Dietary Tryptophan Requirement of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Fish and Feeding Management
2.3. Sample Collection
2.4. Measurement Methods
2.4.1. Proximate Composition of Feed and Fish
2.4.2. Digestive Enzyme Activity
2.4.3. Serum Biochemical Indices
2.4.4. Tryptophan and Protein Metabolism-Related Parameters
2.4.5. Total RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR Analysis
2.5. Calculation
2.6. Statistical Analysis
3. Results
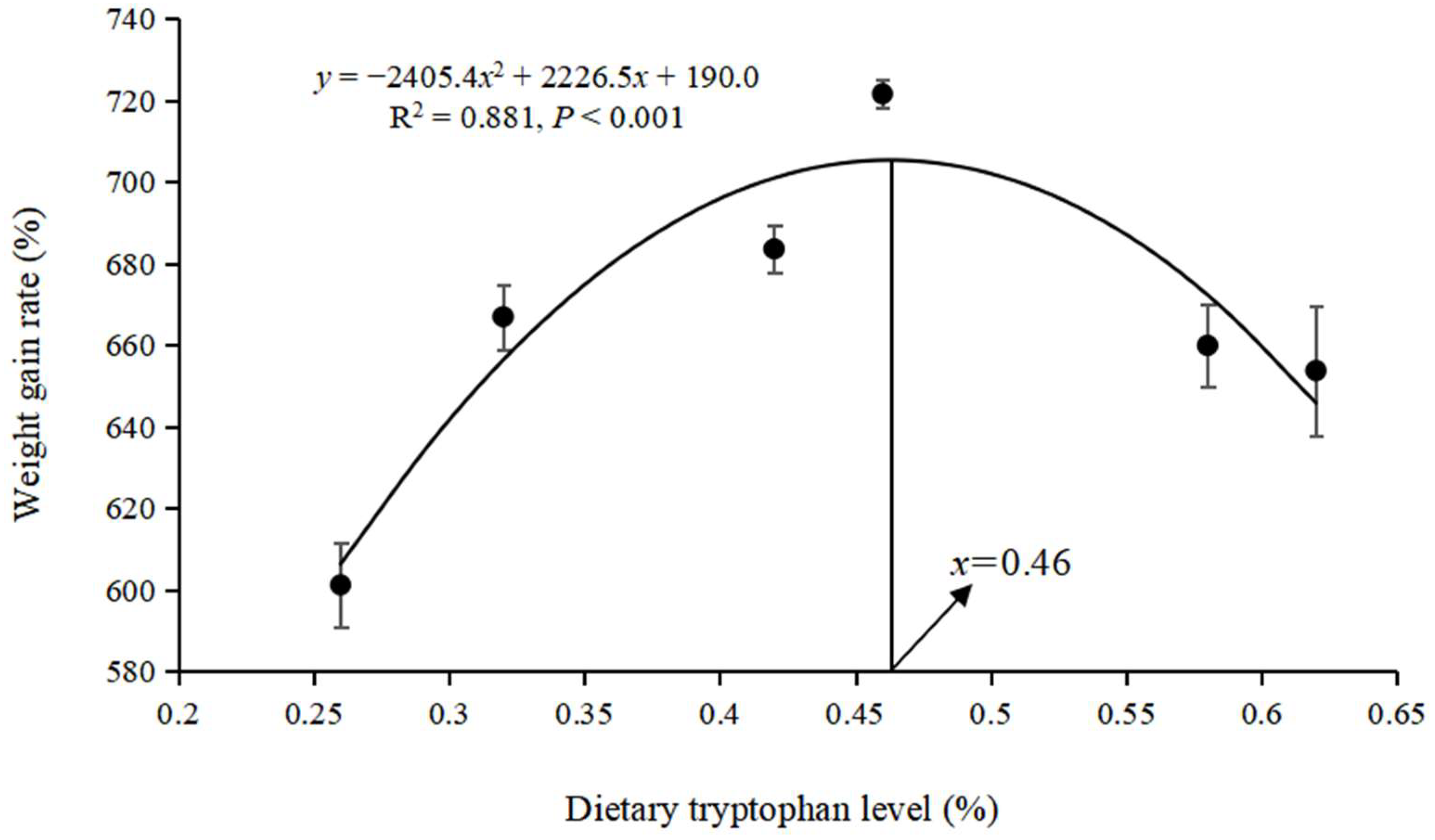
3.1. Growth Performance and Body Index
3.2. Proximate Composition of Fish Body
3.3. Intestinal Digestive Enzyme Activities
3.4. Serum Biochemical Indices
3.5. Activities of Tryptophan Metabolism-Related Enzymes
3.6. Activities of Protein Metabolism-Related Enzymes
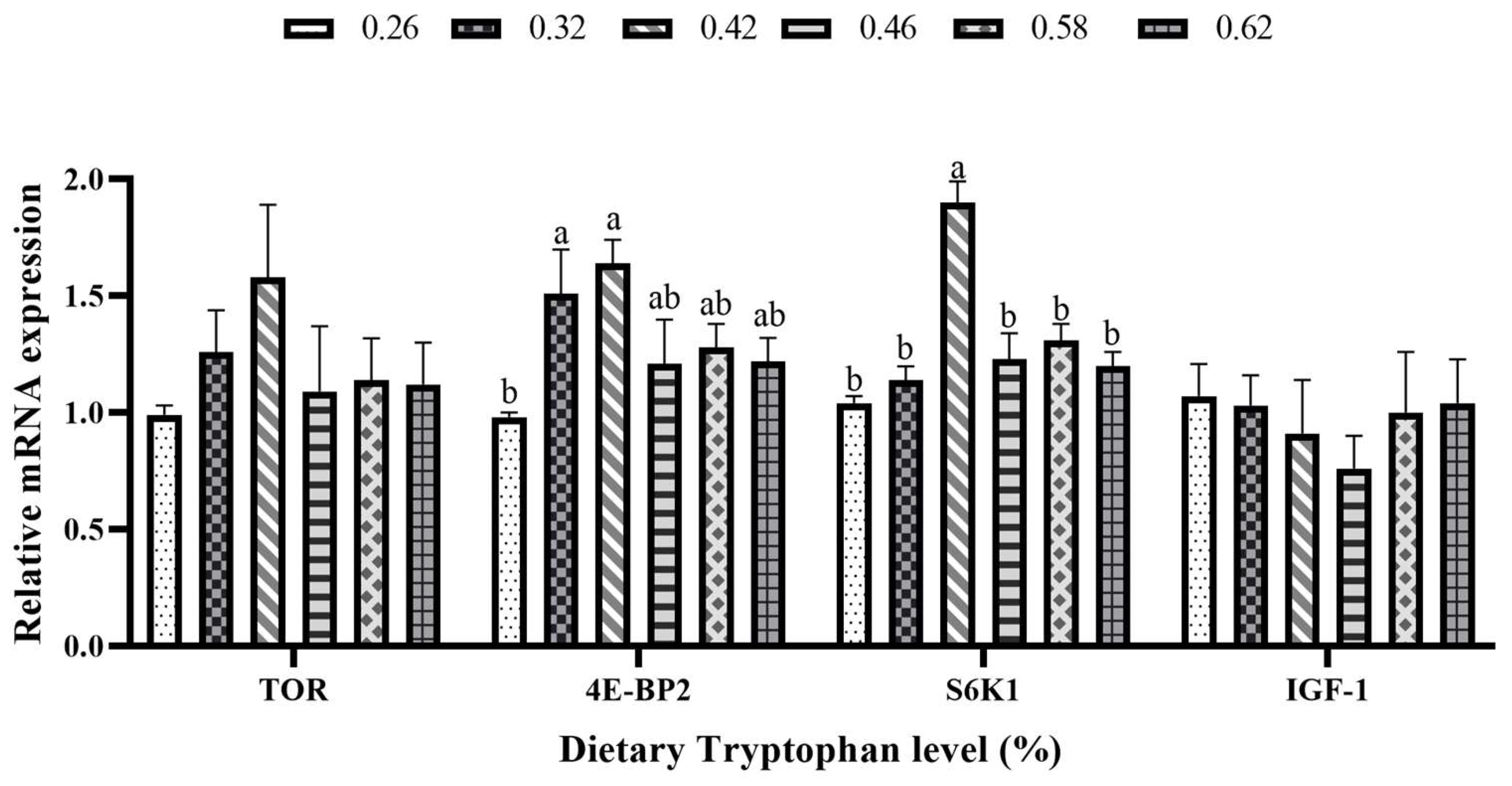
3.7. The Relative mRNA Expression Levels of Mammalian Target of Rapamycin and Amino Acid Response Pathways in Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hseu, J.R.; Lu, F.I.; Su, H.M.; Wang, L.S.; Tsai, C.L.; Hwang, P.P. Effect of Exogenous Tryptophan on Cannibalism, Survival and Growth in Juvenile Grouper, Epinephelus coioides. Aquaculture 2003, 218, 251–263. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Pérez-Jiménez, A.; Costas, B.; Azeredo, R.; Gesto, M. Physiological Roles of Tryptophan in Teleosts: Current Knowledge and Perspectives for Future Studies. Rev. Aquacult. 2019, 11, 3–24. [Google Scholar] [CrossRef]
- Fagbenro, O.A.; Nwanna, L.C. Dietary Tryptophan Requirement of the African Catfish, Clarias gariepinus. J. Appl. Aquac. 1999, 9, 65–72. [Google Scholar] [CrossRef]
- Akiyama, T.; Arai, S.; Murai, T.; Nose, T. Tryptophan Requirement of Chum Salmon Fry. Nippon. Suisan Gakk. 1985, 51, 1005–1008. [Google Scholar] [CrossRef][Green Version]
- NRC. Nutrient Requirements of Fish and Shrimp; National Research Council: Washington, DC, USA, 2011. [Google Scholar]
- Farhat; Khan, M.A. Dietary L-Tryptophan Requirement of Fingerling Stinging Catfish, Heteropneustes fossilis (Bloch). Aquac. Res. 2014, 45, 1224–1235. [Google Scholar] [CrossRef]
- Han, Y.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Gao, J. Optimum Supplementations and Interactive Effects of Methionine and Tryptophan on Growth and Health Status of Juvenile Japanese Flounder Paralichthys olivaceus. Aquacult. Sci. 2013, 61, 239–251. [Google Scholar] [CrossRef]
- Wilson, R.P.; Allen, O.W.; Robinson, E.H.; Poe, W.E. Tryptophan and Threonine Requirements of Fingerling Channel Catfish. J. Nutr. 1978, 108, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.J.; Cowey, C.B.; Coloso, R.M.; Adron, J.W. Dietary Requirements of Rainbow Trout for Tryptophan, Lysine and Arginine Determined by Growth and Biochemical Measurements. Fish Physiol. Biochem. 1986, 2, 161–169. [Google Scholar] [CrossRef]
- Fatma Abidi, S.; Khan, M.A. Dietary Tryptophan Requirement of Fingerling Rohu, Labeo rohita (Hamilton), Based on Growth and Body Composition. J. World Aquacult. Soc. 2010, 41, 700–709. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Dong, Y.; Gao, Y.; Yao, W.; Zhou, Z. Effects of Dietary Lysine Levels on Growth, Feed Utilization and Related Gene Expression of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Aquaculture 2019, 502, 153–161. [Google Scholar] [CrossRef]
