A New Method to Detect Buffalo Mastitis Using Udder Ultrasonography Based on Deep Learning Network
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
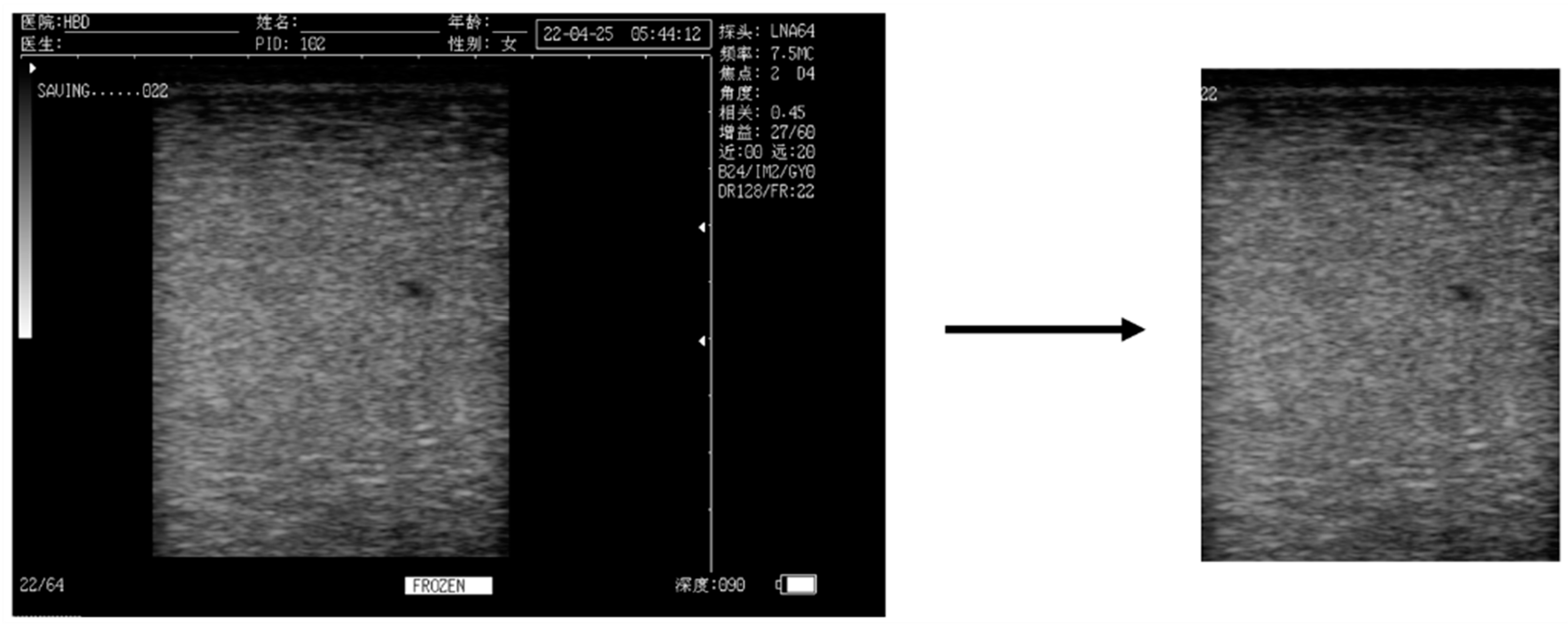
2.1. Data
2.2. Establishment of a Deep Learning Network
2.2.1. Data Set Composition
2.2.2. Establishment of a Mastitis Detection Network Based on Udder Ultrasound Images
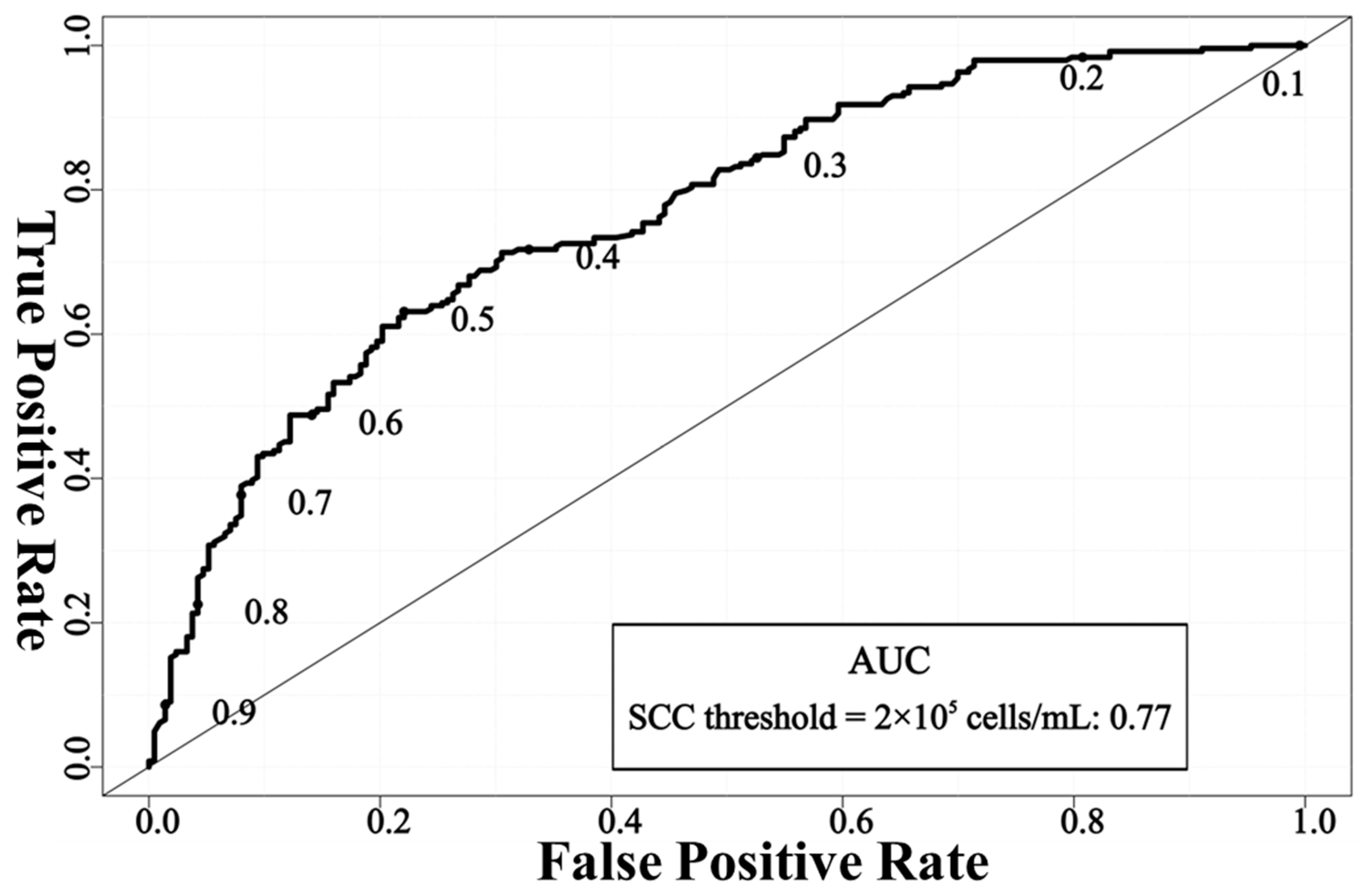
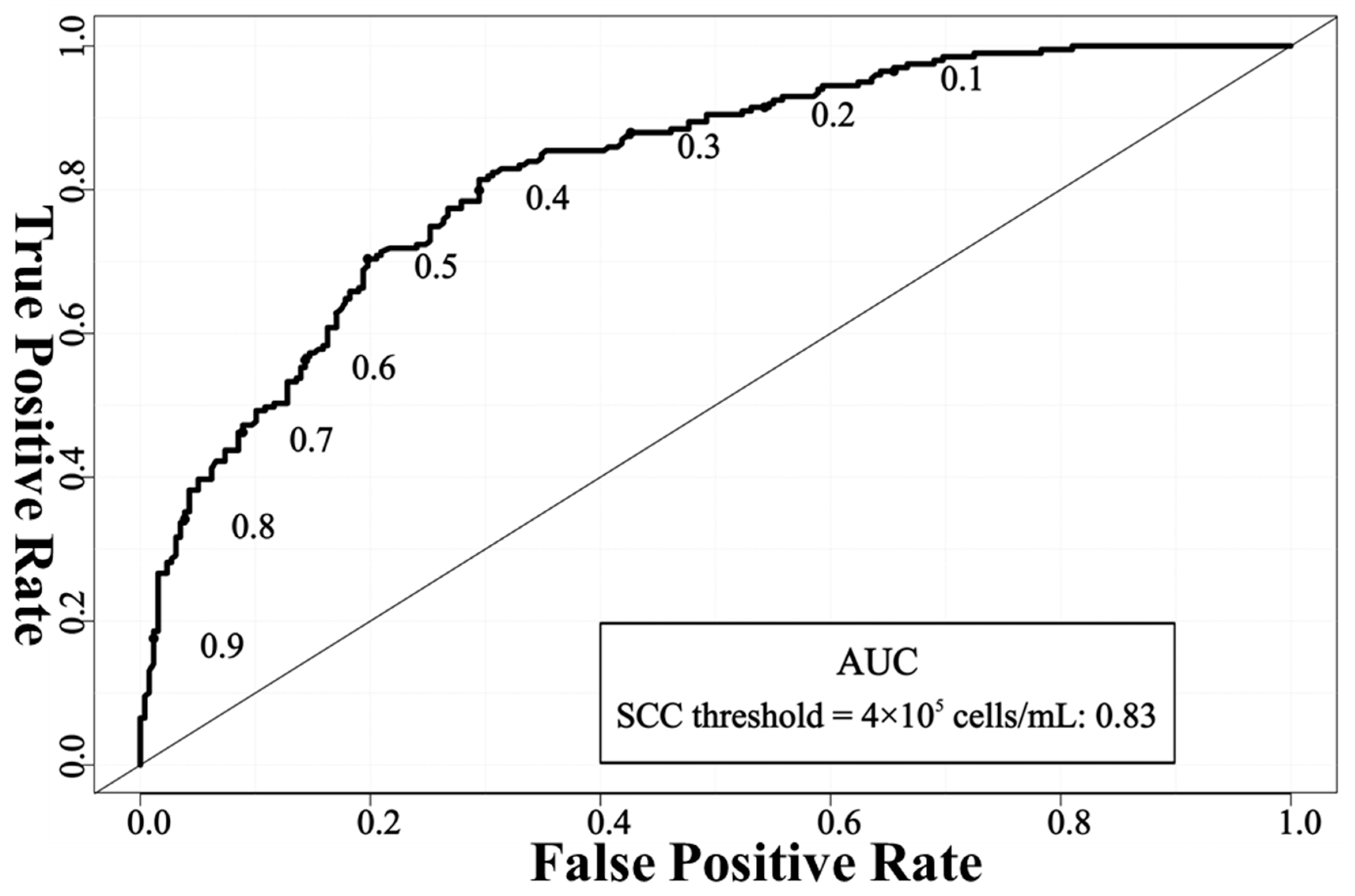
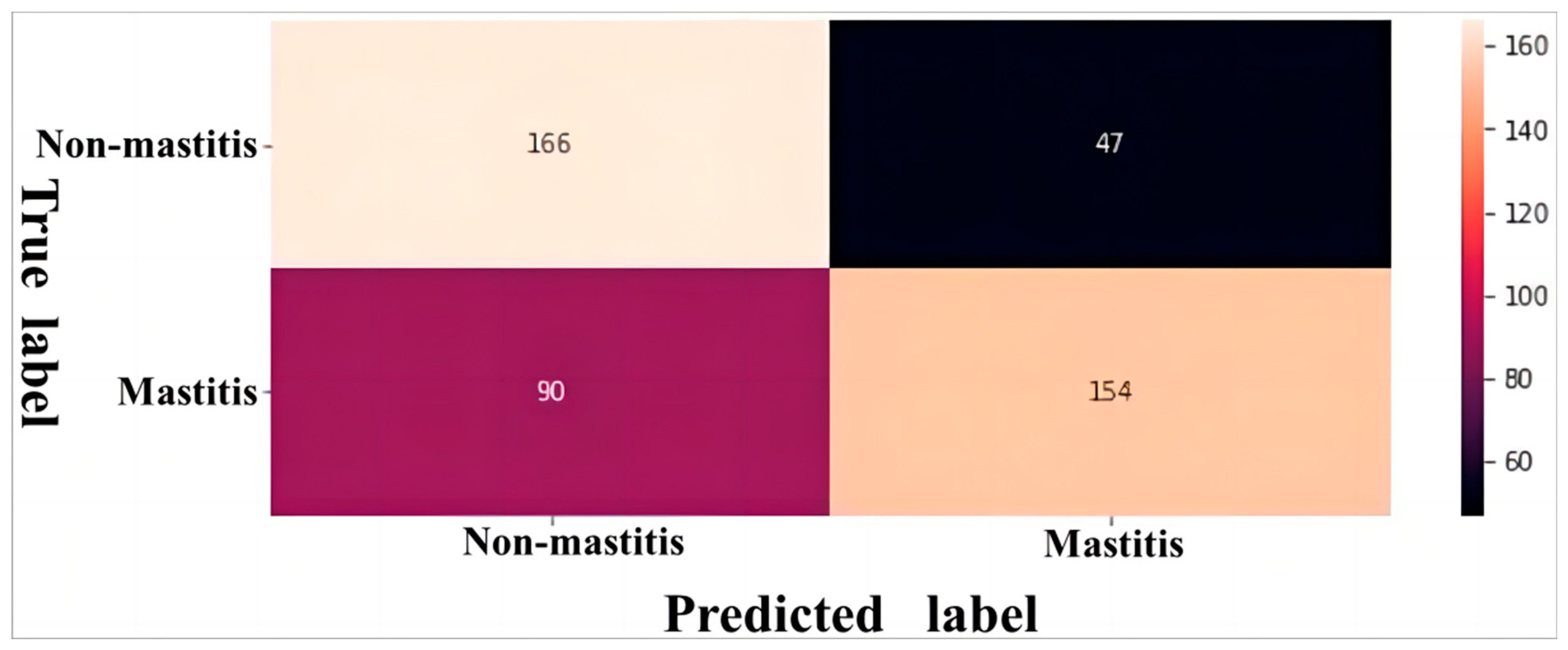
2.3. Model Performance Evaluation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rehman, S.U.; Hassan, F.-U.; Luo, X.; Li, Z.; Liu, Q. Whole-genome sequencing and characterization of buffalo genetic resources: Recent advances and future challenges. Animals 2021, 11, 904. [Google Scholar] [CrossRef]
- Ahmad, S.; Gaucher, I.; Rousseau, F.; Beaucher, E.; Piot, M.; Grongnet, J.F.; Gaucheron, F. Effects of acidification on physico-chemical characteristics of buffalo milk: A comparison with cow’s milk. Food Chem. 2008, 106, 11–17. [Google Scholar] [CrossRef]
- Mejares, C.T.; Huppertz, T.; Chandrapala, J. Thermal processing of buffalo milk—A review. Int. Dairy J. 2022, 129, 105311. [Google Scholar] [CrossRef]
- Naveena, B.; Kiran, M. Buffalo meat quality, composition, and processing characteristics: Contribution to the global economy and nutritional security. Anim. Front. 2014, 4, 18–24. [Google Scholar] [CrossRef]
