1. Introduction
Eggs are an important source of quality protein for the human diet. However, eggs are high in cholesterol and saturated fatty acids (SFAs), and it is claimed that these contribute to coronary heart disease [
1]. For this reason, numerous studies have been carried out on reducing the cholesterol and SFA content of egg yolk and enriching omega-3 FA by manipulating the diets of laying hens [
2].
It is well known that omega-3 FAs, especially alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), have many benefits for human health, including preventing cardiovascular diseases [
3] and improving immune functions and fertility [
4], as well as anti-inflammatory, antitumor, and antiviral properties [
5]. Eggs contain low levels of n-3 FA, but the FA profile of egg yolk fats could be improved by adding natural sources of n-3 FA to the diets of hens. Previous reports have shown that table eggs can be enriched with n-3 FA by adding flax seeds [
2,
6,
7], hemp seeds [
8,
9,
10,
11], or hemp seed cake [
11] to the diet.
The significant increase in hemp seed production in European countries [
12,
13,
14] and the valuable nutritional content create opportunities to use hemp seeds as a valuable ingredient in animal feed. Whole hemp seeds contain approximately 25% crude protein (CP), 33–35% fat, 34% carbohydrates, crude fiber, vitamins, minerals, and functional components [
8,
11,
15]. Hemp seed fats are high in PUFAs (78.61% of total FAs), including linoleic acid (54.80%) and α-linolenic acid (18.63%) [
15]. The main reason why hemp seeds could be used in hens’ feed is the relatively high level of ALA (18–19%) [
15], surpassed only by flax seeds (55–57%) [
6]. The introduction of 8% hemp seeds in the diets of laying hens led to enriched omega-3 FAs in egg yolks, specifically ALA, EPA, and DHA [
11]. In addition, studies by Neijat et al. [
16] proved that introducing hemp seeds into hens’ feed, even at high levels (30%), had no negative effects on egg production, general egg sensory qualities, or bird health.
The disadvantage of enriching eggs with omega-3 FAs is increased susceptibility to lipid peroxidation in the yolks, which would affect the nutritional and sensory quality of the eggs, as well as consumer safety. Increasing the PUFA content in the diets of laying hens by using various oil seeds and vegetable oils was found to increase the incidence of liver hemorrhage [
17], probably due to the oxidative rancidity of unsaturated FA. Additionally, eggs enriched in omega-3 FAs have been noted to have a fishy odor, which originates from the oxidation of PUFAs in the yolks [
18]. Therefore, it is necessary to enrich eggs with antioxidant compounds (vitamin E, carotenoids, and phenols) along with PUFA enrichment to reduce the oxidation of unsaturated FAs and provide a good source of antioxidants to consumers [
19,
20].
An important, still little-studied source of natural antioxidants is fruit pomace. Following the processing of fruit by squeezing or crushing to obtain the juice, pomace results as a by-product. Fruit pomace contains parts of the pulp, peel, and seeds and represents 20–40% of the processed fruit mass [
21]. Annually, more than 500 million tons of fruit pomace is produced globally [
22], for which no sustainable unified management strategy has been developed. Adding these by-products to the diets of laying hens would reduce the need for synthetic antioxidants, which can have a negative impact on the health of both poultry and humans, and it could contribute to enriching the natural antioxidants in eggs, which would have a positive effect on the perception and acceptance of eggs by consumers [
23]. This sustainable agri-food system promotes the from-farm-to-fork strategy, which is designed to build a fair, healthy, and environmentally friendly food system. In addition, the utilization of fruit pomace in poultry feed fits perfectly into the contemporary concept of a circular bioeconomy and, at the same time, represents a strategy for environmental protection and the sustainable development of poultry production [
21].
Berries, such as blackcurrants and rosehips, are rich in essential fatty acids, polyphenols, tocopherols, carotenes, and vitamin C, hence their high antioxidant capacity. The pulp that remains as a by-product after juicing is still a good source of bioactive compounds (phenols, vitamins, provitamins, and essential fatty acids) [
24], and it could be used in the diets of laying hens to naturally improve the nutritional quality of table eggs [
22]. In Romania, significant amounts of by-products obtained from the processing of forest fruits result annually. According to INS data (National Institute of Statistics), in 2022 in Romania, over 5000 ha was cultivated with forest fruits (blackberries, currants, blueberries, raspberries, and strawberries), and over 9000 tons of forest fruits was harvested from spontaneous flora and 4000 tons of rosehips [
25].