- Li, X.; Mu, W.; Wu, X.; Dong, Y.; Zhou, Z.; Wang, X.; Ma, L.; Ye, B.; Geng, L. The Optimum Methionine Requirement in Diets of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂): Effects on Survival, Growth Performance, Gut Micromorphology and Immunity. Aquaculture 2020, 520, 735014. [Google Scholar] [CrossRef]
- Wu, M.; Wu, X.; Lu, S.; Gao, Y.; Yao, W.; Li, X.; Dong, Y.; Jin, Z. Dietary Arginine Affects Growth, Gut Morphology, Oxidation Resistance and Immunity of Hybrid Grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂) Juveniles. Br. J. Nutr. 2018, 120, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Xiaoyi, W.; Bo, Y.; Lina, G.; Zhiyu, Z.; Xiao, W.; Wei, M. The Optimum Threonine Requirement in Diets of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Aquacult. Nutr. 2021, 27, 829–840. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Wu, X.; Gao, Y.; Li, X.; Dong, Y.; Yao, W. Effects of Dietary Leucine Levels on Growth, Feed Utilization, Neuro-Endocrine Growth Axis and TOR-Related Signaling Genes Expression of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Aquaculture 2019, 504, 172–181. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.; Li, X.; Dong, Y.; Wang, X.; Mu, W.; Iii, D.M.G.; Zhang, Y. The Optimum Dietary Isoleucine Requirement of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Aquacult. Nutr. 2020, 26, 1295–1310. [Google Scholar] [CrossRef]
- Wu, M.; Lu, S.; Wu, X.; Jiang, S.; Luo, Y.; Yao, W.; Jin, Z. Effects of Dietary Amino Acid Patterns on Growth, Feed Utilization and Hepatic IGF-I, TOR Gene Expression Levels of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) Juveniles. Aquaculture 2017, 468, 508–514. [Google Scholar] [CrossRef]
- Dou, X.; Liu, Y.; Cao, Y.; Zhang, Y.; Fu, X.; Deng, J.; Tan, B. Effects of Dietary Lysine Level on Growth Performance and Protein Metabolism in Juvenile Leopard Coral Grouper (Plectropomus leopardus). Aquacult. Nutr. 2023, 2023, 1017222. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2006. [Google Scholar]
- Wang, R.; Mohammadi, M.; Mahboubi, A.; Taherzadeh, M.J. In-vitro Digestion Models: A Critical Review for Human and Fish and a Protocol for In-vitro Digestion in Fish. Bioengineered 2021, 12, 3040–3064. [Google Scholar] [CrossRef]
- Castillo-Lopez, E.; Espinoza-Villegas, R.E.; Viana, M.T. In Vitro Digestion Comparison from Fish and Poultry By-Product Meals from Simulated Digestive Process at Different Times of the Pacific Bluefin Tuna, Thunnus orientalis. Aquaculture 2016, 458, 187–194. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kvålseth, T.O. Cautionary Note about R2. Am. Stat. 1985, 39, 279–285. [Google Scholar] [CrossRef]
