, D.P.; Shubham, G.; Kumar, M.S.; Reena, A.; Manishi, M.; Kumar, N.S.; Periasamy, K.; Singh, K.R. Identification of polymorphism in fatty acid binding protein 3 (FABP3) gene and its association with milk fat traits in riverine buffalo (Bubalus bubalis). Trop. Anim. Health Prod. 2016, 48, 849–853. [Google Scholar]
- Wang, C.; Zhao, J.; Feng, X.; Zhao, W.; Ma, R.; Yu, B.; Xue, L.; Wang, H.; Chen, Y.; Zhang, J.; et al. bta-miR-224 regulates milk fat metabolism by targeting FABP4 in bovine mammary epithelial cells. Genomics 2024, 116, 110955. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xue, J.; Shan, X.; Qiu, L.; Miao, Y. Functional roles for AGPAT6 in milk fat synthesis of buffalo mammary epithelial cells. Anim. Biotechnol. 2022, 34, 11–12. [Google Scholar] [CrossRef]
- Ramos, M.C.G.; Souza, F.P.A.d.; Diniz, P.M.G.C.; Cruz, R.I.; Ferraz, L.F.C.; Thalia, Z.; Vercesi, F.A.E.; Tomita, B.F.Â.; Santos, C.M.R.; Sundfeld, G.M.A. Phenotypic variation in milk fatty acid composition and its association with stearoyl-CoA desaturase 1 (SCD1) gene polymorphisms in Gir cows. J. Anim. Breed. Genet. = Z. Fur Tierz. Und Zucht. 2023, 140, 532–548. [Google Scholar]
- Li, N.; Zhao, F.; Wei, C.; Liang, M.; Zhang, N.; Wang, C.; Li, Q.-Z.; Gao, X.-J. Function of SREBP1 in the Milk Fat Synthesis of Dairy Cow Mammary Epithelial Cells. Int. J. Mol. Sci. 2014, 15, 16998–17013. [Google Scholar] [CrossRef]
- Amalfitano, N.; Mota, L.F.M.; Rosa, G.M.; Cecchinato, A.; Bittante, G. Role of CSN2, CSN3, and BLG genes and the polygenic background in the cattle milk protein profile. J. Dairy Sci. 2022, 105, 6001–6020. [Google Scholar] [CrossRef]
- Molee, A.; Poompramun, C.; Mernkrathoke, P. Effect of casein genes—Beta-LGB, DGAT1, GH, and LHR—On milk production and milk composition traits in crossbred Holsteins. Genet. Mol. Res. GMR 2015, 14, 2561–2571. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, G.; Zha, X.; Ma, X.; La, Y.; Wu, X.; Guo, X.; Chu, M.; Bao, P.; Yan, P.; et al. Polymorphisms Within the IQGAP2 and CRTAC1 Genes of Gannan Yaks and Their Association with Milk Quality Characteristics. Foods 2024, 13, 3720. [Google Scholar] [CrossRef]
- Pambuko, G.; Vanessa, R.; Widyastuti, R.; Prastowo, S. FSHR gene polymorphism and its association to reproductive traits in Friesian Holstein cattle. IOP Conf. Ser. Earth Environ. Sci. 2024, 1341, 012023. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.; Chen, Y.; Ding, H.; Fang, Y.; Ma, X.; Liu, H.; Guo, J.; Zhao, J.; Wang, J.; et al. ALKBH5 Reduces BMP15 mRNA Stability and Regulates Bovine Puberty Initiation Through an m6A-Dependent Pathway. Int. J. Mol. Sci. 2024, 25, 11605. [Google Scholar] [CrossRef]
- Umesh, S.; Rani, A.; Sushil, K.; Rajib, D.; Thiruvothur, V.R.; Shaily, S.; Singh, S.G.; Bharat, S.R.; Kotikalapudi, S.M.; Nitin, V.R.P. Association of bovine KISS1 single nucleotide polymorphisms with reproductive traits in Indian Cattle. Reprod. Domest. Anim. = Zuchthyg. 2020, 55, 922–930. [Google Scholar]
- Gupta, J.P.; Bhushan, B.; Asaf, V.N.M.; Kumar, A.; Ranjan, S.; Panigrahi, M.; Kumar, A.; Kumar, P. Association and expression analysis of single nucleotide polymorphisms of CD14 gene with somatic cell score in crossbred cattle. Gene Rep. 2018, 12, 255–260. [Google Scholar] [CrossRef]
