Immunophysiological State of Dogs According to the Immunoregulatory Index of Their Blood and Spleens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Condition, Research Ethics and Procedures
2.2. Statistical Analyses of Data
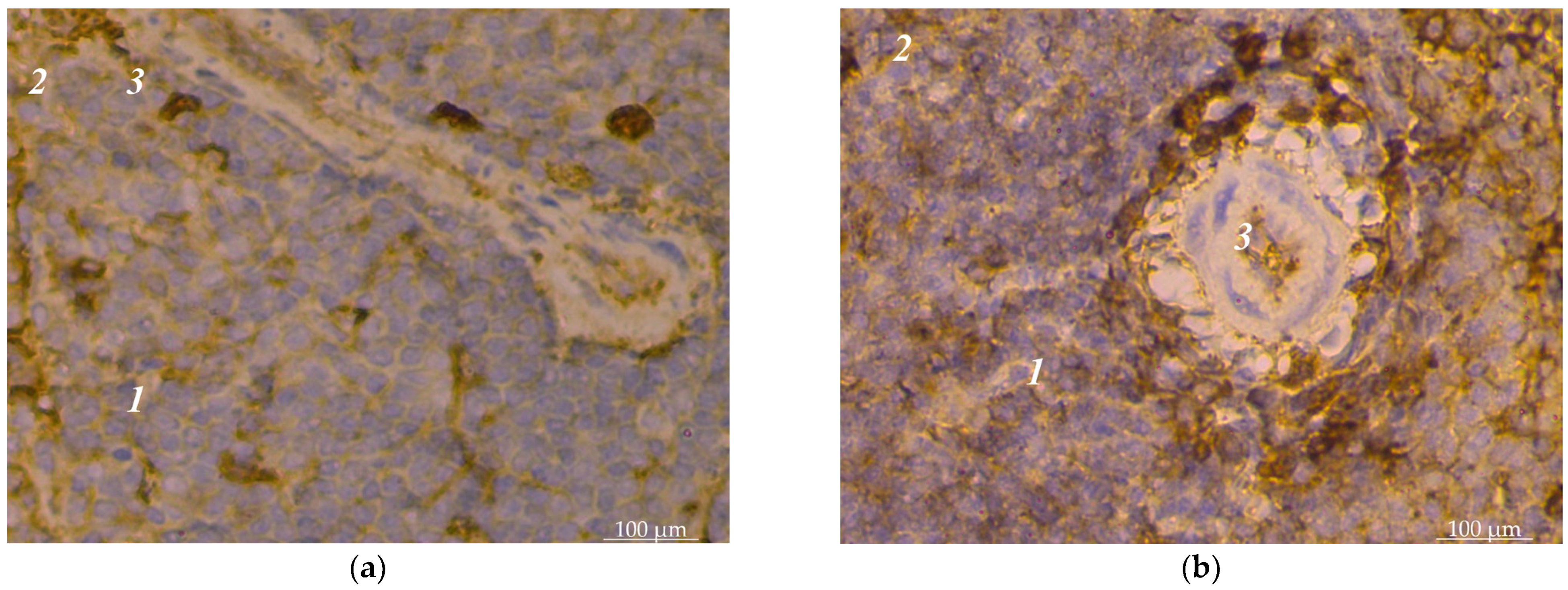
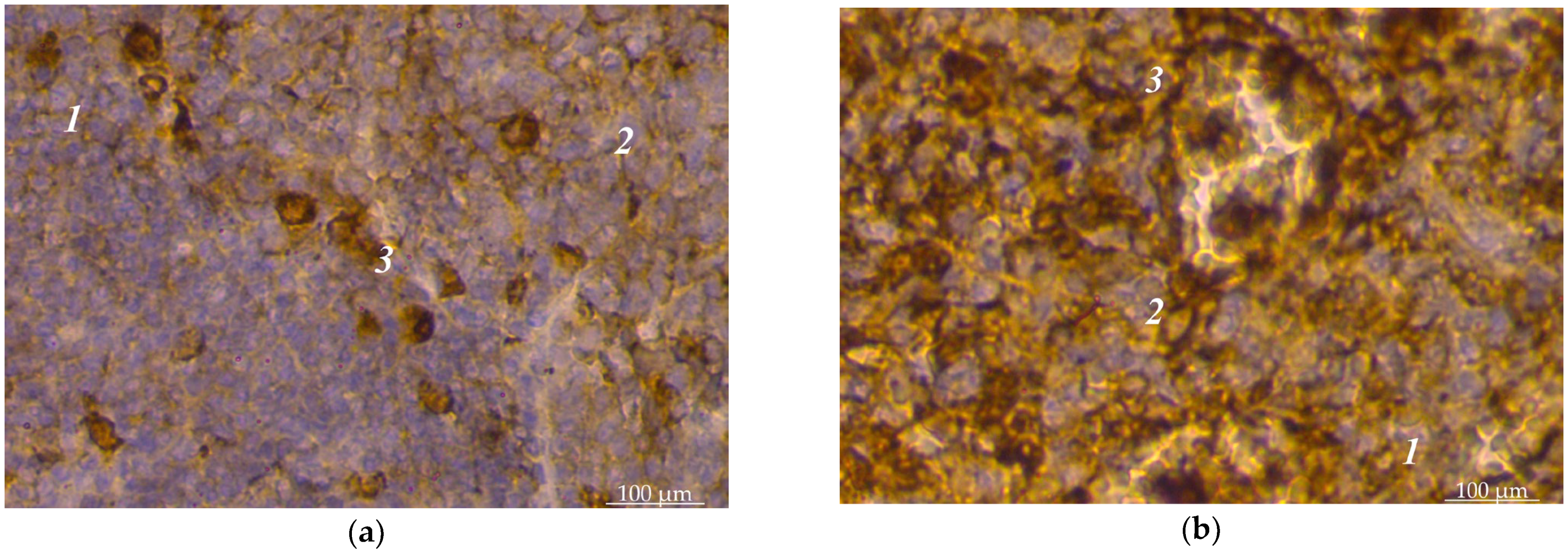
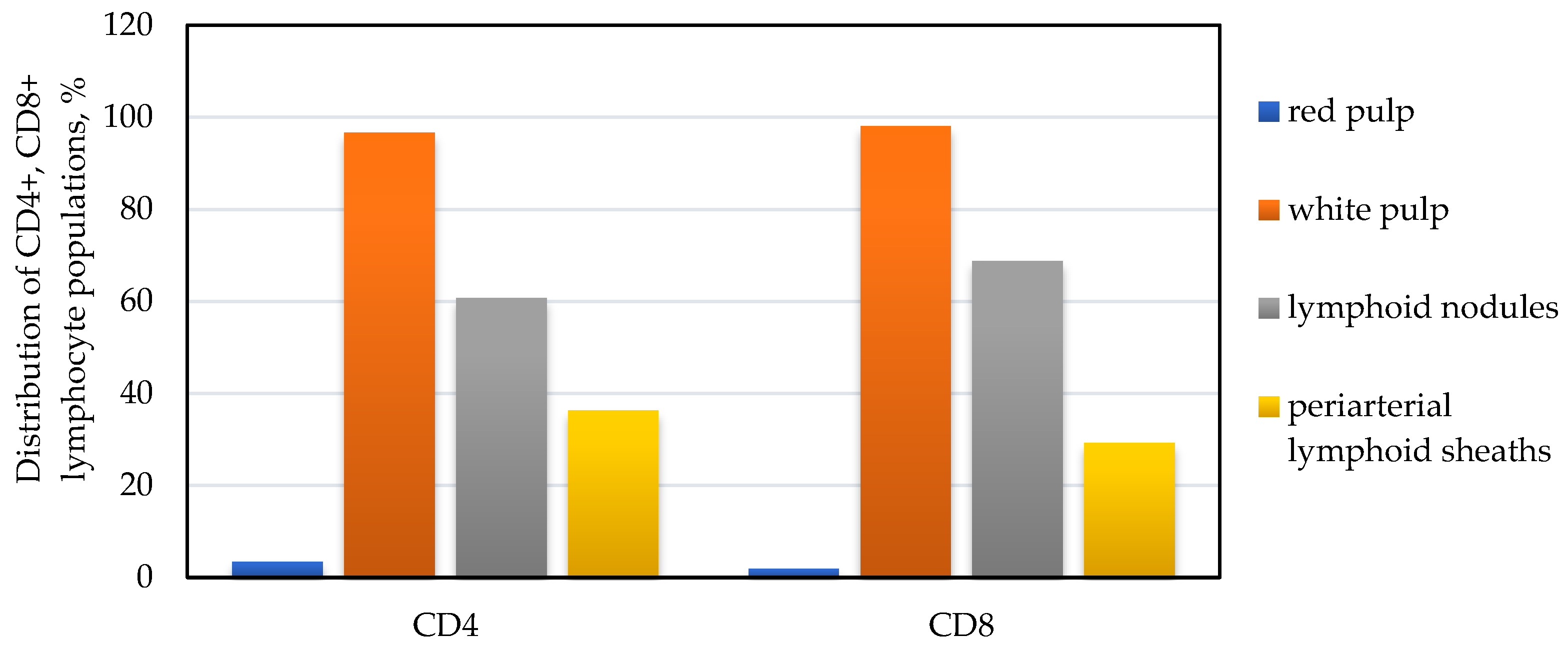
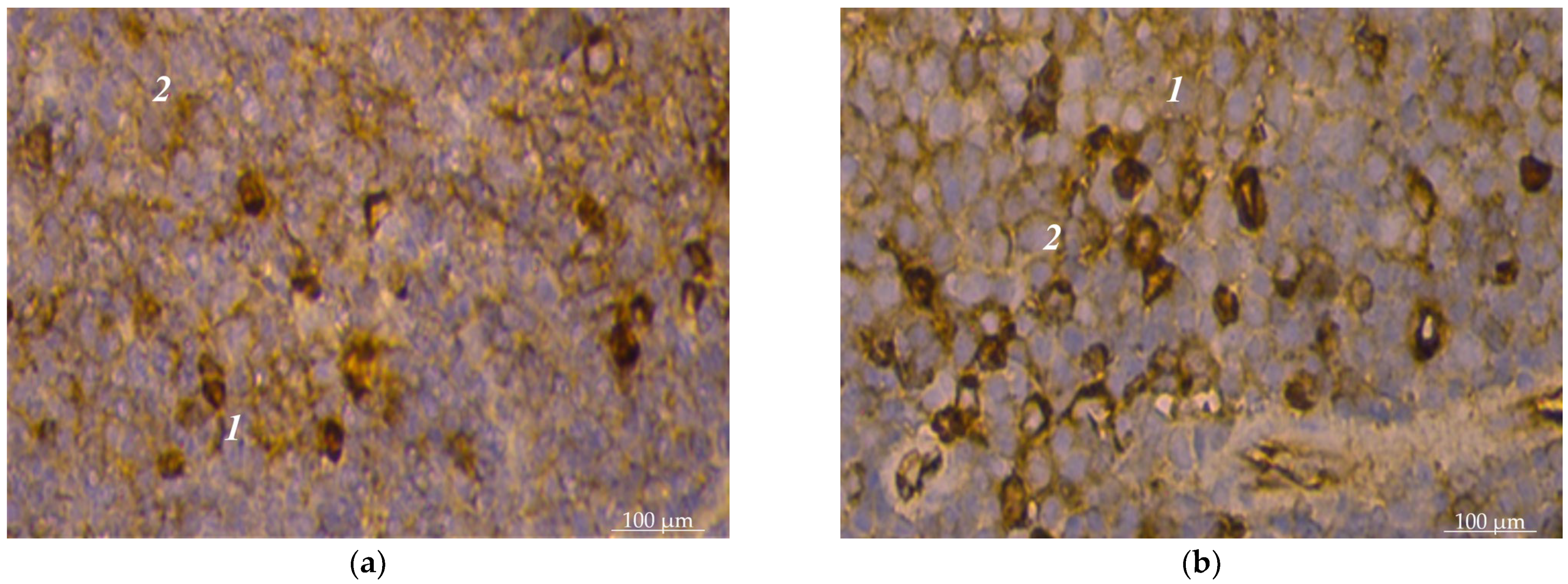
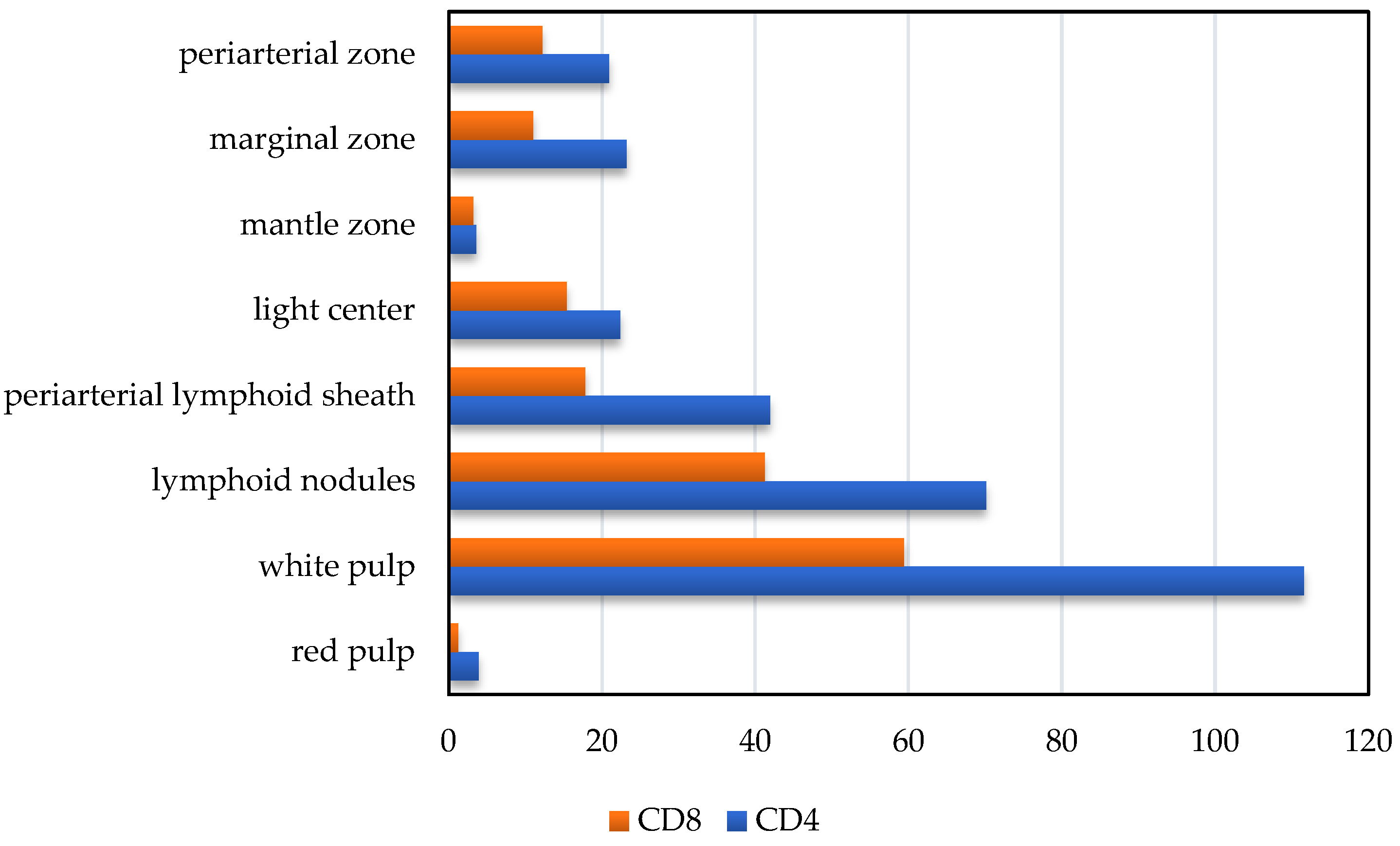
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konovalova, T.S. Indicators of the immunological status in maies with acne vulgaris. Ukr. J. Dermatol. Venereol. Cosmetol. 2014, 2, 42–52. [Google Scholar]
- Wężyk, D.; Romanczuk, K.; Rodo, A.; Kavalevich, D.; Bajer, A. Haematological indices and immune response profiles in dogs naturally infected and co-infected with Dirofilaria repens and Babesia canis. Sci. Rep. 2023, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Hrushko, V.V. Indicators of humoral and cellular immunity in athletes of high professional skill after the competitive period. Immunol. Allergol. Sci. Pract. 2018, 3, 48–52. [Google Scholar]