- Akiyama, T.; Kabuto, H.; Hiramatsu, M.; Murai, T.; Mori, K. Effect of Dietary 5-Hydroxy-L-Tryptophan for Prevention of Scoliosis in Tryptophan-Deficient Chum Salmon Fry. Nippon. Suisan Gakk. 1989, 55, 99–104. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahmad, I.; Malla, B.A. Effects of Dietary Tryptophan Levels on Growth Performance, Plasma Profile, Intestinal Antioxidant Capacity and Growth Related Genes in Rainbow Trout (Oncorhynchus mykiss) Fingerlings. Aquaculture 2024, 585, 740710. [Google Scholar] [CrossRef]
- Pewitt, E.; Castillo, S.; Velásquez, A.; Gatlin, D.M. The Dietary Tryptophan Requirement of Juvenile Red Drum, Sciaenops ocellatus. Aquaculture 2017, 469, 112–116. [Google Scholar] [CrossRef]
- Kim, K.; Kayes, T.B.; Amundson, C.H. Effects of Dietary Tryptophan Levels on Growth, Feed/Gain, Carcass Composition and Liver Glutamate Dehydrogenase Activity in Rainbow Trout (Salmo gairdneri). Comp. Biochem. Physiol. B 1987, 88, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I. Dietary Amino Acid L-Tryptophan Requirement of Fingerling Indian Catfish, Heteropneustes fossilis (Bloch), Estimated by Growth and Haemato-biochemical Parameters. Fish Physiol. Biochem. 2012, 38, 1195–1209. [Google Scholar] [CrossRef]
- Miao, S.; Chang, E.; Han, B.; Zhang, X.; Liu, X.; Zhou, Z.; Zhou, Y. Dietary Tryptophan Requirement of Northern Snakehead, Channa argus (Cantor, 1842). Aquaculture 2021, 542, 736904. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.; Xu, S.; Xie, J.; Xiang, K.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Zhao, J.; et al. Dietary Tryptophan Affects Growth Performance, Digestive and Absorptive Enzyme Activities, Intestinal Antioxidant Capacity, and Appetite and GH–IGF Axis-Related Gene Expression of Hybrid Catfish (Pelteobagrus vachelli♀ × Leiocassis longirostris♂). Fish Physiol. Biochem. 2019, 45, 1627–1647. [Google Scholar] [CrossRef]
- Tang, L.; Feng, L.; Sun, C.-Y.; Chen, G.-F.; Jiang, W.-D.; Hu, K.; Liu, Y.; Jiang, J.; Li, S.-H.; Kuang, S.-Y.; et al. Effect of Tryptophan on Growth, Intestinal Enzyme Activities and TOR Gene Expression in Juvenile Jian Carp (Cyprinus carpio var. Jian): Studies in Vivo and in Vitro. Aquaculture 2013, 412-413, 23–33. [Google Scholar] [CrossRef]
- Konturek, S.J.; Radecki, T.; Thor, P.; Dembinski, A. Release of Cholecystokinin by Amino Acids. Exp. Biol. Med. 1973, 143, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Aldman, G.; Grove, D.; Holmgren, S. Duodenal Acidification and Intra-Arterial Injection of CCK8 Increase Gallbladder Motility in the Rainbow Trout, Oncorhynchus mykiss. Gen. Comp. Endocr. 1992, 86, 20–25. [Google Scholar] [CrossRef]
- Jaworek, J. Ghrelin and Melatonin in the Regulation of Pancreatic Exocrine Secretion and Maintaining of Integrity. J. Physiol. Pharmacol. 2006, 57, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Mai, K.; He, G.; Xue, M.; Xie, S.Q.; Kaushik, S.J. Protein and Amino Acids. In Fish Nutrition, 4th ed.; Hardy, R.W., Kaushik, S.J., Eds.; Academic Press: Salt Lake City, UT, USA, 2022; Volume 4, pp. 181–302. [Google Scholar]
