Epidemiology and Molecular Characterization of Zoonotic Gastrointestinal Protozoal Infection in Zoo Animals in China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Genomic DNA Extraction
2.3. PCR Amplification
2.4. Sequencing and Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
3.1. Prevalence and Species Distribution of Cryptosporidium spp.
3.2. Prevalence and Genotype Distribution of G. duodenalis
3.3. Prevalence and Gene Subtype Distribution of Blastocystis spp.
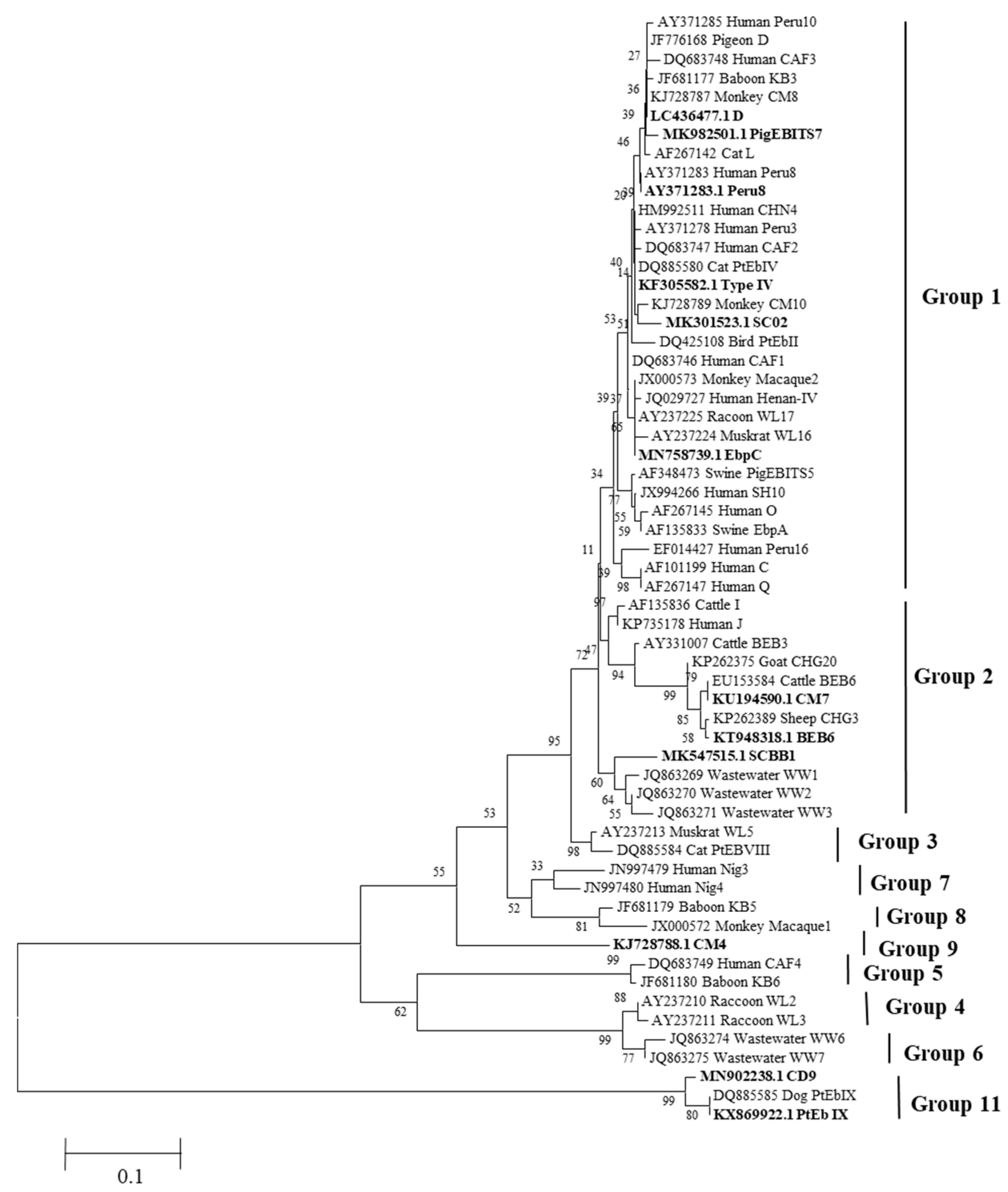
3.4. Prevalence and Genotype Distribution of E. bieneusi
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bengis, R.G.; Leighton, F.A.; Fischer, J.R.; Artois, M.; Mörner, T.; Tate, C.M. The role of wildlife in emerging and re-emerging zoonoses. Rev. Sci. Tech. 2004, 23, 497–511. [Google Scholar]
- Karim, M.R.; Li, J.; Rume, F.I.; Sumon, S.M.R.; Selim, A.S.M.; Hoda, N.; Zhang, L. Occurrence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis among captive mammals in the Bangladesh National Zoo. Parasitol. Int. 2021, 84, 102414. [Google Scholar] [CrossRef] [PubMed]
- Levecke, B.; Dorny, P.; Geurden, T.; Vercammen, F.; Vercruysse, J. Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet. Parasitol. 2007, 148, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Pielok, Ł.A.; Kłudkowska, M.; Frąckowiak, K.; Stefaniak, J. Parasitic infections among patients hospitalized in the Tropical and Parasitic Clinic of Poznan University of Medical Sciences, Poland between 2015 and 2018. Is there a relationship between protozoa infection and gastrointestinal symptoms? Prz. Gastroenterol. 2022, 17, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Smith, A. Zoonotic enteric protozoa. Vet. Parasitol. 2011, 182, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Plutzer, J.; Lassen, B.; Jokelainen, P.; Djurković-Djaković, O.; Kucsera, I. Review of Cryptosporidium and Giardia in the eastern part of Europe, 2016. Euro Surveill 2018, 23, 16–00825. [Google Scholar] [CrossRef] [PubMed]
- Upcroft, J.A.; McDonnell, P.A.; Gallagher, A.N.; Chen, N.; Upcroft, P. Lethal Giardia from a wild-caught sulphur-crested cockatoo (Cacatua galerita) established in vitro chronically infects mice. Parasitology 1997, 114, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shen, Y.; Yin, J.; Yuan, Z.; Jiang, Y.; Xu, Y.; Pan, W.; Hu, Y.; Cao, J. Prevalence and genetic characterization of Cryptosporidium, Enterocytozoon, Giardia and Cyclospora in diarrheal outpatients in China. BMC Infect. Dis. 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Suankratay, C.; Thiansukhon, E.; Nilaratanakul, V.; Putaporntip, C.; Jongwutiwes, S. Disseminated infection caused by novel species of Microsporidium. Thailand. Emerg. Infect. Dis. 2012, 18, 302–304. [Google Scholar] [CrossRef]