- Muslimova, Z.; Abdualiyeva, A.; Shaugimbayeva, N.; Orynkhanov, K.; Ussenbekov, Y. Genotyping of Holstein Cows by SELL, MX1 and CXCR1 Gene Loci Associated With Mastitis Resistance. Reprod. Domest. Anim. = Zuchthyg. 2024, 59, e14713. [Google Scholar] [CrossRef] [PubMed]
- Kamaldeep; Magotra, A.; Pander, B.L.; Dalal, D.S.; Malik, B.S.; Garg, A.R.; Malik, A. Evaluation of candidate genotype of immune gene MBL1 associated with udder health and performance traits in dairy cattle and buffalo of India. Trop. Anim. Health Prod. 2021, 53, 429. [Google Scholar] [CrossRef]
- Lim, J.W.; Lee, J.H.; Nejad, J.G.; Lee, H.G. Effects of L-leucine and sodium acetate on milk protein synthesis under heat stress conditions in bovine mammary epithelial cells in vitro. J. Therm. Biol. 2024, 126, 103975. [Google Scholar]
- Vilela, P.B.; Bonvino, S.N.; Afonso, d.F.L.; Zerlotti, M.M.E.; Silveira, R.E.; Paro, P.C.C. Expression of candidate genes for residual feed intake in tropically adapted Bos taurus and Bos indicus bulls under thermoneutral and heat stress environmental conditions. J. Therm. Biol. 2021, 99, 102998. [Google Scholar]
- Xu, L.; Idrees, M.; Joo, M.D.; Sidrat, T.; Wei, Y.; Song, S.H.; Li, K.L.; Kong, I.K. Constitutive Expression of TERT Enhances β-Klotho Expression and Improves Age-Related Deterioration in Early Bovine Embryos. Int. J. Mol. Sci. 2021, 22, 5327. [Google Scholar] [CrossRef]
- Oleszycka, E.; Kwiecień, K.; Grygier, B.; Cichy, J.; Kwiecińska, P. The many faces of DGAT1. Life Sci. 2024, 362, 123322. [Google Scholar] [CrossRef]
- Anonymous. Thematic Review Series: Glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. Int. News Fats Oils Relat. Mater. Inform 2009, 20, 175. [Google Scholar]
- Elzaki, S.; Korkuć, P.; Arends, D.; Reissmann, M.; Brockmann, G.A. Effects of DGAT1 on milk performance in Sudanese Butana × Holstein crossbred cattle. Trop. Anim. Health Prod. 2022, 54, 142. [Google Scholar] [CrossRef]
- Zahoor, K.M.; Yulin, M.; Jiaying, M.; Jianxin, X.; Yue, L.; Shuai, L.; Adnan, K.; Muhammad, K.I.; Zhijun, C. Association of DGAT1 With Cattle, Buffalo, Goat, and Sheep Milk and Meat Production Traits. Front. Vet. Sci. 2021, 8, 712470. [Google Scholar]
- Mou, M.A.; Deb, G.K.; Hridoy, M.F.A.; Alam, M.A.; Barai, H.R.; Haque, M.A.; Bhuiyan, M.S.A. Detection of Polymorphisms in FASN, DGAT1, and PPARGC1A Genes and Their Association with Milk Yield and Composition Traits in River Buffalo of Bangladesh. Animals 2024, 14, 1945. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, S.; Li, Z.; Zhang, S.; Hua, G.; Salzano, A.; Campanile, G.; Gasparrini, B.; Liang, A.; Yang, L. DGAT1 polymorphism in Riverine buffalo, Swamp buffalo and crossbred buffalo. J. Dairy Res. 2018, 85, 412–415. [Google Scholar] [CrossRef]
- Spelman, R.J.; Ford, C.A.; McElhinney, P.; Gregory, G.C.; Snell, R.G. Characterization of the DGAT1 Gene in the New Zealand Dairy Population. J. Dairy Sci. 2002, 85, 3514–3517. [Google Scholar] [CrossRef]
- Bovenhuis, H.; Visker, M.H.P.W.; Poulsen, N.A.; Sehested, J.; Valenberg, H.J.F.v.; Arendonk, J.A.M.v.; Larsen, L.B.; Buitenhuis, A.J. Effects of the diacylglycerol o-acyltransferase 1 (DGAT1) K232A polymorphism on fatty acid, protein, and mineral composition of dairy cattle milk. J. Dairy Sci. 2016, 99, 3113–3123. [Google Scholar] [CrossRef]