- Gorissen, S.H.; Crombag, J.J.; Senden, J.M.; Waterval, W.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J. Protein Content and Amino Acid Composition of Commercially Available Plant-based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Proud, C.G. The mTOR Pathway in the Control of Protein Synthesis. Physiology 2006, 21, 362–369. [Google Scholar] [CrossRef]
- Corradetti, M.; Guan, K.-L. Upstream of the Mammalian Target of Rapamycin: Do All Roads Pass through mTOR? Oncogene 2006, 25, 6347–6360. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, M.N.; Shan, J.; Kilberg, M.S. Dynamic Changes in Genomic Histone Association and Modification during Activation of the ASNS and ATF3 Genes by Amino Acid Limitation. Biochem. J. 2013, 449, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Sheffield-Moore, M.; Zhang, X.-J.; Volpi, E.; Wolf, S.E.; Aarsland, A.; Ferrando, A.A.; Wolfe, R.R. Amino Acid Ingestion Improves Muscle Protein Synthesis in the Young and Elderly. Am. J. Physiol.-Endocrinol. Metab. 2004, 286, E321–E328. [Google Scholar] [CrossRef]
- Zid, B.M.; Rogers, A.N.; Katewa, S.D.; Vargas, M.A.; Kolipinski, M.C.; Lu, T.A.; Benzer, S.; Kapahi, P. 4E-BP Extends Lifespan upon Dietary Restriction by Enhancing Mitochondrial Activity in Drosophila. Cell 2009, 139, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; He, G. Advances in Amino Acid Perception and Metabolic Regulation. Chin. J. Anim. Nutr. 2015, 27, 342–351, (In Chinese with English abstract). [Google Scholar]
- Romano, A.; Parrinello, N.L.; Todoerti, K.; La Cava, P.; Puglisi, F.; Giallongo, C.; Tibullo, D.; Camiolo, G.; Sapienza, G.; Del Fabro, V.; et al. Tryptophan Deprivation Promotes an Adaptive Response and Contributes to Bioenergetics in Multiple Myeloma. Blood 2018, 132, 4511. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Larsen, B.K.; Pedersen, P.B. Nitrogen Waste from Rainbow Trout (Oncorhynchus mykiss) with Particular Focus on Urea. Aquacult. Eng. 2015, 65, 2–9. [Google Scholar] [CrossRef]
- Hu, D.; Liu, J.; Yu, W.; Li, C.; Huang, L.; Mao, W.; Lu, Z. Tryptophan Intake, Not Always the More the Better. Front. Nutr. 2023, 10, 1140054. [Google Scholar] [CrossRef]
- Jin, Y. Dietary Tryptophan Requirements of Juvenile Pacific White Shrimp, Litopenaeus vannamei (Boone) Reared in Low-Salinity Water. Aquacult. Int. 2017, 25, 955–968. [Google Scholar] [CrossRef]
- Lemaire, P.; Drai, P.; Mathieu, A.; Lemaire, S.; Carrière, S.; Giudicelli, J.; Lafaurie, M. Changes with Different Diets in Plasma Enzymes (GOT, GPT, LDH, ALP) and Plasma Lipids (Cholesterol, Triglycerides) of Sea-Bass (Dicentrarchus labrax). Aquaculture 1991, 93, 63–75. [Google Scholar] [CrossRef]
- Okolo, C.C.; Uju, C.N.; Chibuzor, S.D.; Ezema, K.J.; Kolndadacha, O.D.; Ibrahim, A.; Aronu, C.J. The Effects of Treatments with Multistrain Probiotics on Serum Aminotransferases, AST-ALT Ratio, Body Weight and Survivability Scores of Rats Experimentally Infected with Trypanosoma brucei. Comp. Clin. Pathol. 2020, 29, 1229–1236. [Google Scholar] [CrossRef]