- El Debaky, H.A.; Kutchy, N.A.; Ul-Husna, A.; Indriastuti, R.; Akhter, S.; Purwantara, B.; Memili, E. Potential of water buffalo in world agriculture: Challenges and opportunities. Appl. Anim. Sci. 2019, 35, 255–268. [Google Scholar] [CrossRef]
- El-Ashker, M.; Gwida, M.; Tomaso, H.; Monecke, S.; Ehricht, R.; El-Gohary, F.; Hotzel, H. Staphylococci in cattle and buffaloes with mastitis in Dakahlia Governorate, Egypt. J. Dairy Sci. 2015, 98, 7450–7459. [Google Scholar] [CrossRef] [PubMed]
- Fagiolo, A.; Lai, O. Mastitis in buffalo. Ital. J. Anim. Sci. 2007, 6 (Suppl. 2), 200–206. [Google Scholar] [CrossRef]
- Viguier, C.; Arora, S.; Gilmartin, N.; Welbeck, K.; O’Kennedy, R. Mastitis detection: Current trends and future perspectives. Trends Biotechnol. 2009, 27, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Panchal, I.; Sawhney, I.; Sharma, A.; Dang, A. Classification of healthy and mastitis Murrah buffaloes by application of neural network models using yield and milk quality parameters. Comput. Electron. Agric. 2016, 127, 242–248. [Google Scholar] [CrossRef]
- EZ Kotb, E.; El-Fattah, A.; Ola, A.; Azab, A.M.; Leil, A.Z. Ultrasonography, histopathological udder alterations and bacteriological investigations for diagnosis of mastitic goats. J. Appl. Vet. Sci. 2020, 5, 77–86. [Google Scholar] [CrossRef]
- Fasulkov, I.; Karadaev, M.; Vasilev, N.; Simeonov, R.; Urumova, V.; Mladenova, E. Ultrasound and histopathological investigations of experimentally induced Staphylococcus aureus mastitis in goats. Small Rumin. Res. 2015, 129, 114–120. [Google Scholar] [CrossRef]