- Dunaeva, I.P.; Chernyavskaya, I.V.; Dorosh, A.G.; Karachentsev, Y.I. Association of circulating adiponectin level with immunological indices and peripheral blood coagulation properties in type 2 diabetic patients with non-alcoholic fatty liver disease. Endokrynologia 2017, 22, 37–44. [Google Scholar]
- Borisova, I.S. The prognosis of pneumonia in patients with impaired immunity against the background of oncohematological pathology. Fam. Med. 2017, 6, 38–44. [Google Scholar] [CrossRef]
- Goltsev, A.N.; Bondarovich, N.A.; Kuznyakov, A.V.; Ostankov, M.V.; Ostankova, L.V.; Chelombit’ko, O.V. Determination of Immunity T-cell Link State and Content of Cancer Stem Cells as Criterion to Estimate Efficiency of Preventive Breast Cancer Therapy with Cryopreserved Fetal Liver Cells. Probl. Cryobiol. Cryomedicine 2014, 24, 238–248. [Google Scholar] [CrossRef]
- Koval, H.; Lutsenko, O.; Bondarovych, M.; Ostankov, M.; Goltsev, A. The Role of Cord Blood in the Regulation of the Cellular and Humoral Link of Immunity in Experimental Atopic Dermatitis. Innov. Biosyst. Bioeng. 2021, 5, 167–177. [Google Scholar] [CrossRef]
- Bibyk, V.V. Study of the state of the cellular link of immunity in women with cervical intraepithelial neoplasia of moderate severity at the first level of medical care. Ukr. Med. Alm. 2013, 16, 22–25. [Google Scholar]