- Gong, X.W.; Xu, Y.J.; Yang, Q.H.; Liang, Y.J.; Zhang, Y.P.; Wang, G.L.; Li, Y.Y. Effects of Soothing Liver and Invigorating Spleen Recipes on the IKKβ-NF-κB Signaling Pathway in Kupffer Cells of Nonalcoholic Steatohepatitis Rats. Evid.-Based Compl. Alt. 2015, 2015, 687690. [Google Scholar] [CrossRef] [PubMed]
- Sener, A.; Owen, A.; Malaisse-Lagae, F.; Malaisse, W.J. The Stimulus-Secretion Coupling of Amino Acid-Induced Insulin Release XI. Kinetics of Deamination and Transamination Reactions. Horm. Metab. Res. 1982, 14, 405–409. [Google Scholar] [CrossRef]
- Coffee, C.J. AMP Deaminase from Rat Skeletal Muscle. Method. Enzymol. 1978, 51, 490–497. [Google Scholar] [CrossRef]
- Tng, Y.Y.M.; Wee, N.L.J.; Ip, Y.K.; Chew, S.F. Postprandial Nitrogen Metabolism and Excretion in Juvenile Marble Goby, Oxyeleotris marmorata (Bleeker, 1852). Aquaculture 2008, 284, 260–267. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, E.R.; Rebhun, S.; Eckel, M. Characterization of an Indoleamine 2,3-Dioxygenase Induced by Gamma-Interferon in Cultured Human Fibroblasts. J. Interferon Res. 1986, 6, 267–279. [Google Scholar] [CrossRef]
- Duan, X.; Luan, Y.; Wang, Y.; Wang, X.; Su, P.; Li, Q.; Pang, Y.; He, J.; Gou, M. Tryptophan Metabolism Can Modulate Immunologic Tolerance in Primitive Vertebrate Lamprey Via IDO-kynurenine-AHR Pathway. Fish Shellfish Immunol. 2023, 132, 108485. [Google Scholar] [CrossRef] [PubMed]
- Britan, A.; Maffre, V.; Tone, S.; Drevet, J.R. Quantitative and Spatial Differences in the Expression of Tryptophan-Metabolizing Enzymes in Mouse Epididymis. Cell Tissue Res. 2006, 324, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Klaessens, S.; Stroobant, V.; De Plaen, E.; Van den Eynde, B.J. Systemic Tryptophan Homeostasis. Front. Mol. Biosci. 2022, 9, 897929. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host. Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]


| Dietary Tryptophan Level (%) | ||||||
|---|---|---|---|---|---|---|
| Ingredient | 0.26 | 0.32 | 0.42 | 0.46 | 0.58 | 0.62 |
| Fish meal | 27.00 | 27.00 | 27.00 | 27.00 | 27.00 | 27.00 |
| Corn gluten meal | 6.40 | 6.40 | 6.40 | 6.40 | 6.40 | 6.40 |
| Brewer’s yeast meal | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Wheat flour | 23.38 | 23.38 | 23.38 | 23.38 | 23.38 | 23.38 |
| Amino acid mixture 1 | 23.27 | 23.27 | 23.27 | 23.27 | 23.27 | 23.27 |
| Guar gum | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Fish oil | 3.60 | 3.60 | 3.60 | 3.60 | 3.60 | 3.60 |
| Soybean oil | 3.70 | 3.70 | 3.70 | 3.70 | 3.70 | 3.70 |
| Soybean phospholipid | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Ca(H2PO4)2 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin C | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Ethoxyquin | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Y2O3 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Coated L-tryptophan (90.3%) 2 | 0.00 | 0.11 | 0.22 | 0.33 | 0.44 | 0.55 |
| Coated L-alanine (90.3%) 2 | 0.55 | 0.44 | 0.33 | 0.22 | 0.11 | 0.00 |
| Vitamin premix 3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mineral premix 3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Proximate composition | ||||||
| Dry matter (% DM) | 89.86 | 88.76 | 88.29 | 88.98 | 88.65 | 90.65 |