- Mansour, R.F. Deep-learning-based automatic computer-aided diagnosis system for diabetic retinopathy. Biomed. Eng. Lett. 2018, 8, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Chugh, G.; Kumar, S.; Singh, N. Survey on machine learning and deep learning applications in breast cancer diagnosis. Cogn. Comput. 2021, 13, 1451–1470. [Google Scholar] [CrossRef]
- Hyde, R.M.; Down, P.M.; Bradley, A.J.; Breen, J.E.; Hudson, C.; Leach, K.A.; Green, M.J. Automated prediction of mastitis infection patterns in dairy herds using machine learning. Sci. Rep. 2020, 10, 4289. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Yang, X.; Lei, B.; Liu, L.; Li, S.X.; Ni, D.; Wang, T. Deep learning in medical ultrasound analysis: A review. Engineering 2019, 5, 261–275. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Mohammadi-Dehcheshmeh, M.; Ebrahimie, E.; Petrovski, K.R. Comprehensive analysis of machine learning models for prediction of sub-clinical mastitis: Deep Learning and Gradient-Boosted Trees outperform other models. Comput. Biol. Med. 2019, 114, 103456. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Min, Y.; Choi, B. Real-time temperature monitoring for the early detection of mastitis in dairy cattle: Methods and case researches. Comput. Electron. Agric. 2019, 162, 119–125. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, X.; He, Z.; Feng, Y.; Liu, G. Accurate detection of dairy cow mastitis with deep learning technology: A new and comprehensive detection method based on infrared thermal images. Animal 2022, 16, 100646. [Google Scholar] [CrossRef]