Loetscher et al. [
26] reported that adding 25 g/kg of dried and ground rosehip fruit to feed significantly slowed the process of lipid oxidation in the meat of broiler chickens. Similarly, Grigorova et al. [
27] found that, by adding rosehips to the feed of laying hens (0.5%), the color of the yolk improved significantly, and the level of malondialdehyde (MDA, a marker of lipid peroxidation) in the yolk decreased significantly during storage in a refrigerator or at room temperature for 30 days. Similar results were reported by Vlaicu et al. [
6], who supplemented the diets of laying hens with 30 g/kg of dried rosehips. The antioxidant effects of blackcurrant pomace were demonstrated in a study on turkeys [
28].
The limited number of studies that tested the addition of dried fruit pomace to the diets of laying hens [
29,
30], but especially the lack of studies using dried blackcurrant (DB) or dried rosehip (DR) pomace as a source of natural antioxidants in PUFA-enriched feed for laying hens, prompted the authors to conduct this study. The purpose of this study was to evaluate the effects of including hemp seeds (8%) alone or with dried fruit pomace (DB or DR) (3%) in the diets of layer hens on the hens’ performance, as well as the cholesterol, fatty acid profile, antioxidant content, and lipid oxidative status of the yolks of fresh or stored eggs (refrigerated at 4 °C for 28 days). In this experiment, we evaluated the hypothesis that the simultaneous inclusion of hemp seeds and dried fruit pomace in the diets of laying hens increases the concentration of PUFA and natural antioxidants in the yolk and improves the oxidative stability of the egg yolk without affecting egg production.
2. Materials and Methods
2.1. Ethical Approval
The experimental protocol was approved by the Ethics Committee of the University of Oradea and complied with the legislative regulations (Law 206/2004, Directive 2010/63/EU, Law 43/2014) regarding the use of animals for scientific purposes.
2.2. Experimental Materials
The hemp seeds (Jubileu variety) used in this study came from a culture approved for the production of seeds intended for obtaining commercial vegetable oil. Dried blackcurrant (DB) and dried rosehip (DR) pomace were produced by pressing (Bucher HPX presse; Bücher-Unipektin, Niederweningen, Switzerland) at a commercial fruit processing factory in Zalău (Sălaj county, Romania). Immediately after the pomace was obtained, it was dried in a convection oven at 60 °C and then ground using a universal hammer mill with a 1 mm mesh. The dried fruit pomace was kept under vacuum in dark-colored foil bags until use.
Before being added to the diets of laying hens, the hemp seeds and dried fruit pomace (DB and DR) were analyzed for their proximate composition (dry matter (DM), crude protein (CP), ether extract (EE), neutral detergent fiber (NDF), and acid detergent fiber (ADF)) and antioxidant content (α-tocopherol, β-carotene, and total phenols).
2.3. Experimental Design
An 8-week experiment was conducted with 128 Tetra SL laying hens 35 weeks of age (initial body weight: 1694.2 ± 87.63 g), which were purchased from a commercial farm (SC Rosbro Avicom SRL, Bihor, Romania). They were divided into 4 homogeneous groups of 32 hens each (8 replicates/group with 4 hens/replicate). Each group of hens was randomly assigned to 1 of 4 experimental diets (treatments): a standard diet for laying hens based on corn, wheat, and soybean meal (control diet, C); standard diet containing 8% ground hemp seed (H); hemp seed diet containing 3% dried blackcurrant pomace (HB); and hemp seed diet containing 3% dried rosehip pomace (HR). Application doses of 3% for the dried fruit pomace were established in accordance with previous studies that demonstrated that the optimal dose of sources of natural antioxidants (tomato waste, dehydrated carrots, rosehip meal, dehydrated sea buckthorn pomace, and dehydrated kapia peppers) incorporated into the diet of laying hens is 2–3% [
6,
7]. In addition, Konca et al. [
30] reported that rosehips can act as a pro-oxidant at high concentrations of 5% in the diet of laying quail.
The hens were raised in a shelter equipped with Zucami three-tier metallic cages (60 cm width × 60 cm length × 40 cm height) at a density of 4 hens/cage (900 cm2/hen) and a controlled microclimate (temperature: 20–22 °C; humidity 65–68%), which allowed food intake and egg production to be recorded separately for each replicate (cage). Access to food and water was provided ad libitum. Before the start of the experiment, the hens were adapted to their cages and experimental diets for a period of 2 weeks.