- Bobbo, T.; Tiezzi, F.; Penasa, M.; Marchi, M.D.; Cassandro, M. Short communication: Association analysis of diacylglycerol acyltransferase (DGAT1) mutation on chromosome 14 for milk yield and composition traits, somatic cell score, and coagulation properties in Holstein bulls. J. Dairy Sci. 2018, 101, 8087–8091. [Google Scholar] [CrossRef] [PubMed]
- Argov-Argaman, N.; Mida, K.; Cohen, B.-C.; Visker, M.; Hettinga, K. Milk fat content and DGAT1 genotype determine lipid composition of the milk fat globule membrane. PLoS ONE 2017, 8, e68707. [Google Scholar] [CrossRef] [PubMed]
- DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J. Clin. Investig. 2011, 121, 1667. [CrossRef][Green Version]
- El-Komy, S.M.; Saleh, A.A.; Abdel-Hamid, T.M.; El-Magd, M.A. Association of GHR Polymorphisms with Milk Production in Buffaloes. Animals 2020, 10, 1203. [Google Scholar] [CrossRef]
- Sakamoto, K.; Komatsu, T.; Kobayashi, T.; Rose, M.T.; Aso, H.; Hagino, A.; Obara, Y. Growth hormone acts on the synthesis and secretion of α-casein in bovine mammary epithelial cells. J. Dairy Res. 2005, 72, 264–270. [Google Scholar] [CrossRef]
- Maj, A.; Oprzadek, J.; Oprzadek, A.; Dymnicki, E.; Zwierzchowski, L. Polymorphism in the 5′-noncoding region of the bovine growth hormone receptor gene and its association with meat production traits in cattle. Anim. Res. 2004, 53, 503–514. [Google Scholar] [CrossRef]
- Andrzej, M.; Lech, Z. Molecular evolution of coding and non-coding sequences of the growth hormone receptor (GHR) gene in the family Bovidae. Folia Biol. 2006, 54, 31–36. [Google Scholar]
- Waters, S.M.; McCabe, M.S.; Howard, D.J.; Giblin, L.; Magee, D.A.; MacHugh, D.E.; Berry, D.P. Associations between newly discovered polymorphisms in the Bos taurus growth hormone receptor gene and performance traits in Holstein-Friesian dairy cattle. Anim. Genet. 2011, 42, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.P.; Govignon-Gion, A.; Ferrand, M.; Gelé, M.; Pourchet, D.; Amigues, Y.; Fritz, S.; Boussaha, M.; Capitan, A.; Rocha, D.; et al. Whole-genome scan to detect quantitative trait loci associated with milk protein composition in 3 French dairy cattle breeds. J. Dairy Sci. 2016, 99, 8203–8215. [Google Scholar] [CrossRef]
- Sarah, B.; Jong-Joo, K.; Sirja, M.; Anne, S.-K.; Anne, C.; Paulette, B.; Nadine, C.; Christine, F.; Bernard, G.; Dave, J.; et al. Molecular dissection of a quantitative trait locus: A phenylalanine-to-tyrosine substitution in the transmembrane domain of the bovine growth hormone receptor is associated with a major effect on milk yield and composition. Genetics 2003, 163, 253–266. [Google Scholar]
- Rahmatalla, S.A.; Müller, U.; Strucken, E.M.; Reissmann, M.; Brockmann, G.A. The F279Y polymorphism of the GHR gene and its relation to milk production and somatic cell score in German Holstein dairy cattle. J. Appl. Genet. 2011, 52, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.N.; He, P.J.; Zhu, J.; Lei, Z.M.; Liu, Z.; Wu, J.P. The effect of polymorphism F279Y of GHR gene on milk production trait in Chinese Holstein cattle. Zhongguo Ying Yong Sheng Li Xue Za Zhi = Zhongguo Yingyong Shenglixue Zazhi = Chin. J. Appl. Physiol. 2013, 29, 400–404. [Google Scholar]
- Perry, J.K.; Mohankumar, K.M.; Emerald, B.S.; Mertani, H.C.; Lobie, P.E. The contribution of growth hormone to mammary neoplasia. J. Mammary Gland Biol. Neoplasia 2008, 13, 131–145. [Google Scholar] [CrossRef]