- Minervino, A.H.H.; Zava, M.; Vecchio, D.; Borghese, A. Bubalus bubalis: A short story. Front. Vet. Sci. 2020, 7, 570413. [Google Scholar] [CrossRef]
- Neculai-Valeanu, A.-S.; Ariton, A.-M. Udder health monitoring for prevention of bovine mastitis and improvement of milk quality. Bioengineering 2022, 9, 608. [Google Scholar] [CrossRef]
- Kasai, S.; Prasad, A.; Kumagai, R.; Takanohashi, K. Scanning electrochemical microscopy-somatic cell count as a method for diagnosis of bovine mastitis. Biology 2022, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Le, Q. Efficientnet: Rethinking model scaling for convolutional neural networks. Proc. Mach. Learn. Res. 2019, 97, 6105–6114. [Google Scholar]
- Woo, S.; Park, J.; Lee, J.-Y.; Kweon, I.S. Cbam: Convolutional block attention module. In Proceedings of the European Conference on Computer Vision (ECCV), Munich, Germany, 8–14 September 2018; pp. 3–19. [Google Scholar]
- Leng, Z.; Tan, M.; Liu, C.; Cubuk, E.D.; Shi, X.; Cheng, S.; Anguelov, D. Polyloss: A polynomial expansion perspective of classification loss functions. arXiv 2022, arXiv:2204.12511. [Google Scholar]
- Wang, H.; Shen, W.; Zhang, Y.; Gao, M.; Zhang, Q.; Xiaohui, A.; Du, H.; Qiu, B. Diagnosis of dairy cow diseases by knowledge-driven deep learning based on the text reports of illness state. Comput. Electron. Agric. 2023, 205, 107564. [Google Scholar] [CrossRef]