Feed was offered once a day at 8:00 a.m., separately for each replicate, in an amount limited to 120 g/hen (400 g/replicate), to reduce feed selection behavior so that the hens would consume almost all food provided. Throughout the experiment, the hens had a schedule of 16 h of light and 8 h of darkness.
2.4. Dietary Treatments
Appropriate software (HYBRIMIN
® Futter 5) was used to formulate the diets (
Table 1) according to the feeding requirements of laying hens [
31]. In contrast to diet C, experimental diet H included 8% hemp seeds as a source of PUFAs, while an additional 3% dried blackcurrant pomace was present in diet HB and 3% dried rosehip pomace was present in diet HR, as sources of natural antioxidants. All diets were isocaloric and isonitrogenous, containing 17.5% CP and 2750 kcal/kg metabolizable energy (ME). The ME of the diets was adjusted using sunflower oil.
For good homogenization, whole (intact) hemp seeds were first mixed with wheat grains and then ground before formulating the diets [
16]. The diets were stored during the experiment in labeled bags in a cool and dry room.
2.5. Performance Parameters
Egg production was recorded daily, and egg weight was determined 3 times per week. Recordings were made for each replicate. Egg mass was calculated based on laying rate and egg weight. Feed consumption was measured each week by weighing the feed at the beginning and end of the period.
2.6. Egg Sampling
To determine the physical characteristics of the eggs, 16 eggs/treatment (2 eggs/replicate) were collected twice during the experiment (weeks 6 and 8 of the experimental period). After weighing, the eggs were broken, and the weights of their components were determined; based on these, the percentages of white, yolk, and shell were calculated. Eggshells from the same eggs were washed and left at room temperature for 2 days, after which they were weighed using an electronic scale (Mettler-Toledo LLC, Columbus, OH, USA).
In the last week of the experiment, a total of 256 eggs were taken, 64 eggs for each dietary treatment (2 eggs/replicate × 8 replicates × 4 days); 128 of these eggs (32/treatment) were processed and analyzed as fresh eggs, and 128 eggs (32/treatment) were stored in a refrigerator at 4 °C for 28 days. The eggs were broken, and the yolks were separated. The containers with yolk samples were wrapped with aluminum foil to protect them from light and were frozen at −80 °C until the laboratory analysis of the FA profile, antioxidant content (α-tocopherol, retinol, β-carotene, and total phenols), and lipid oxidative status (antioxidant capacity and MDA concentration) of the yolks.
Yolk pigmentation and cholesterol content were determined in 24 eggs per treatment (3 eggs per replicate), recorded at the beginning of the last week of the experimental period. The yolk color was determined using the Roché scale (1–15 points).
2.7. Chemical Analyses
2.7.1. Proximate Chemical Composition of Feed
Samples of the hemp seeds, the dried blackcurrant pomace, the dried rosehip pomace, and each experimental diet (150 g) were analyzed for DM following the gravimetric method [
33], CP following the Kjeldahl method (N × 6.25, Kjeltec Auto 1030 Analyzer; Foss Tecator AB, Höganäs, Sweden), EE by petroleum ether extraction using method 920.39 (SOXTHERM, C. Gerhardt GmbH, Königswinter, Germany) [
33], and NDF and ADF by means of the method described by Van Soest et al. [
34] using an ANKOM 220 analyzer (ANKOM Technology, Fairport, NY, USA). All determinations were performed in triplicate.