| Crude protein (% DM) | 49.98 | 49.13 | 49.85 | 49.38 | 49.53 | 50.38 |
| Crude lipid (% DM) | 12.04 | 12.62 | 12.39 | 12.00 | 12.31 | 12.34 |
| Crude ash (% DM) | 8.02 | 8.09 | 8.08 | 8.04 | 7.93 | 8.11 |
| Dietary Tryptophan Level (%) | ||||||
|---|---|---|---|---|---|---|
| 0.26 | 0.32 | 0.42 | 0.46 | 0.58 | 0.62 | |
| Essential amino acids (EAA) | ||||||
| Tryptophan | 0.26 | 0.32 | 0.42 | 0.46 | 0.58 | 0.62 |
| Methionine | 1.11 | 1.08 | 1.07 | 1.05 | 1.07 | 1.16 |
| Lysine | 3.01 | 2.92 | 2.94 | 2.99 | 2.94 | 2.99 |
| Threonine | 1.90 | 1.87 | 1.70 | 1.73 | 1.87 | 1.94 |
| Isoleucine | 1.67 | 1.60 | 1.64 | 1.66 | 1.64 | 1.68 |
| Leucine | 3.14 | 3.10 | 3.05 | 3.10 | 3.16 | 3.22 |
| Phenylalanine | 1.64 | 1.60 | 1.62 | 1.65 | 1.66 | 1.68 |
| Arginine | 2.60 | 2.51 | 2.51 | 2.59 | 2.52 | 2.60 |
| Histidine | 0.85 | 0.77 | 0.77 | 0.81 | 0.80 | 0.80 |
| Valine | 1.74 | 1.69 | 1.77 | 1.76 | 1.73 | 1.72 |
| ∑EAA | 17.92 | 17.46 | 17.49 | 17.80 | 17.97 | 18.41 |
| Non-essential amino acids (NEAA) | ||||||
| Aspartate | 5.47 | 5.33 | 5.28 | 5.36 | 5.44 | 5.62 |
| Glutamate | 7.31 | 7.20 | 7.01 | 7.13 | 7.22 | 7.44 |
| Glycine | 3.85 | 3.76 | 3.71 | 3.96 | 3.83 | 3.88 |
| Alanine | 3.49 | 3.34 | 3.25 | 3.21 | 3.13 | 3.09 |
| Cystine | 0.52 | 0.52 | 0.54 | 0.57 | 0.50 | 0.55 |
| Serine | 1.62 | 1.60 | 1.33 | 1.31 | 1.64 | 1.70 |
| Proline | 4.11 | 4.05 | 4.06 | 3.98 | 5.04 | 4.39 |
| Tyrosine | 1.26 | 1.21 | 1.18 | 1.22 | 1.14 | 1.19 |
| ∑NEAA | 27.63 | 27.01 | 26.36 | 26.74 | 27.94 | 27.86 |
| Genes | Forward and Reverse Primer Sequences (5’-3’) | Efficiency of Primers (%) | Genbank Accession No. |
|---|---|---|---|
| TOR | F: CAAGGTTTCTTCCGCTCCATCTCC R: CTCCACCAGGGCTTCATTCACTTC | 93.80 | JN850959.1 |
| S6K1 | F: TCACCTCCCGCACTCCTAAAGAC R: GCTTGACCTTCTCCACTGAACCTTC | 103.54 | XM_033643204.1 |
| 4E-BP2 | F: TAACTCTCCCATTGCCCAGACTCC R: GTGGTTGTTGGCTTCGTTCTTCTTG | 109.18 | XM_033613907.1 |
| GCN2 | F: AGGAGGACTGTCTCGTGGTGAAC R: GAGTGTGGTTGGTGAGGCTTTGG | 100.71 | XM_042500953.1 |
| ATF3 | F: CCAAACACCCGAGGATGAGAGAAAC R: AGGAGGAGGCGGAGGAGGAG | 107.71 | XM_042503803.1 |
| ATF4a | F: TGGAGCAGACGATGGCAAAGATG R: CGGATGAGCAGGAACCAATGAGG | 104.66 | XM_042485343.1 |
| ASNS | F: GCTCCATCTGTATGACCGCTTTG R: GCAAGGAATCCATCGTCTGTGAG | 98.03 | XM_033636649.1 |
| IGF-1 | F: TGCGGCGTCTGGAGATGTAC R: AGGTAAAGGTCTCTTGGGTGCTC | 94.92 | XM_033614181.1 |
| β-actin | F: TTCACCACCACAGCCGAGAGG R: GAGGAGGAGGAGGCAGCAGTG | 90.94 | AY510710.2 |
| Dietary Tryptophan Level (%) | FBW (g) | WGR (%) | SGR (%/d) | FIR (%) | FCR | PER | SR (%) | CF (g/cm3) | VSI (%) | HSI (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.26 | 73.59 ± 0.62 d | 601.21 ± 5.89 d | 2.87 ± 0.01 d | 1.89 ± 0.00 | 0.86 ± 0.00 | 2.36 ± 0.01 bc | 86.66 ± 3.84 | 2.89 ± 0.02 | 10.51 ± 0.65 b | 3.94 ± 0.08 |
| 0.32 | 80.49 ± 0.83 bc | 666.91 ± 7.91 bc | 2.98 ± 0.01 bc | 1.87 ± 0.00 | 0.83 ± 0.00 | 2.44 ± 0.01 ab | 87.77 ± 2.22 | 2.92 ± 0.04 | 11.97 ± 0.19 a | 3.97 ± 0.27 |