- Cui, Y.; Sun, X.; Jin, L.; Yu, G.; Li, Q.; Gao, X.; Ao, J.; Wang, C. MiR-139 suppresses β-casein synthesis and proliferation in bovine mammary epithelial cells by targeting the GHR and IGF1R signaling pathways. BMC Vet. Res. 2017, 13, 350. [Google Scholar] [CrossRef]
- Cordero, A.; Pellegrini, P.; Sanz-Moreno, A.; Trinidad, E.M.; Serra-Musach, J.; Deshpande, C.; Dougall, W.C.; Pujana, M.A.; González-Suárez, E. Rankl Impairs Lactogenic Differentiation Through Inhibition of the Prolactin/Stat5 Pathway at Midgestation. Stem Cells 2016, 34, 1027–1039. [Google Scholar] [CrossRef]
- Oakes, S.R.; Rogers, R.L.; Naylor, M.J.; Ormandy, C.J. Prolactin regulation of mammary gland development. J. Mammary Gland Biol. Neoplasia 2008, 13, 13–28. [Google Scholar] [CrossRef]
- Hennighausen, L.; Robinson, G.W.; Wagner, K.-U.; Liu, X. Prolactin Signaling in Mammary Gland Development. J. Biol. Chem. 1997, 272, 7567–7569. [Google Scholar] [CrossRef] [PubMed]
- Mapes, J.; Li, Q.; Kannan, A.; Anandan, L.; Laws, M.; Lydon, J.P.; Bagchi, I.C.; Bagchi, M.K. CUZD1 is a critical mediator of the JAK/STAT5 signaling pathway that controls mammary gland development during pregnancy. PLoS Genet. 2017, 13, e1006654. [Google Scholar] [CrossRef]
- Mehmannavaz, Y.; Amirinia, C.; Bonyadi, M.; Torshizi, R. Effects of bovine prolactin gene polymorphism within exon 4 on milk related traits and genetic trends in Iranian Holstein bulls. Afr. J. Biotechnol. 2009, 8, 19. [Google Scholar]
- Elena, I.D.; Eugeniu, M.A.; Valentin, M.C.; Ionel, N.R.; Toma, C.L.; Mihai, C.; Cristian, G.A. Polymorphism of the Prolactin (PRL) Gene and Its Effect on Milk Production Traits in Romanian Cattle Breeds. Vet. Sci. 2023, 10, 275. [Google Scholar] [CrossRef]
- Corina, P.; Viorel, H.; Ionica, I.; Luminita, C. Etiology of Mastitis and Antimicrobial Resistance in Dairy Cattle Farms in the Western Part of Romania. Antibiotics 2022, 11, 5. [Google Scholar] [CrossRef]
- Salgado-Lora, M.G.; Medina-Estrada, I.; López-Meza, J.E.; Ochoa-Zarzosa, A. Prolactin and Estradiol are Epigenetic Modulators in Bovine Mammary Epithelial Cells during Staphylococcus aureus Infection. Pathogens 2020, 9, 520. [Google Scholar] [CrossRef]
- Antonio, B.M.M.; Guadalupe, S.L.M.; Edmundo, L.M.J.; Alejandra, O.Z. Prolactin regulates H3K9ac and H3K9me2 epigenetic marks and miRNAs expression in bovine mammary epithelial cells challenged with Staphylococcus aureus. Front. Microbiol. 2022, 13, 990478. [Google Scholar]
- Beishova, I.; Belaya, A.; Kuzhebayeva, U.; Ulyanova, T.; Ulyanov, V.; Beishov, R.; Ginayatov, N.; Kovalchuk, A.; Kharzhau, A.; Sidarova, A. Association of polymorphic variants of prolactin (PRL) and beta-lactoglobulin (BLG) genes with resistance/susceptibility to mastitis in holstein cows. Braz. J. Biol. = Rev. Brasleira De Biol. 2024, 84, e284961. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, J.; Sun, D.; Ma, P.; Ding, X.; Yu, Y.; Zhang, Q. Genome wide association studies for milk production traits in Chinese Holstein population. PLoS ONE 2010, 5, e13661. [Google Scholar] [CrossRef]
- Liang, Z.; Prakapenka, D.; VanRaden, P.M.; Jiang, J.; Ma, L.; Da, Y. A Million-Cow Genome-Wide Association Study of Three Fertility Traits in U.S. Holstein Cows. Int. J. Mol. Sci. 2023, 24, 10496. [Google Scholar] [CrossRef]