- Deng, L.; Chai, Y.; Zhou, Z.; Liu, H.; Zhong, Z.; Hu, Y.; Fu, H.; Yue, C.; Peng, G. Epidemiology of Blastocystis sp. infection in China: A systematic review. Parasite 2019, 26, 41. [Google Scholar] [CrossRef]
- Rauff-Adedotun, A.A.; Meor Termizi, F.H.; Shaari, N.; Lee, I.L. The Coexistence of Blastocystis spp. in Humans, Animals and Environmental Sources from 2010–2021 in Asia. Biology 2021, 10, 990. [Google Scholar] [CrossRef]
- Lee, L.I.; Chye, T.T.; Karmacharya, B.M.; Govind, S.K. Blastocystis sp.: Waterborne zoonotic organism, a possibility? Parasit. Vectors 2012, 5, 130. [Google Scholar] [CrossRef]
- Ahsani, M.R.; Mohammad Abadi, M.R.; Shamsodini Bafti, M.; Ezatkhah, M.; Hasani Derakhshan, M.; Esmailzadeh Koshkooeh, A. Application of triplex PCR technique in identification of clostridium perfringens b, c and d types. Iran J. Pharm. Res. 2010, 2, 185–190. [Google Scholar]
- Mohammadabadi, M.R.; Omarovich Shaikhaev, G.; Efimovna Sulimova, G.; Rahman, O.; Mozafari, M.R. Detection of bovine leukemia virus proviral DNA in Yaroslavsl, Mongolian and black pied cattle by PCR. Cell Mol. Biol. 2004, 9, 766–768. [Google Scholar]
- Xiao, L.; Singh, A.; Limor, J.; Graczyk, T.K.; Gradus, S.; Lal, A. Molecular characterization of Cryptosporidium oocysts in samples of raw surface water and wastewater. Appl. Environ. Microbiol. 2001, 67, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Lan, X.; Dan, J.; Ren, Z.; Cao, S.; Shen, L.; Deng, J.; Zuo, Z.; Yu, S.; et al. Occurrence and multilocus genotyping of Giardia duodenalis in captive non-human primates from 12 zoos in China. PLoS ONE 2020, 15, e0228673. [Google Scholar] [CrossRef] [PubMed]
- Scicluna, S.M.; Tawari, B.; Clark, C.G. DNA barcoding of Blastocystis. Protist 2006, 157, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Buckholt, M.A.; Lee, J.H.; Tzipori, S. Prevalence of Enterocytozoon bieneusi in swine: An 18-month survey at a slaughterhouse in Massachusetts. Appl. Environ. Microbiol. 2002, 68, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Zhang, S.; Jian, F.; Li, J.; Zhou, C.; Zhang, L.; Sun, M.; Yang, G.; Zou, F.; Dong, H.; et al. Multilocus typing of Cryptosporidium spp. and Giardia duodenalis from non-human primates in China. Int. J. Parasitol. 2014, 44, 1039–1047. [Google Scholar] [CrossRef]
- Feng, Y.; Ryan, U.M.; Xiao, L. Genetic Diversity and Population Structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef]
- Li, N.; Xiao, L.; Alderisio, K.; Elwin, K.; Cebelinski, E.; Chalmers, R.; Santin, M.; Fayer, R.; Kvac, M.; Ryan, U.; et al. Subtyping Cryptosporidium ubiquitum, a zoonotic pathogen emerging in humans. Emerg. Infect. Dis. 2014, 20, 217–224. [Google Scholar] [CrossRef]
- Zou, Y.; Li, X.D.; Meng, Y.M.; Wang, X.L.; Wang, H.N.; Zhu, X.Q. Prevalence and multilocus genotyping of Giardia duodenalis in zoo animals in three cities in China. Parasitol. Res. 2022, 121, 2359–2366. [Google Scholar] [CrossRef]