- Horalskyi, L.P.; Ragulya, M.R.; Glukhova, N.M.; Sokulskiy, I.M.; Kolesnik, N.L.; Dunaievska, O.F.; Gutyj, B.V.; Goralska, I.Y. Morphology and specifics of morphometry of lungs and myocardium of heart ventricles of cattle, sheep and horses. Regul. Mech. Biosyst. 2022, 13, 53–59. [Google Scholar] [CrossRef]
- Marshall, G.D., Jr. The adverse effects of psychological stress on immunoregulatory balance: Applications to human inflammatory diseases. Immunol. Allergy Clin. North Am. 2011, 31, 133–140. [Google Scholar] [CrossRef]
- Broshkov, M.M. Dynamic of indicators cellular and humoral immunity in puppies depending on the degree of the body stressing. Sci. Messenger LNU Vet. Med. Biotechnol. Ser. Vet. Sci. 2014, 16, 41–46. [Google Scholar]
- Dunaeva, I.P.; Dorosh, O.G.; Karachentsev, Y.I. Nutrient correction in type 2 diabetes patients with non-alcoholic fatty liver disease. Wschod. Czas. Nauk. (East Eur. Sci. J.) 2016, 6, 41–53. [Google Scholar]
- Stepanov, Y.M.; Filippova, O.Y. The features of the changes of T- and B-cell immunity depending on the body mass index in the patients with the nonalcoholic fatty liver disease in combination with the obesity and the pathology of biliary tract. Ukr. Ther. J. 2016, 4, 46–54. [Google Scholar]