- Mohamed, A.I.; Xubin, L.; Mudasir, N.; Idriss, A.A.A.; Tianle, X.; Husien, Y.M.; Yongjiang, M.; Zhangping, Y. Genome-Wide Association Study Identifies Candidate Genes Associated with Feet and Leg Conformation Traits in Chinese Holstein Cattle. Animals 2021, 11, 2259. [Google Scholar] [CrossRef]
- Klein, S.L.; Scheper, C.; May, K.; König, S. Genetic and nongenetic profiling of milk β-hydroxybutyrate and acetone and their associations with ketosis in Holstein cows. J. Dairy Sci. 2020, 103, 10332–10346. [Google Scholar] [CrossRef]
- Freebern, E.; Santos, D.J.; Fang, L.; Jiang, J.; Parker Gaddis, K.L.; Liu, G.E.; VanRaden, P.M.; Maltecca, C.; Cole, J.B.; Ma, L. GWAS and fine-mapping of livability and six disease traits in Holstein cattle. BMC Genom. 2020, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, S.; Schenkel, F.; Fleming, A.; Kroezen, V.; Sargolzaei, M.; Baes, C.; Cánovas, A.; Squires, J.; Miglior, F. Genome-wide association analysis for β-hydroxybutyrate concentration in Milk in Holstein dairy cattle. BMC Genet. 2019, 20, 58. [Google Scholar] [CrossRef]
- Choi, Y.; Davis, M.E.; Chung, H. Effects of genetic variants in the promoter region of the bovine adiponectin (ADIPOQ) gene on marbling of Hanwoo beef cattle. Meat Sci. 2015, 105, 57–62. [Google Scholar] [CrossRef]
- Ribeca, C.; Bonfatti, V.; Cecchinato, A.; Albera, A.; Gallo, L.; Carnier, P. Effect of polymorphisms in candidate genes on carcass and meat quality traits in double muscled Piemontese cattle. Meat Sci. 2014, 96, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.L.; Bishop, S.C.; McCorquodale, C.; Williams, J.L.; Wiener, P. Associations between single nucleotide polymorphisms in multiple candidate genes and carcass and meat quality traits in a commercial Angus-cross population. Meat Sci. 2010, 86, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Orrù, L.; Cifuni, G.F.; Piasentier, E.; Corazzin, M.; Bovolenta, S.; Moioli, B. Association analyses of single nucleotide polymorphisms in the LEP and SCD1 genes on the fatty acid profile of muscle fat in Simmental bulls. Meat Sci. 2010, 87, 344–348. [Google Scholar] [CrossRef]
- Da Silva, R.C.G.; Ferraz, J.B.S.; Meirelles, F.V.; Eler, J.P.; Balieiro, J.C.D.C.; Cucco, D.D.C.; Mattos, E.C.; Rezende, F.M.D.; Silva, S.D.L. Association of single nucleotide polymorphisms in the bovine leptin and leptin receptor genes with growth and ultrasound carcass traits in Nellore cattle. Genet. Mol. Res. GMR 2012, 11, 3721–3728. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Z.; Zhang, L.; Zhang, Q.; Yu, Z.; Li, J.; Yang, R. Association of the leptin gene E2-169T>C and E3-299T>A mutations with carcass and meat quality traits of the Chinese Simmental-cross steers. Gene 2013, 518, 443–448. [Google Scholar] [CrossRef]
- Melucci, L.M.; Panarace, M.; Feula, P.; Villarreal, E.L.; Grigioni, G.; Carduza, F.; Soria, L.A.; Mezzadra, C.A.; Arceo, M.E.; Mazzucco, J.P.; et al. Genetic and management factors affecting beef quality in grazing Hereford steers. Meat Sci. 2012, 92, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Casas, E.; Duan, Q.; Schneider, M.J.; Shackelford, S.D.; Wheeler, T.L.; Cundiff, L.V.; Reecy, J.M. Polymorphisms in calpastatin and mu-calpain genes are associated with beef iron content. Anim. Genet. 2014, 45, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Cinzia, R.; Valentina, B.; Alessio, C.; Andrea, A.; Fabio, M.; Luigi, G.; Paolo, C. Association of polymorphisms in calpain 1, (mu/I) large subunit, calpastatin, and cathepsin D genes with meat quality traits in double-muscled Piemontese cattle. Anim. Genet. 2013, 44, 193–196. [Google Scholar]