2.7.2. Yolk Cholesterol Content
The cholesterol in yolk samples was determined using a Perkin-Elmer gas chromatograph (Shelton, MA, USA) according to AOAC [
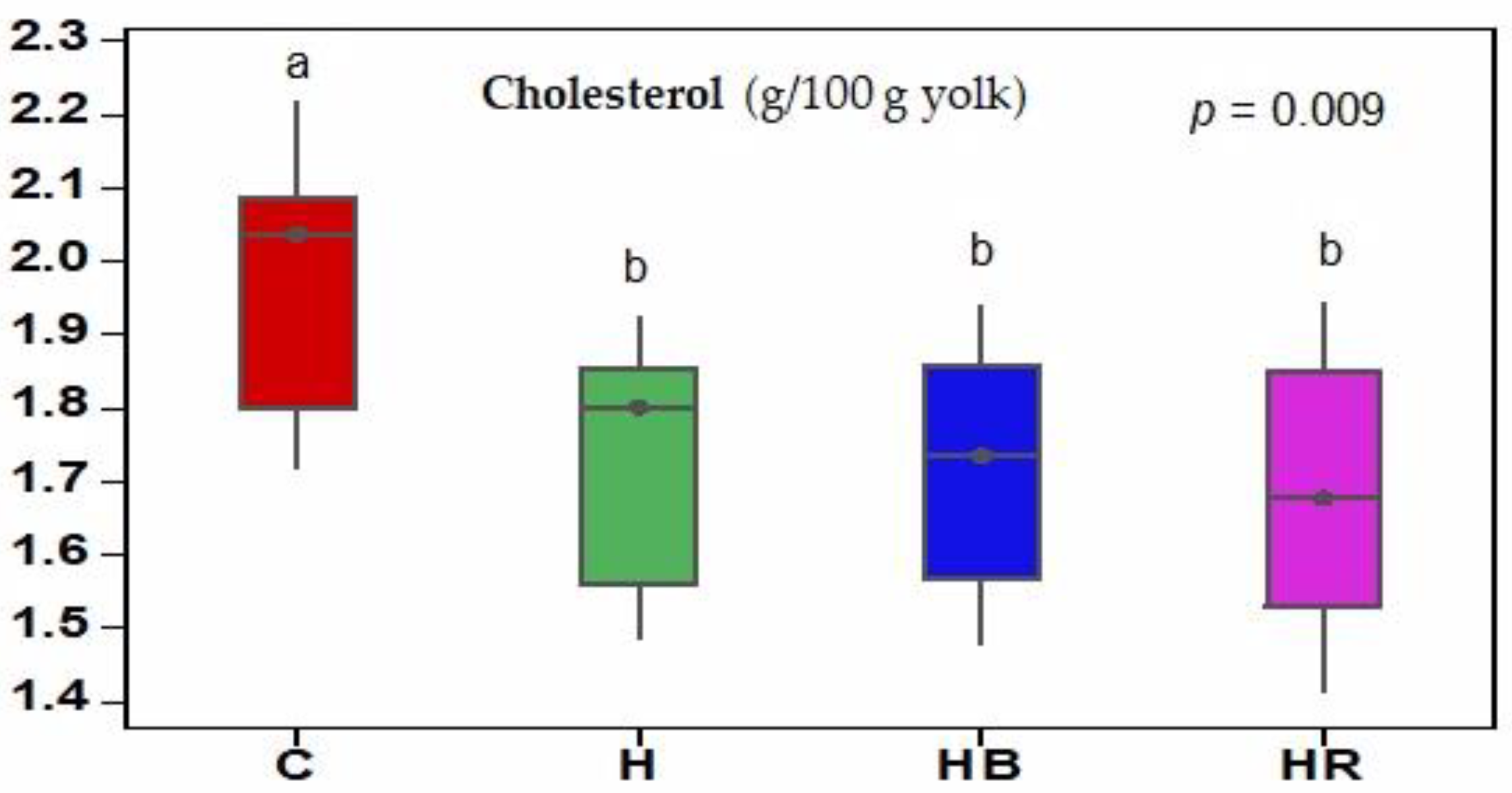
35]. Dried yolk samples (65 °C) were saponified with methanolic KOH solution (50 mL) in a water bath for 1 h. Next, the samples were treated with petroleum ether, concentrated on a rotary evaporator, and brought to neutral pH with distilled water. After removing the petroleum ether, the residue was treated with chloroform (5 mL). Aliquots of 1 μL of the obtained extracts were injected into an HP-5 GC fused silica capillary column (30 m × 0.32 mm ID, 0.1 µm film thickness; J&W GC Columns, Agilent, Santa Clara, CA, USA) and analyzed on a detector with flame ionization (FID). Cholesterol was identified by comparing the peak areas with those obtained from the laboratory standard solution. The cholesterol concentration is expressed as g/100 g yolk.