| 0.42 | 82.24 ± 0.61 b | 683.53 ± 5.84 b | 3.01 ± 0.00 b | 1.84 ± 0.00 | 0.80 ± 0.00 | 2.48 ± 0.02 ab | 85.55 ± 2.22 | 2.98 ± 0.04 | 12.38 ± 0.49 a | 4.17 ± 0.31 |
| 0.46 | 86.23 ± 0.37 a | 721.53 ± 3.54 a | 3.10 ± 0.00 a | 1.87 ± 0.06 | 0.85 ± 0.03 | 2.52 ± 0.06 a | 86.66 ± 5.08 | 3.01 ± 0.03 | 12.90 ± 0.28 a | 4.62 ± 0.30 |
| 0.58 | 79.75 ± 0.60 c | 659.88 ± 5.73 c | 2.98 ± 0.01 bc | 1.95 ± 0.04 | 0.87 ± 0.01 | 2.33 ± 0.05 bc | 86.66 ± 1.92 | 2.93 ± 0.04 | 11.69 ± 0.34 ab | 4.01 ± 0.14 |
| 0.62 | 79.11 ± 0.97 c | 653.71 ± 9.21 c | 2.97 ± 0.01 c | 1.87 ± 0.01 | 0.89 ± 0.03 | 2.22 ± 0.07 c | 89.99 ± 3.33 | 2.86 ± 0.02 | 11.64 ± 0.17 ab | 4.01 ± 0.13 |
| ANOVA | ||||||||||
| p-value | <0.001 | <0.001 | <0.001 | 0.334 | 0.168 | 0.010 | 0.949 | 0.151 | 0.020 | 0.341 |
| Regression | ||||||||||
| Model | SOP | SOP | SOP | / | / | SOP | / | / | SOP | / |
| p-value | <0.001 | <0.001 | <0.001 | / | / | <0.001 | / | / | 0.002 | / |
| Adj. R2 | 0.802 | 0.802 | 0.769 | / | / | 0.613 | / | / | 0.513 | / |
| OPTI INCL | 0.463 | 0.463 | 0.465 | / | / | 0.414 | / | / | 0.461 | / |
| Dietary Tryptophan Level (%) | Moisture (%) | Crude Protein (%) | Crude Lipid (%) | Crude Ash (%) |
|---|---|---|---|---|
| 0.26 | 72.68 ± 0.42 | 59.53 ± 0.58 | 25.21 ± 0.21 ab | 14.53 ± 0.06 |
| 0.32 | 72.65 ± 0.31 | 60.16 ± 0.67 | 25.51 ± 0.14 a | 13.91 ± 0.39 |
| 0.42 | 72.32 ± 0.17 | 61.80 ± 1.76 | 25.48 ± 0.11 a | 14.30 ± 0.16 |
| 0.46 | 72.37 ± 0.33 | 61.73 ± 2.71 | 24.66 ± 0.18 b | 14.33 ± 0.16 |
| 0.58 | 72.93 ± 0.22 | 59.99 ± 1.59 | 25.18 ± 0.18 ab | 14.15 ± 0.19 |
| 0.62 | 72.66 ± 0.33 | 56.56 ± 1.08 | 25.22 ± 0.16 ab | 14.09 ± 0.26 |
| ANOVA | ||||
| p-value | 0.760 | 0.272 | 0.049 | 0.544 |
| Regression | ||||
| Model | / | / | NR | / |
| p-value | / | / | 0.637 | / |
| Adj. R2 | / | / | / | / |
| OPTI INCL | / | / | / | / |
| Dietary Tryptophan Level (%) | Trypsin (U/mg Protein) | Lipase (U/g Protein) | Amylase (U/mg Protein) |
|---|---|---|---|
| 0.26 | 277.22 ± 28.14 b | 2.00 ± 0.14 b | 0.51 ± 0.00 d |
| 0.32 | 237.24 ± 32.52 b | 2.46 ± 0.12 b | 0.55 ± 0.00 c |
| 0.42 | 213.78 ± 3.30 b | 2.94 ± 0.21 ab | 0.83 ± 0.01 a |
| 0.46 | 445.40 ± 3.95 a | 3.44 ± 0.35 a | 0.67 ± 0.01 b |
| 0.58 | 272.04 ± 14.13 b | 2.71 ± 0.46 ab | 0.42 ± 0.00 e |
| 0.62 | 286.19 ± 39.29 b | 2.22 ± 0.25 b | 0.36 ± 0.00 f |
| ANOVA | |||
| p-value | <0.001 | 0.043 | <0.001 |
| Regression | |||
| Model | NR | SOP | SOP |
| p-value | 0.523 | 0.004 | <0.001 |
| Adj. R2 | / | 0.457 | 0.798 |
| OPTI INCL | / | 0.456 | 0.418 |
| Dietary Tryptophan Level (%) | BUN (nmol/L) | TAA (μmol/mL) | AST (U/L) | ALT (U/L) | AST/ALT |
|---|---|---|---|---|---|
| 0.26 | 61.47 ± 4.82 a | 26.76 ± 1.01 cd | 359.14 ± 21.06 c | 754.59 ± 25.71 a | 0.47 ± 0.03 c |
| 0.32 | 61.47 ± 4.32 a | 29.05 ± 0.93 bc | 402.39 ± 44.09 bc | 507.33 ± 33.87 d | 0.81 ± 0.13 bc |
| 0.42 | 68.39 ± 0.86 a | 41.02 ± 0.64 a | 412.71 ± 40.91 bc | 552.72 ± 58.35 cd | 0.76 ± 0.12 bc |
| 0.46 | 65.80 ± 1.73 a | 32.05 ± 2.06 b | 430.01 ± 55.36 bc | 480.99 ± 39.84 d | 0.91 ± 0.18 b |
| 0.58 | 42.42 ± 2.29 b | 23.93 ± 2.03 d | 507.06 ± 30.28 b | 665.48 ± 4.04 ab | 0.76 ± 0.04 bc |