- SeungHwan, L.; SeungChang, K.; HanHa, C.; SooHyun, C.; HyeongCheol, K.; DaJeong, L.; BongHwan, C.; ChangGwan, D.; Aditi, S.; Gondro, C.; et al. Mutations in calpastatin and micro -Calpain are associated with meat tenderness, flavor and juiciness in Hanwoo (Korean cattle): Molecular modeling of the effects of substitutions in the calpastatin/ micro -Calpain complex. Meat Sci. 2014, 96, 1501–1508. [Google Scholar]
- Joo, K.H.; Aditi, S.; Hyun, L.S.; Ho, L.D.; Jeong, L.D.; Min, C.Y.; Suk, Y.B.; Hwan, L.S. Genetic association of PLAG1, SCD, CYP7B1 and FASN SNPs and their effects on carcass weight, intramuscular fat and fatty acid composition in Hanwoo steers (Korean cattle). Anim. Genet. 2017, 48, 251–252. [Google Scholar]
- Bartoň, L.; Bureš, D.; Kott, T.; Řehák, D. Associations of polymorphisms in bovine DGAT1, FABP4, FASN, and PPARGC1A genes with intramuscular fat content and the fatty acid composition of muscle and subcutaneous fat in Fleckvieh bulls. Meat Sci. 2016, 114, 18–23. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.A.; Kim, Y.K.; Kim, H.J.; Lee, D.H.; Lee, S.H.; Yoon, H.B.; Lee, S.H. Genome-wide association study and prediction of genomic breeding values for fatty-acid composition in Korean Hanwoo cattle using a high-density single-nucleotide polymorphism array 1. J. Anim. Sci. 2018, 96, 4063–4075. [Google Scholar] [CrossRef]
- Mazzucco, J.P.; Goszczynski, D.E.; Ripoli, M.V.; Melucci, L.M.; Pardo, A.M.; Colatto, E.; Rogberg-Muñoz, A.; Mezzadra, C.A.; Depetris, G.J.; Giovambattista, G.; et al. Growth, carcass and meat quality traits in beef from Angus, Hereford and cross-breed grazing steers, and their association with SNPs in genes related to fat deposition metabolism. Meat Sci. 2016, 114, 121–129. [Google Scholar] [CrossRef]
- Dongyep, O.; Yoonseok, L.; Boomi, L.; Jungsou, Y.; Euiryong, C.; Younyoung, K.; Chaeyoung, L. Fatty acid composition of beef is associated with exonic nucleotide variants of the gene encoding FASN. Mol. Biol. Rep. 2012, 39, 4083–4090. [Google Scholar]
- Rempel, L.A.; Casas, E.; Shackelford, S.D.; Wheeler, T.L. Relationship of polymorphisms within metabolic genes and carcass traits in crossbred beef cattle. J. Anim. Sci. 2012, 90, 1311–1316. [Google Scholar] [CrossRef]
- Liu, H.; Tian, W.; Zan, L.; Wang, H.; Cui, H. Mutations of MC4R gene and its association with economic traits in Qinchuan cattle. Mol. Biol. Rep. 2010, 37, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Jiyeon, S.; Sang, S.D.; Do, P.K.; Kyo, L.H.; Sik, K.H. Identification and analysis of MC4R polymorphisms and their association with economic traits of Korean cattle (Hanwoo). Mol. Biol. Rep. 2012, 39, 3597–3601. [Google Scholar]
- Haruna, I.L.; Ekegbu, U.J.; Ullah, F.; Amirpour-Najafabadi, H.; Zhou, H.; Hickford, J.G.H. Genetic variations and haplotypic diversity in the Myostatin gene of New Zealand cattle breeds. Gene 2020, 740, 144400. [Google Scholar] [CrossRef]
- Martínez, A.; Aldai, N.; Celaya, R.; Osoro, K. Effect of breed body size and the muscular hypertrophy gene in the production and carcass traits of concentrate-finished yearling bulls. J. Anim. Sci. 2010, 88, 1229–1239. [Google Scholar] [CrossRef]