- Cai, W.; Ryan, U.; Xiao, L.; Feng, Y. Zoonotic giardiasis: An update. Parasitol. Res. 2021, 120, 4199–4218. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and Giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Li, J.; Qi, M.; Chang, Y.; Wang, R.; Li, T.; Dong, H.; Zhang, L. Molecular Characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi in Captive Wildlife at Zhengzhou Zoo, China. J. Eukaryot. Microbiol. 2015, 62, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Wang, R.; Yu, F.; Li, T.; Dong, H.; Li, D.; Zhang, L.; Li, J.; Jian, F.; Zhang, S.; et al. Multi-locus analysis of Giardia duodenalis from nonhuman primates kept in zoos in China: Geographical segregation and host-adaptation of assemblage B isolates. Infect. Genet. Evol. 2015, 30, 82–88. [Google Scholar] [CrossRef]
- Ryan, U.; Cacciò, S.M. Zoonotic potential of Giardia. Int. J. Parasitol. 2013, 43, 943–956. [Google Scholar] [CrossRef]
- Wang, S.S.; Wang, R.J.; Fan, X.C.; Liu, T.L.; Zhang, L.X.; Zhao, G.H. Prevalence and genotypes of Enterocytozoon bieneusi in China. Acta Trop. 2018, 183, 142–152. [Google Scholar] [CrossRef]
- Santín, M.; Fayer, R. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res. Vet. Sci. 2011, 90, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiao, L. Ecological and public health significance of Enterocytozoon bieneusi. One Health 2020, 12, 100209. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, Y.; Santin, M. Host Specificity of Enterocytozoon bieneusi and Public Health Implications. Trends Parasitol. 2019, 35, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zheng, S.; Wang, Y.; Wang, K.; Wang, Y.; Gazizova, A.; Han, K.; Yu, F.; Chen, Y.; Zhang, L. Occurrence and molecular characterization of Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi, and Blastocystis sp. in captive wild animals in zoos in Henan, China. BMC Vet. Res. 2021, 17, 332. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, L.; Yu, X.; Zhong, Z.; Wang, Q.; Liu, X.; Niu, L.; Xie, N.; Deng, J.; Lei, S.; et al. Multilocus genotypes and broad host-range of Enterocytozoon bieneusi in captive wildlife at zoological gardens in China. Parasit. Vectors 2016, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Dong, H.; Li, T.; Yu, F.; Li, D.; Zhang, L.; Li, J.; Wang, R.; Li, S.; Li, X.; et al. Predomination and new genotypes of Enterocytozoon bieneusi in captive nonhuman primates in zoos in China: High genetic diversity and zoonotic significance. PLoS ONE 2015, 10, e0117991. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhou, Z.; Liu, H.; Deng, L.; Bi, B.; Chai, Y.; Zhong, Z.; Hu, Y.; Fu, H.; Peng, G. New genotypes and molecular characterization of Enterocytozoon bieneusi in captive black bears in China. Int. J. Parasitol. Parasites Wildl. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Cian, A.; El Safadi, D.; Osman, M.; Moriniere, R.; Gantois, N.; Benamrouz-Vanneste, S.; Delgado-Viscogliosi, P.; Guyot, K.; Li, L.L.; Monchy, S.; et al. Molecular Epidemiology of Blastocystis sp. in Various Animal Groups from Two French Zoos and Evaluation of Potential Zoonotic Risk. PLoS ONE 2017, 12, e0169659. [Google Scholar] [CrossRef] [PubMed]
- Song, J.K.; Yin, Y.L.; Yuan, Y.J.; Tang, H.; Ren, G.J.; Zhang, H.J.; Li, Z.X.; Zhang, Y.M.; Zhao, G.H. First genotyping of Blastocystis sp. in dairy, meat, and cashmere goats in northwestern China. Acta Trop. 2017, 176, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Alfellani, M.A.; Taner-Mulla, D.; Jacob, A.S.; Imeede, C.A.; Yoshikawa, H.; Stensvold, C.R.; Clark, C.G. Genetic diversity of blastocystis in livestock and zoo animals. Protist 2013, 164, 497–509. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Clark, C.G. Current status of Blastocystis: A personal view. Parasitol. Int. 2016, 65, 763–771. [Google Scholar] [CrossRef]
- Böhm-Gloning, B.; Knobloch, J.; Walderich, B. Five subgroups of Blastocystis hominis from symptomatic and asymptomatic patients revealed by restriction site analysis of PCR-amplified 16S-like rDNA. Trop. Med. Int. Health 1997, 2, 771–778. [Google Scholar] [CrossRef]
| Pathogen | Gene Locus | Prime Sequence (5′-3′) | Expected Product Size (bp) | Annealing Temperature (°C) | References |
|---|---|---|---|---|---|
| Cryptosporidium spp. | SSU rRNA | SSU-F2: TTCTAGAGCTAATACATGC | ~1325 | 55 | [15] |
| SSU-R2: CCCATTTCCTTCGAAACAGGA | |||||
| SSU-F3: GGAAGGGTTGTATTTATTAGATAAAG | ~840 | 55 | |||
| SSU-F4: CTCATAAGGTGCTGAAGGAGTA | |||||
| G. duodenalis | tpi | AL3543: AAATIATGCCTGCTCGTCG | ~605 | 55 | [16] |
| AL3546: CAAACCTTITCCGCAAACC | |||||
| AL3544: CCCTTCATCGGIGGTAACTT | ~530 | 55 | |||
| AL3545: GTGGCCACCACICCCGTGCC | |||||
| bg | G7: AAGCCCGACGACCTCACCCGCAGTGC | 753 | 55 | ||
| G759: GAGGCCGCCCTGGATCTTCGAGACGAC | |||||
| B-F: GAA CGA ACG AGA TCG AGG TCCG | 511 | 55 | |||
| B-R: CTCGACGAGCTTCGTGTT | |||||
| Blastocystis spp. | SSU rRNA | RD5: ATCTGGTTGATCCTGCCAGT | ~600 | 58 | [17] |
| BhRDr: GAGCTTTTTAACTGCAACAACG | |||||
| E. bieneusi | ITS | EBITS3: GATGGTCATAGGGATGAAGAGCTT | ~435 | 57 | [18] |
| EBITS4: TATGCTTAAGTCCAGGGAG | |||||
| EBITS1: AGGGATGAAGAGCTTCGGCTCTG | ~392 | 55 | |||
| EBITS2.4: AGTGATCCTGTATTAGGGATATT |
| Location | Sample Size | No. of Positive Samples | Type of Assemblage | Genotype | |
|---|---|---|---|---|---|
| tpi (n) | bg (n) | ||||
| Guiyang | 49 | 8 | E (2), B (4) | E (5) | E (4), B (2), E + B (2) |
| Beijing | 101 | 10 | B (5) | E (5), B (3) | E (5), B (5) |
| Shijiazhuang | 69 | 2 | B (1) | E (1), B (1) | E (1), B (1) |
| Tangshan | 66 | 3 | B (2) | E (1) | E (1), B (2) |