| 0.62 | 59.50 ± 3.97 a | 23.71 ± 1.28 d | 1002.93 ± 41.21 a | 640.51 ± 16.46 bc | 1.57 ± 0.10 a |
| ANOVA | |||||
| p-value | 0.002 | <0.001 | <0.001 | 0.001 | 0.001 |
| Regression | |||||
| Model | NR | SOP | L | SOP | L |
| p-value | 0.087 | <0.001 | 0.001 | 0.003 | 0.003 |
| Adj. R2 | / | 0.599 | 0.503 | 0.476 | 0.407 |
| OPTI INCL | / | 0.423 | / | 0.445 | / |
| Dietary Tryptophan Level (%) | 5-HTPDC (U/mg Protein) | TPH (U/mg Protein) | TDO (U/mg Protein) | IDO (IU/mg Protein) |
|---|---|---|---|---|
| 0.26 | 152.73 ± 3.10 b | 77.68 ± 1.66 a | 25.98 ± 0.15 d | 17.41 ± 0.06 b |
| 0.32 | 155.79 ± 5.98 b | 33.08 ± 1.32 e | 29.33 ± 1.48 c | 17.06 ± 0.22 b |
| 0.42 | 156.82 ± 5.62 b | 40.03 ± 0.57 d | 37.69 ± 0.88 b | 16.73 ± 0.29 b |
| 0.46 | 284.66 ± 7.70 a | 43.12 ± 1.86 d | 41.30 ± 0.66 a | 25.85 ± 0.38 a |
| 0.58 | 119.22 ± 5.69 c | 52.35 ± 2.05 c | 39.90 ± 0.78 ab | 11.84 ± 0.21 c |
| 0.62 | 128.63 ± 3.93 c | 57.24 ± 0.97 b | 31.76 ± 1.44 c | 8.25 ± 0.27 d |
| ANOVA | ||||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
| Regression | ||||
| Model | SOP | SOP | SOP | SOP |
| p-value | 0.023 | 0.001 | <0.001 | <0.001 |
| Adj. R2 | 0.314 | 0.540 | 0.794 | 0.622 |
| OPTI INCL | 0.429 | 0.451 | 0.483 | 0.402 |
| Dietary Tryptophan Level (%) | AST (U/g Protein) | ALT (U/g Protein) | AMPD (U/mg Protein) | GDH (U/g Protein) |
|---|---|---|---|---|
| 0.26 | 15.54 ± 1.25 b | 16.80 ± 0.71 a | 43.22 ± 0.94 b | 19.12 ± 1.09 c |
| 0.32 | 9.22 ± 0.60 c | 11.79 ± 0.99 bc | 41.81 ± 2.73 b | 21.52 ± 0.88 c |
| 0.42 | 8.90 ± 0.23 c | 9.59 ± 0.50 c | 38.12 ± 0.96 b | 27.19 ± 0.85 b |
| 0.46 | 12.85 ± 1.00 bc | 11.72 ± 1.07 bc | 98.20 ± 2.51 a | 30.52 ± 1.31 a |
| 0.58 | 16.66 ± 0.72 b | 13.59 ± 0.58 b | 43.60 ± 1.53 b | 19.80 ± 0.52 c |
| 0.62 | 21.83 ± 2.83 a | 12.81 ± 0.87 b | 39.10 ± 1.87 b | 11.83 ± 0.55 d |
| ANOVA | ||||
| p-value | <0.001 | 0.001 | <0.001 | <0.001 |
| Regression | ||||
| Model | SOP | SOP | NR | SOP |
| p-value | <0.001 | 0.002 | 0.098 | <0.001 |
| Adj. R2 | 0.722 | 0.510 | / | 0.851 |
| OPTI INCL | 0.399 | 0.460 | / | 0.425 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Dong, X.; Yang, Q.; Chi, S.; Zhang, S.; Tan, B.; Deng, J. Dietary Tryptophan Requirement of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂). Animals 2025, 15, 104. https://doi.org/10.3390/ani15010104
Chen J, Dong X, Yang Q, Chi S, Zhang S, Tan B, Deng J. Dietary Tryptophan Requirement of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂). Animals. 2025; 15(1):104. https://doi.org/10.3390/ani15010104
Chicago/Turabian StyleChen, Jiaxian, Xiaohui Dong, Qihui Yang, Shuyan Chi, Shuang Zhang, Beiping Tan, and Junming Deng. 2025. "Dietary Tryptophan Requirement of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂)" Animals 15, no. 1: 104. https://doi.org/10.3390/ani15010104
APA StyleChen, J., Dong, X., Yang, Q., Chi, S., Zhang, S., Tan, B., & Deng, J. (2025). Dietary Tryptophan Requirement of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂). Animals, 15(1), 104. https://doi.org/10.3390/ani15010104