- Raes, K.; De Smet, S.; Demeyer, D. Effect of double-muscling in Belgian Blue young bulls on the intramuscular fatty acid composition with emphasis on conjugated linoleic acid and polyunsaturated fatty acids. Anim. Sci. 2001, 73, 253–260. [Google Scholar] [CrossRef]
- Fiems, L.O. Double Muscling in Cattle: Genes, Husbandry, Carcasses and Meat. Animals 2012, 2, 472–506. [Google Scholar] [CrossRef]
- Robinson, D.L.; Cafe, L.M.; McIntyre, B.L.; Geesink, G.H.; Barendse, W.; Pethick, D.W.; Thompson, J.M.; Polkinghorne, R.; Greenwood, P.L. Production and processing studies on calpain-system gene markers for beef tenderness: Consumer assessments of eating quality. J. Anim. Sci. 2012, 90, 2850–2860. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.D.; Goes, R.H.d.T.e.B.d.; Mancio, A.B. Maciez da carne bovina. Ciência Anim. Bras. 2006, 6, 135. [Google Scholar]
- Shackelford, S.D.; Koohmaraie, M.; Cundiff, L.V.; Gregory, K.E.; Rohrer, G.A.; Savell, J.W. Heritabilities and phenotypic and genetic correlations for bovine postrigor calpastatin activity, intramuscular fat content, Warner-Bratzler shear force, retail product yield, and growth rate1. J. Anim. Sci. 1994, 72, 857–863. [Google Scholar] [CrossRef]
- Geary, T.W.; McFadin, E.L.; MacNeil, M.D.; Grings, E.E.; Short, R.E.; Funston, R.N.; Keisler, D.H. Leptin as a predictor of carcass composition in beef cattle. J. Anim. Sci. 2003, 81, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Gui, L.; Wang, H.; Zan, L. Association and expression analyses of the Ucp2 and Ucp3 gene polymorphisms with body measurement and meat quality traits in Qinchuan cattle. J. Genet. 2016, 95, 939–946. [Google Scholar] [CrossRef]
- Molinari, P.C.; Bromfield, J.J. Inflammatory responses of bovine endometrial epithelial cells are increased under in vitro heat stress conditions. J. Therm. Biol. 2023, 114, 103564. [Google Scholar] [CrossRef]
- Poleti, M.D.; DeRijk, R.H.; Rosa, A.F.; Moncau, C.T.; Oliveira, P.S.; Coutinho, L.L.; Eler, J.P.; Balieiro, J.C.C. Genetic variants in glucocorticoid and mineralocorticoid receptors are associated with concentrations of plasma cortisol, muscle glycogen content, and meat quality traits in male Nellore cattle. Domest. Anim. Endocrinol. 2015, 51, 105–113. [Google Scholar] [CrossRef]
- Wang, L.; Sun, H.Z.; Guan, L.L.; Liu, J.X. Short communication: Relationship of blood DNA methylation rate and milk performance in dairy cows. J. Dairy Sci. 2019, 102, 5208–5211. [Google Scholar] [CrossRef]
- Geng, Q.; Lin, W.; Yang, L.; Hu, X.; Qiu, X. Rumen-protected guanidinoacetic acid improves growth performance in beef cattle under chronic heat stress by reshaping gut microbiota and modulating serum metabolism. Front. Microbiol. 2025, 16, 1529596. [Google Scholar] [CrossRef]
- Tian, R.; Mahmoodi, M.; Tian, J.; Koshkoiyeh, S.E.; Zhao, M.; Saminzadeh, M.; Li, H.; Wang, X.; Li, Y.; Esmailizadeh, A. Leveraging Functional Genomics for Understanding Beef Quality Complexities and Breeding Beef Cattle for Improved Meat Quality. Genes 2024, 15, 1104. [Google Scholar] [CrossRef]