- Yanase, J.; Triantaphyllou, E. A systematic survey of computer-aided diagnosis in medicine: Past and present developments. Expert Syst. Appl. 2019, 138, 112821. [Google Scholar] [CrossRef]
- Duarte, C.M.; Freitas, P.P.; Bexiga, R. Technological advances in bovine mastitis diagnosis: An overview. J. Vet. Diagn. Investig. 2015, 27, 665–672. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.; Liu, G.; Ning, Y.; Li, J. Dairy Cow Mastitis Detection by Thermal Infrared Images Based on CLE-UNet. Animals 2023, 13, 2211. [Google Scholar] [CrossRef]
- Thompson, J.; Nunn, S.L.E.; Sarkar, S.; Clayton, B. Diagnostic Screening of Bovine Mastitis Using MALDI-TOF MS Direct-Spotting of Milk and Machine Learning. Vet. Sci. 2023, 10, 101. [Google Scholar] [CrossRef]
- Abdul Ghafoor, N.; Sitkowska, B. MasPA: A machine learning application to predict risk of mastitis in cattle from AMS sensor data. AgriEngineering 2021, 3, 575–583. [Google Scholar] [CrossRef]
- Dhoble, A.S.; Ryan, K.T.; Lahiri, P.; Chen, M.; Pang, X.; Cardoso, F.C.; Bhalerao, K.D. Cytometric fingerprinting and machine learning (CFML): A novel label-free, objective method for routine mastitis screening. Comput. Electron. Agric. 2019, 162, 505–513. [Google Scholar] [CrossRef]
- Xudong, Z.; Xi, K.; Ningning, F.; Gang, L. Automatic recognition of dairy cow mastitis from thermal images by a deep learning detector. Comput. Electron. Agric. 2020, 178, 105754. [Google Scholar] [CrossRef]
- Liu, G.; Guo, J. Bidirectional LSTM with attention mechanism and convolutional layer for text classification. Neurocomputing 2019, 337, 325–338. [Google Scholar] [CrossRef]