2.7.3. Feed and Egg Yolk Fatty Acid Analysis
To determine the FA composition of the feed and egg yolk, we used standard fatty acid methyl ester (FAME) gas chromatography techniques [
33]. The fat was extracted with petroleum ether and stored at −20 °C in Eppendorf tubes until laboratory analysis. In the first step, the extracted fat (100 mg) was saponified with 2N KOH (100 μL) and hexane (3 mL). After vigorous shaking for 1 min, the mixture was centrifuged for 5 min at 5000 rpm. A chromatographic analysis was performed with a gas chromatograph (Shimadzu GC-2010 Plus, Tokyo, Japan) equipped with FID and an HP-88 column (100 m long, 0.25 mm diameter, and 0.20 μm film thickness). Helium was the carrier gas (2 mL/min), and the split rate was 1:50. The injector temperature was 240 °C. The temperature program for the oven was set at 100 °C for 1 min, then 100 to 170 °C at 6.5 °C/min, 170 to 220 °C for 10 min at 3 °C/min, and finally 230 °C for 5 min. Each FA was identified using external standards (Supelco 37 Component FAME mix; Supelco Inc., Bellefonte, PA, USA) by comparing retention times. The results are expressed as % FA of total FA.
2.7.4. Determination of Antioxidant Compounds
To determine the contents of retinol and α-tocopherol in the feed and yolk samples, the method described in EC Regulation [
36] was used, using a high-performance liquid chromatograph (HPLC) equipped with a PDA-UV detector (Finnigan Surveyor Plus, Thermo Scientific, Waltham, MA, USA) at 325 nm for retinol and 292 nm for tocopherol. A HyperSil BDS C18 column with a silica gel size of 250 × 4.6 nm and particle size of 5 µm (Thermo Scientific, Waltham, MA, USA) was used. The mobile phase was methanol–water (96% methanol and 4% ultrapure water) at a flow rate of 1.5 mL/min.
For the determination of carotene, an HPLC equipped with a PDA-UV detector (Finnigan Surveyor Plus, Thermo Scientific, Waltham, MA, USA) at a wavelength of 450 nm and a C18 column (250 × 4.60 nm, particle size 5 µm) (Nucleodur, Macherey-Nagel, Düren, Germany) was used. The mobile phase was 100% acetone at a flow rate of 0.8 mL/min.
Total phenolic content (TPC) was measured spectrophotometrically according to the Folin–Ciocalteu method as described by Velioglu et al. [
37]. Briefly, samples of methanolic extract (0.1 mL) were mixed with Folin–Ciocalteu reagent (0.1 mL) and distilled water (0.8 mL), and then they were homogenized and incubated for 5 min at room temperature. Next, 0.5 mL sodium carbonate solution (20%) was added and incubated at room temperature for 30 min. The samples were stored for 1 h in the dark, and then the absorbance at 750 nm was measured. Gallic acid was used as a standard to obtain a calibration curve, and the results are expressed in milligrams per gallic acid equivalent (mg GAE/g).
2.7.5. Lipid Oxidative Status of the Yolk
The antioxidant activity was determined by the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) method, as described by Gaffney et al. [
38]. Briefly, the ABTS radical cation (ABTS
•+) was obtained by mixing ABTS solution (7.0 mmol/L) with potassium persulfate (2.45 mmol/L) at a ratio of 1:1 (ABTS:potassium persulfate). The mixture was kept in the dark at room temperature for 15 h and diluted with ethanol to an absorbance of 0.7 ± 0.05. The homogenized yolk samples were treated with the prepared mixture, and, after 30 min of storage at room temperature, the absorbance of the mixture was measured at 734 nm. The results are expressed as μmol Trolox equivalent (TE)/g egg yolk.
The oxidative stability of the yolk was evaluated based on thiobarbituric acid reactive substances (TBARSs), using the method described by Mierlita [
11]. Briefly, egg yolk was mixed with trichloroacetic acid and centrifuged at 5500×
g at 4 °C for 15 min. The supernatant was mixed with a thiobarbituric acid solution (pH 2.5), after which the tubes were placed in a water bath (90 °C) for 30 min. After cooling, distilled water was added, and the mixture was centrifuged again. The colored product formed by the reaction of lipid peroxidation products with thiobarbituric acid was measured spectrophotometrically at 534 nm. To calculate the concentration of malondialdehyde (µg MDA/g yolk), the values obtained were compared with the standard curve prepared by using standard MDA tetrabutylammonium salt (Sigma-Aldrich, Buchs, Switzerland).
2.8. Estimation of Health-Related Lipid Quality Indices
Based on the fatty acid composition of the egg yolk, health indices and fatty acid metabolism indices were calculated using appropriate equations, as they were previously validated in other reports [
2,
6,
15,
39].