- Cole, J.B.; VanRaden, P.M. Symposium review: Possibilities in an age of genomics: The future of selection indices 1. J. Dairy Sci. 2018, 101, 3686–3701. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Guo, Q.; Deng, H.; Luo, J.; Yi, K.; Sun, A.; Chen, K.; Shen, Q. Muscle Fatty Acids, Meat Flavor Compounds and Sensory Characteristics of Xiangxi Yellow Cattle in Comparison to Aberdeen Angus. Animals 2022, 12, 1161. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Ferreira, J.C.P.; Kastelic, J.; Kasimanickam, V. Application of Genomic Selection in Beef Cattle Disease Prevention. Animals 2025, 15, 277. [Google Scholar] [CrossRef]
- Kaur, D.; Virk, A.K. Smart neck collar: IoT-based disease detection and health monitoring for dairy cows. Discov. Internet Things 2025, 5, 12. [Google Scholar] [CrossRef]
- Schnidrig, G.A.; Struchen, R.; Schärrer, S.; Heim, D.; Hadorn, D.; Regula, G.S.; Paternoster, G. Improved cattle farm classification: Leveraging machine learning and linked national datasets. Front. Vet. Sci. 2025, 12, 1517173. [Google Scholar] [CrossRef]
- Kittichai, V.; Kaewthamasorn, M.; Arnuphaprasert, A.; Jomtarak, R.; Naing, K.M.; Tongloy, T.; Chuwongin, S.; Boonsang, S. A deep contrastive learning-based image retrieval system for automatic detection of infectious cattle diseases. J. Big Data 2025, 12, 2. [Google Scholar] [CrossRef]
- Dilger, A.C. Gene editing in livestock: Advancements, opportunities and challenges. J. Anim. Sci. 2019, 97, 60. [Google Scholar] [CrossRef]
- Villamil, P.R.; Beaton, B.P.; Krisher, R.L. Gene editing in livestock: Innovations and applications. Anim. Reprod. 2024, 21, e20240054. [Google Scholar] [CrossRef]

| Gene Names | Main Function | References |
|---|---|---|
| FABP3, PPARG, AGPAT6, SCD1, DGAT1, SREBP1 | Regulation of milk fat metabolism | [31,32,33,34,35] |
| CSN2, CSN3, LGB | Regulation of milk protein metabolism | [36,37] |
| IQGAP2 | Regulation of lactose metabolism | [38] |
| FSHR, PRLR, BMP15, KISS1 | Influence reproductive function | [28,39,40,41] |
| CD14, CXCR1, MBL1 | Modulation of immune function | [42,43,44] |
| HSP70, NR3C1 | Improvement in stress capacity | [45,46] |
| TERT | Regulation of rate of aging | [47] |
| Gene Name | Trait | Reference |
|---|---|---|
| ADIPOQ | Marble pattern | [86] |
| PRKAG3 | Muscle pH | [87] |
| Cartilage length | [88] | |
| LEP | Fatty acid composition | [89] |
| Eye muscle area | [90] | |
| Muscle pH | [91] | |
| Marble pattern | [92] | |
| CAST | Muscle iron content | [93] |
| Muscle pH | [94] | |
| Juiciness | [95] | |
| FASN | MUFA | [96] |
| SFA | [97] | |
| C14:0 | [98] | |
| C16:0 | [99] | |
| C18:0 | [100] | |
| Carcass weight | [101] | |
| MC4R | Muscle pH | [87] |
| Carcass weight | [102] | |
| Marble pattern | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cui, X.; Chen, Z. Innovations in Cattle Breeding Technology: Prospects in the Era of Gene Editing. Animals 2025, 15, 1364. https://doi.org/10.3390/ani15101364
Wang Y, Cui X, Chen Z. Innovations in Cattle Breeding Technology: Prospects in the Era of Gene Editing. Animals. 2025; 15(10):1364. https://doi.org/10.3390/ani15101364
Chicago/Turabian StyleWang, Yu, Xiangshun Cui, and Zhi Chen. 2025. "Innovations in Cattle Breeding Technology: Prospects in the Era of Gene Editing" Animals 15, no. 10: 1364. https://doi.org/10.3390/ani15101364
APA StyleWang, Y., Cui, X., & Chen, Z. (2025). Innovations in Cattle Breeding Technology: Prospects in the Era of Gene Editing. Animals, 15(10), 1364. https://doi.org/10.3390/ani15101364