- Guo, M.-H.; Xu, T.-X.; Liu, J.-J.; Liu, Z.-N.; Jiang, P.-T.; Mu, T.-J.; Zhang, S.-H.; Martin, R.R.; Cheng, M.-M.; Hu, S.-M. Attention mechanisms in computer vision: A survey. Comput. Vis. Media 2022, 8, 331–368. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, H.; Yang, W.; Xu, X.; Jiang, W.; Lai, X. Nonlocal convolutional block attention module VNet for gliomas automatic segmentation. Int. J. Imaging Syst. Technol. 2022, 32, 528–543. [Google Scholar] [CrossRef]
- Farag, M.M.; Fouad, M.; Abdel-Hamid, A.T. Automatic severity classification of diabetic retinopathy based on densenet and convolutional block attention module. IEEE Access 2022, 10, 38299–38308. [Google Scholar] [CrossRef]
- Šimundić, A.-M. Measures of diagnostic accuracy: Basic definitions. EJIFCC 2009, 19, 203. [Google Scholar] [PubMed]
- Dos Santos, S.K.; Oliveira, M.G.; Noriler, E.P.; Vrisman, D.P.; Borges, L.P.B.; Santos, V.J.C.; Coutinho, L.N.; Teixeira, P.P.M. Mammary gland ultrasound evaluation of Jersey cattle breed. Acta Sci. Vet. 2016, 44, 5. [Google Scholar] [CrossRef]
- Zhang, X.; Ahmad, M.J.; An, Z.; Niu, K.; Wang, W.; Nie, P.; Gao, S.; Yang, L. Relationship between somatic cell counts and mammary gland parenchyma ultrasonography in buffaloes. Front. Vet. Sci. 2022, 9, 842105. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, O.M.; Aslam, S.; Khan, M.A.; Mushtaq, H.; Hassan, M.; Ijaz, M. Healthy, sub-clinical, and clinical mastitis in Holstein-Friesian cattle: A comparative echotextural and electrical conductivity study. S. Afr. J. Anim. Sci. 2023, 53, 221–230. [Google Scholar]