| Xingtai | 115 | 1 | - | D (1) | D (1) |
| Total | 400 | 24 | E (2) B (12) | E (12), B (4), D (1) | E (11), B (10), E + B (2), D (1) |
| Groups of Animals | Animal Species (Common Name/Scientific Name) | Sample Size | No. of Positive Samples | Type of Assemblage |
|---|---|---|---|---|
| Artiodactyla | Giraffe/Giraffa camelopardalis | 5 | 2 | E (2) |
| Milk goat/Capra hircus L. | 3 | 2 | E (1), E + B (1) | |
| Siberian ibex/Capra sibirica | 11 | 2 | E (2) | |
| Argali/Ovis ammon | 15 | 1 | E (1) | |
| Roe deer/Capreolus pygargus | 4 | 2 | E (2) | |
| Addax/Addax nasomaculatus | 3 | 1 | E (1) | |
| Yak/Bos grunniens domesticus | 5 | 1 | E (1) | |
| Carnivora | Masked civet/Paguma larvata | 3 | 1 | D (1) |
| Brown bear/Ursus arctos | 6 | 1 | E + B (1) | |
| Primate | Ring-tailed lemur/Lemur catta | 21 | 9 | E (1), B (8) |
| Chimpanzee/Pan troglodytes | 9 | 2 | B (2) |
| Groups of Animals | Animal Species (Common Name/Scientific Name) | Sample Size | No. of Positive Samples | Positive Rate (%) |
|---|---|---|---|---|
| Proboscidean | Asian elephant/Pan troglodytes | 6 | 1 | 16.7 |
| Artiodactyla | Camel/Camelus bactrianus | 10 | 3 | 30.0 |
| Milk goat/Capra hircus L. | 3 | 1 | 33.3 | |
| Giraffe/Giraffa camelopardalis | 5 | 2 | 40.0 | |
| Siberian ibex/Capra sibirica | 11 | 5 | 45.5 | |
| Ammotragus/Ammotragus lervia | 3 | 3 | 100.0 | |
| Argali/Ovis ammon | 15 | 6 | 40.0 | |
| Roe deer/Capreolus pygargus | 4 | 2 | 50.0 | |
| River deer/Hydropotes inermis | 11 | 9 | 81.8 | |
| Addax/Addax nasomaculatus | 3 | 3 | 100.0 | |
| Giant eland/Tragelaphus derbianus | 7 | 3 | 42.9 | |
| Alpaca/Vicugna pacos | 24 | 3 | 12.5 | |
| Red deer/Cervus canadensis | 18 | 7 | 38.9 | |
| Yak/Bos grunniens domesticus | 5 | 3 | 60.0 | |
| Fallow deer/Dama dama | 13 | 4 | 30.8 | |
| Blue sheep/Pseudois nayaur | 5 | 3 | 60.0 | |
| Sika deer/Cervus nippon | 18 | 2 | 11.1 | |
| Big-eared sheep/Capra hircus | 6 | 2 | 33.3 | |
| Perissodactyla | Zebra/Equus quagga | 23 | 2 | 8.7 |
| Primates | Golden monkey/ Rhinopithecus | 5 | 2 | 40.0 |
| Mandrill/Mandrillus sphinx | 9 | 7 | 77.8 | |
| Ring-tailed lemur/ Lemur catta | 21 | 11 | 52.4 | |
| Gibbon/Hylobatidae | 10 | 2 | 20.0 | |
| Baboon/Papio | 1 | 1 | 100.0 | |
| Spider monkey/Ateles | 1 | 1 | 100.0 | |
| Black langur/ Trachypithecus francoisi | 2 | 1 | 50.0 | |
| Chimp/Pan troglodytes | 9 | 1 | 11.1 | |
| Patas monkey/Erythrocebus patas | 3 | 1 | 33.3 | |
| Macaque/Macaca mulatta | 10 | 3 | 30.0 | |
| Carnivora | Ocelot/Prionailurus bengalensis | 13 | 1 | 7.7 |
| Manchurian tiger /Panthera tigris ssp. altaica | 6 | 1 | 16.7 | |
| Gray wolf/Canis lupus | 16 | 1 | 6.3 | |
| Avian | Cassowary/Casuarius unappendiculatus | 1 | 1 | 100.0 |
| Gene Subtypes (Population Size) | Host (Population Size) |
|---|---|