- Sokolenko, V.L. Impact of emotional stress on the immune system indices among residents of radiation contaminated areas. Fiziol. Zhurnal 2016, 62, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Sokolenko, M.A.; Moskaliuk, V.D.; Sokolenko, A.A. The influence of herpes viruses on immune status of patients not infected with HIV. Bukovinian Med. Her. 2016, 20, 161–165. [Google Scholar]
- Goncharenko, O.Y.; Belikova, M.V. In the state of immune resistance of an organism of people with different physical training. Fiziol. Zhurnal 2020, 66, 83–88. [Google Scholar] [CrossRef]
- Guzman, E.; Montoya, M. Contributions of Farm Animals to Immunology. Front. Vet. Sci. 2018, 5, 307. [Google Scholar] [CrossRef]
- Davies, G.; Gorman, R.; Greenhough, B.; Hobson-West, P.; Kirk, R.G.W.; Message, R.; Myelnikov, D.; Palmer, A.; Roe, E.; Ashall, V.; et al. Animal research nexus: A new approach to the connections between science, health and animal welfare. Med. Humanit. 2020, 46, 499–511. [Google Scholar] [CrossRef]
- Ruben, R.; Cavatassi, R.; Lipper, L.; Smaling, E.; Winters, P. Towards food systems transformation-five paradigm shifts for healthy, inclusive and sustainable food systems. Food Secur. 2021, 13, 1423–1430. [Google Scholar] [CrossRef]
- Meade, K.G. The increasing relevance of immunobiology within a connected animal science curriculum. Transl. Anim. Sci. 2023, 7, txad007. [Google Scholar] [CrossRef]
- Radzykhovskyi, M.; Sokulskiy, I.; Dyshkant, O.; Antoniuk, A.; Gutyj, B.; Sachuk, R. Experimental study of tropism of cultivated canine parvovirus in the immunogenesis organs of puppies. Regul. Mech. Biosyst. 2022, 13, 241–246. [Google Scholar] [CrossRef]
- Fedorovych, V.V. Natural cow resistance of combined breeds in western region of ukraine. Anim. Breed. Genet. 2014, 48, 156–163. [Google Scholar]
- Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 2020, 380, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Semaniuk, N.V. Dogs patient with have indexes of immunity chronic catarrhal gingivitis. Sci. Messenger LNU Vet. Med. Biotechnol. Ser. Vet. Sci. 2014, 16, 306–311. [Google Scholar]
- Dzetsiukh, T.I. Peculiarities of cellular immunity destruction in animals with acute periodontitis with hypothyroidism. Achiev. Clin. Exp. Med. 2016, 25, 28–30. [Google Scholar] [CrossRef]
- Didenko, V.; Tatarchuk, O.; Melanich, S.; Petishko, O. Features of indicators of the functional state of the liver, immune link, cytokine regulation and carbohydrate metabolism in patients with toxic chronic diffuse liver diseases. Gastroenterology 2021, 54, 88–95. [Google Scholar] [CrossRef]
- Broshkov, M.; Smolyaninov, B.; Stus, S. Immunophysiological dogs state with different indicators of immunoregulatory index. Sci. Tech. Bull. NDC Biosaf. Ecol. Control Agric. Resour. 2015, 4, 118–123. [Google Scholar]
- Selyukov, V.S. The use of service dogs by the criminal enforcement service of Ukraine. Sci. Bull. Public Priv. Law 2020, 6, 263–269. [Google Scholar]
- Yevstafiieva, Y.M.; Buchkovska, V.I. Specific features of using service dogs during operational-investigative and preventive activities. Taurian Sci. Bull. 2020, 121, 127–132. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=74782 (accessed on 1 January 2024).
- Law of Ukraine No. 249. On the Procedure for Carrying out Experiments and Experiments on Animals by Scientific Institutions. 2012. Available online: https://zakon.rada.gov.ua/laws/show/z0416-12#Text (accessed on 1 December 2023).