| Data Set. | SCC Threshold | Healthy Group (n) | Mastitis Group (n) |
|---|---|---|---|
| 1 | 2 × 105 cells/mL | 1424 | 1630 |
| 2 | 4 × 105 cells/mL | 1722 | 1332 |
| SCC Threshold | Accuracy (%) | Specificity (%) | Sensitivity (%) | F1-Score (%) |
|---|---|---|---|---|
| 2 × 105 cells/mL | 70.02 | 77.93 | 63.11 | 69.21 |
| 4 × 105 cells/mL | 75.93 | 80.23 | 70.35 | 71.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, Y.; Zhang, Y.; Yao, Z.; Zou, W.; Nie, P.; Yang, L. A New Method to Detect Buffalo Mastitis Using Udder Ultrasonography Based on Deep Learning Network. Animals 2024, 14, 707. https://doi.org/10.3390/ani14050707
Zhang X, Li Y, Zhang Y, Yao Z, Zou W, Nie P, Yang L. A New Method to Detect Buffalo Mastitis Using Udder Ultrasonography Based on Deep Learning Network. Animals. 2024; 14(5):707. https://doi.org/10.3390/ani14050707
Chicago/Turabian StyleZhang, Xinxin, Yuan Li, Yiping Zhang, Zhiqiu Yao, Wenna Zou, Pei Nie, and Liguo Yang. 2024. "A New Method to Detect Buffalo Mastitis Using Udder Ultrasonography Based on Deep Learning Network" Animals 14, no. 5: 707. https://doi.org/10.3390/ani14050707
APA StyleZhang, X., Li, Y., Zhang, Y., Yao, Z., Zou, W., Nie, P., & Yang, L. (2024). A New Method to Detect Buffalo Mastitis Using Udder Ultrasonography Based on Deep Learning Network. Animals, 14(5), 707. https://doi.org/10.3390/ani14050707