| ST1 (16) | Mandrill (7), ring-tailed lemur (4), cassowary (1), gibbon (1), spider monkey (1), chimp (1), patas monkey (1) |
| ST2 (6) | Ring-tailed lemur (1), baboon (1), gibbon (1), macaque (3) |
| ST3 (3) | Asian elephant (1), patas monkey (1), gray wolf (1) |
| ST4 (3) | Ring-tailed lemur (3) |
| ST5 (21) | Milk goat (1), Siberian ibex (1), Ammotragus (1), argali (2), river deer (3), ocelot (1), ring-tailed lemur (1), zebra (2), yak (3), fallow deer (1), blue sheep (3), red deer (1) |
| ST8 (3) | Camel (1), golden monkey (1), ring-tailed lemur (1) |
| ST10 (31) | Camel (2), giraffe (2), Siberian ibex (1), Ammotragus (1), argali (4), roe deer (1), river deer (1), addax (3), giant eland (3), fallow deer (3), alpaca (1), sika deer (2), big-eared sheep (2), red deer (5) |
| ST13 (2) | Golden monkey (1), black langur (1) |
| ST14 (12) | Siberian ibex (3), Ammotragus (1), roe deer (1), river deer (5), alpaca (2) |
| Unknown ST (1) | Red deer (1) |
| Locations | Sample Size | Infection Rate (%) (Positive Samples/Total Samples) | ||||
|---|---|---|---|---|---|---|
| Total | Cryptosporidium spp. | E. bieneusi | G. duodenalis | Blastocystis spp. | ||
| Guiyang | 49 | 55.1 (27/49) | 2.0 (1/49) | 12.2 (6/49) | 16.3 (8/49) | 24.5 (12/49) |
| Beijing | 101 | 53.5 (54/101) | 1.0 (1/101) | 6.9 (7/101) | 9.9 (10/101) | 35.6 (36/101) |
| Shijiazhuang | 69 | 36.2 (25/69) | 0 (0/69) | 11.6 (8/69) | 2.9 (2/69) | 20.3 (14/69) |
| Tangshan | 66 | 19.8 (13/66) | 0 (0/66) | 6.1 (4/66) | 4.5 (3/66) | 10.6 (7/66) |
| Xingtai | 115 | 51.3 (59/115) | 0 (0/115) | 25.4 (29/115) | 0.9 (1/115) | 25.2 (29/115) |
| Total | 400 | 44.5 (178/400) | 0.5 (2/400) | 13.5 (54/400) | 6 (24/400) | 24.5 (98/400) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, D.; Jiang, T.; Zhang, C.; Ma, L.; Jia, T.; Pei, Y.; Zhu, Z.; Liu, Q.; Liu, J. Epidemiology and Molecular Characterization of Zoonotic Gastrointestinal Protozoal Infection in Zoo Animals in China. Animals 2024, 14, 853. https://doi.org/10.3390/ani14060853
An D, Jiang T, Zhang C, Ma L, Jia T, Pei Y, Zhu Z, Liu Q, Liu J. Epidemiology and Molecular Characterization of Zoonotic Gastrointestinal Protozoal Infection in Zoo Animals in China. Animals. 2024; 14(6):853. https://doi.org/10.3390/ani14060853
Chicago/Turabian StyleAn, Diya, Tingting Jiang, Changsheng Zhang, Lei Ma, Ting Jia, Yanqun Pei, Zifu Zhu, Qun Liu, and Jing Liu. 2024. "Epidemiology and Molecular Characterization of Zoonotic Gastrointestinal Protozoal Infection in Zoo Animals in China" Animals 14, no. 6: 853. https://doi.org/10.3390/ani14060853
APA StyleAn, D., Jiang, T., Zhang, C., Ma, L., Jia, T., Pei, Y., Zhu, Z., Liu, Q., & Liu, J. (2024). Epidemiology and Molecular Characterization of Zoonotic Gastrointestinal Protozoal Infection in Zoo Animals in China. Animals, 14(6), 853. https://doi.org/10.3390/ani14060853