2.9. Statistical Analysis
A one-way analysis of variance (ANOVA) using PROC GLM in SAS [
40] for a completely randomized design was performed to determine the effects of the dietary treatments on the performance of the laying hens and the quality traits, FA profile, antioxidant content, and lipid oxidative status of the egg yolks. The data on the laying performance of the hens and the quality of the eggs were tested only for the effect of diet, and the other data were tested for the type of diet (C, H, HB, or HR) and type of eggs (fresh or stored). The significance between individual means was identified using Tukey’s multiple range test. The final data are provided as mean values ± standard error of the mean (SEM), with a significance level of
p < 0.05.
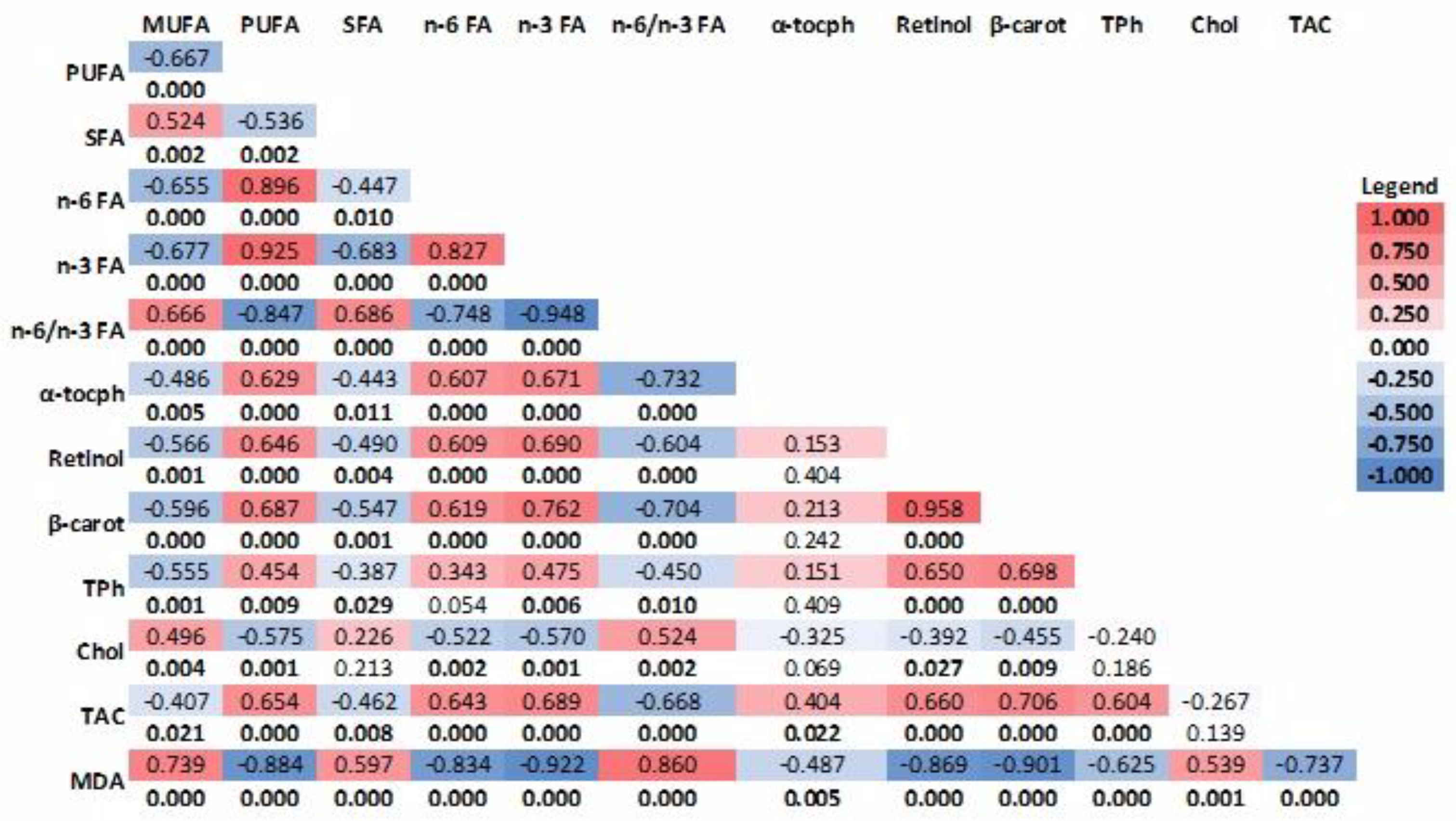
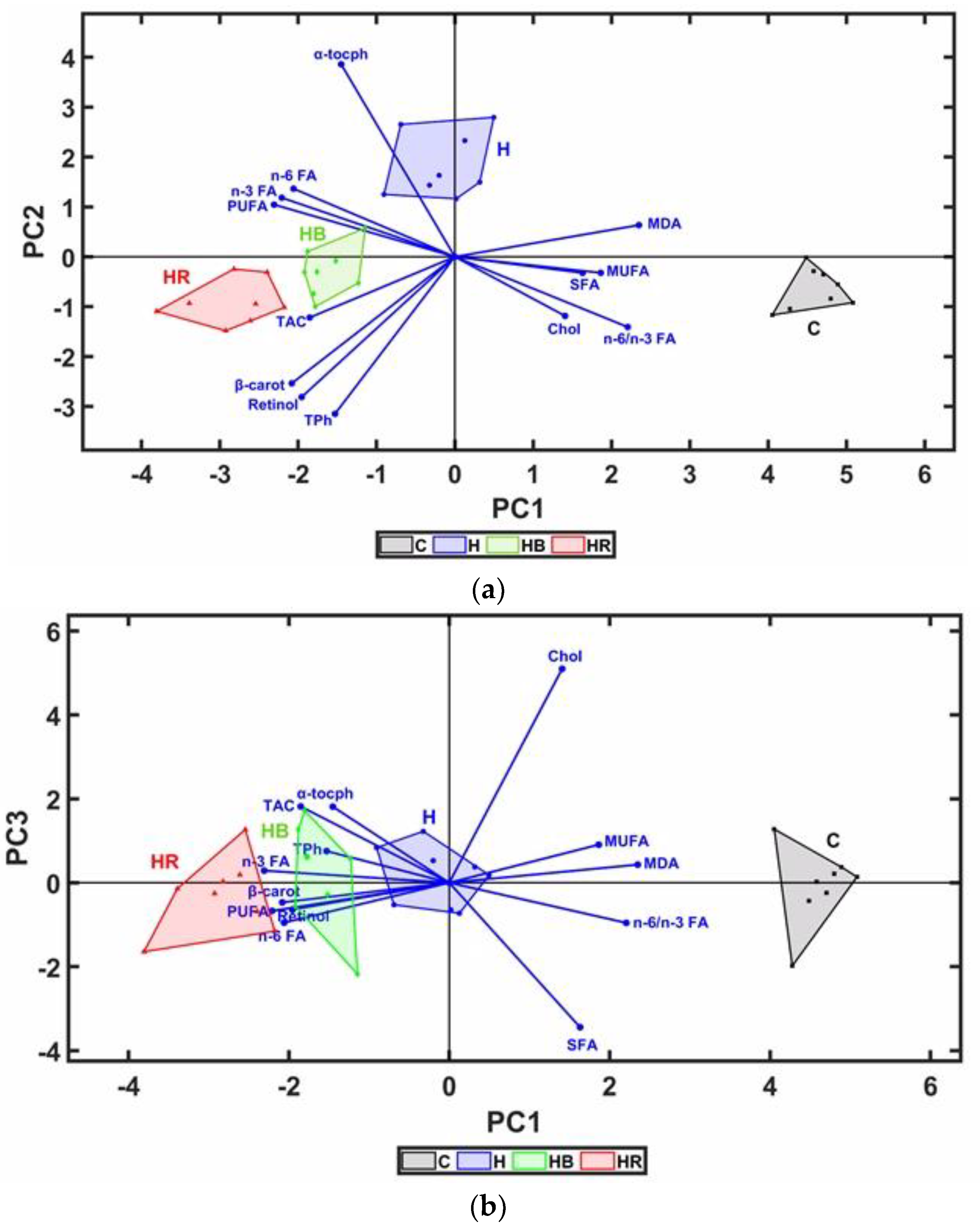
Correlations between variables and the Kruskal–Wallis (p = 0.05) non-parametric test followed by multiple pairwise comparisons (Dunn test, p = 0.05) were calculated with Stata 17.0 SE Standard Edition (StataCorp LLC, StataCorp, 4905 Lakeway Drive, College Station, TX, USA). A principal component analysis (PCA) was computed with a custom-made program developed in MATLAB 2023a 9.14.0 CWL (The MathWorks Inc., 1 Apple Hill Drive, Natick, MA, USA).