- Horalskyi, L.P.; Khomych, V.T.; Kononskyi, O.I. Basics of Histological Technique and Morphofunctional Research Methods in Normal and Pathological Conditions; Polissia: Zhytomyr, Ukraine, 2019; p. 286. [Google Scholar]
- Khomych, V.T. International Veterinary Anatomical Nomenclature; National University of Life and Environmental Sciences of Ukraine: Kyiv, Ukraine, 2005; p. 388, (In Latin, Ukrainian and English). [Google Scholar]
- Khomych, V.T. International Veterinary Histological Nomenclature (Terminological Dictionary); National University of Life and Environmental Sciences of Ukraine: Kyiv, Ukraine, 2019; p. 273. [Google Scholar]
- Marioni, G.; D’Alessandro, E.; Giacomelli, L.; Staffieri, A. CD105 is a marker of tumour vasculature and a potential target for the treatment of head and neck squamous cell carcinoma. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2010, 39, 361–367. [Google Scholar] [CrossRef]
- Barroso, I.; McCarthy, M.I. The Genetic Basis of Metabolic Disease. Cell 2019, 177, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Baker-Groberg, S.M.; Cianchetti, F.A.; Glynn, J.J.; Healy, L.D.; Lam, W.Y.; Nelson, J.W.; Parrish, D.C.; Phillips, K.G.; Scott-Drechsel, D.E.; et al. Measurement science in the circulatory system. Cell. Mol. Bioeng. 2014, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Iismaa, S.E.; Kaidonis, X.; Nicks, A.M.; Bogush, N.; Kikuchi, K.; Naqvi, N.; Harvey, R.P.; Husain, A.; Graham, R.M. Comparative regenerative mechanisms across different mammalian tissues. NPJ Regen. Med. 2018, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 2010, 11, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Cheverda, I.; Zakharenko, M. The morphological composition of the blood and the peculiarities of metabolism in gonadectomized cockerels of the Adler silvery breed. Sci. J. Vet. Med. 2021, 1, 18–26. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, L.; Cao, Y.; Liu, G.; Lin, Q.; Mao, X. Effects of Casein Phosphopeptide-Selenium Complex on the Immune Functions in Beagle Dogs. Animals 2022, 12, 2037. [Google Scholar] [CrossRef]
- Kyrychenko, V.; Broshkov, M. Dynamics of comparative immunogram indicators in female dogs during the estrous cycle. Agrar. Bull. Black Sea Littoral 2023, 105, 5–12. [Google Scholar] [CrossRef]
- Verde, M.T.; Villanueva-Saz, S.; Loste, A.; Marteles, D.; Pereboom, D.; Conde, T.; Fernández, A. Comparison of circulating CD4+, CD8+ lymphocytes and cytokine profiles between dogs with atopic dermatitis and healthy dogs. Res. Vet. Sci. 2022, 145, 13–20. [Google Scholar] [CrossRef]
| Indicator | Control Group of Dogs (n = 56) | Experimental Group of Dogs (n = 46) |
|---|---|---|
| T-helpers, 1012/L | 0.97 ± 0.08 a | 0.84 ± 0.10 a |
| T-suppressors, 1012/L | 0.46 ± 0.05 a | 0.47 ± 0.09 a |
| IRI | 2.1 ± 0.1 b | 1.7 ± 0.13 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunaievska, O.; Sokulskyi, I.; Radzykhovskii, M.; Gutyj, B.; Dyshkant, O.; Khomenko, Z.; Brygadyrenko, V. Immunophysiological State of Dogs According to the Immunoregulatory Index of Their Blood and Spleens. Animals 2024, 14, 706. https://doi.org/10.3390/ani14050706
Dunaievska O, Sokulskyi I, Radzykhovskii M, Gutyj B, Dyshkant O, Khomenko Z, Brygadyrenko V. Immunophysiological State of Dogs According to the Immunoregulatory Index of Their Blood and Spleens. Animals. 2024; 14(5):706. https://doi.org/10.3390/ani14050706
Chicago/Turabian StyleDunaievska, Oksana, Ihor Sokulskyi, Mykola Radzykhovskii, Bogdan Gutyj, Olga Dyshkant, Zoriana Khomenko, and Viktor Brygadyrenko. 2024. "Immunophysiological State of Dogs According to the Immunoregulatory Index of Their Blood and Spleens" Animals 14, no. 5: 706. https://doi.org/10.3390/ani14050706
APA StyleDunaievska, O., Sokulskyi, I., Radzykhovskii, M., Gutyj, B., Dyshkant, O., Khomenko, Z., & Brygadyrenko, V. (2024). Immunophysiological State of Dogs According to the Immunoregulatory Index of Their Blood and Spleens. Animals, 14(5), 706. https://doi.org/10.3390/ani14050706






