Current State of Mugger Populations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Habitat Description and Suitability
3. Country Summaries
3.1. Sri Lanka
3.1.1. Historical Range and Status
3.1.2. Current Range and Status
3.1.3. Habitat Description and Suitability
3.1.4. Threats
3.1.5. Captive Populations
3.1.6. Other Conservation Efforts
3.1.7. Prognosis
3.2. Bangladesh
3.2.1. Historical Range and Status
3.2.2. Current Range and Status
3.2.3. Habitat Description and Suitability
3.2.4. Threats
3.2.5. Captive Populations
3.2.6. Reintroduction Efforts
3.2.7. Other Conservation Efforts
3.2.8. Prognosis
3.3. Iran
3.3.1. Historical Range and Status
3.3.2. Current Range and Status
3.3.3. Habitat Description and Suitability
3.3.4. Threats
3.3.5. Captive Populations
3.3.6. Other Conservation Efforts
3.3.7. Prognosis
3.4. Pakistan
3.4.1. Historical Range and Status
3.4.2. Current Range and Status
3.4.3. Habitat Description and Suitability
3.4.4. Threats
3.4.5. Captive Populations
3.4.6. Reintroduction Efforts
3.4.7. Other Conservation Efforts
3.4.8. Prognosis
3.5. Nepal
3.5.1. Historical Range and Status
3.5.2. Current Range and Status
3.5.3. Habitat Description and Suitability
3.5.4. Threats
3.5.5. Captive Populations
3.5.6. Reintroduction Efforts
3.5.7. Other Conservation Efforts
3.5.8. Prognosis
3.6. India
3.6.1. Historical Range and Status
3.6.2. Current Range and Status
3.6.3. Habitat Description and Suitability
3.6.4. Threats
3.6.5. Captive Populations
3.6.6. Reintroduction Efforts
3.6.7. Other Conservation Efforts
3.6.8. Prognosis
4. Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Silva, A.; Lenin, J. Mugger Crocodile Crocodylus palustris. In Crocodiles. Status Survey and Conservation Action Plan, 3rd ed.; Manolis, S.C., Stevenson, C., Eds.; Crocodile Specialist Group: Darwin, Australia, 2010; pp. 94–98. [Google Scholar]
- Santiapillai, C.; de Silva, M. Status, distribution and conservation of crocodiles in Sri Lanka. Biol. Conserv. 2001, 97, 305–318. [Google Scholar] [CrossRef]
- Mobaraki, A.; Abtin, E. Estimate of Mugger population in Iran. Crocodile Spec. Group Newsl. 2013, 32, 11–21. [Google Scholar]
- Rahim, A.; Gabol, K.; Ahmed, W.; Batool, A. Population Assessment, Threats and Conservation Measures of Marsh Crocodile at Dasht River, Gwadar. Pak. J. Mar. Sci. 2018, 27, 45–53. [Google Scholar]
- Whitaker, R.; Andrews, H.V. Crocodile Conservation, Western Asia Region: An Update. J. Bombay Nat. Hist. Soc. 2003, 100, 432–445. [Google Scholar]
- Zafar, M.; Malik, M.F. A review on status and conservation of mugger crocodile. Environment 2018, 19, 21. [Google Scholar] [CrossRef]
- Fellows, S. A Survey of the Abundance, Population Structure, and Distribution of Mugger Crocodiles (Crocodylus palustris) using day Ground Surveys in District Bhopal and its impact on Community. JOJ Wildl. Biodivers. Juniper Publ. 2019, 1, 14–22. [Google Scholar]
- Singh, Y.; Gurjwar, R.K.; Lodhi, R.; Rao, R.J. People perception on wildlife in national Chambal sanctuary, Madhya Pradesh, India. Int. J. Biol. Sci. 2020, 2, 8–15. [Google Scholar] [CrossRef]
- Pimm, S.L.; Raven, P. Biodiversity: Extinction by numbers. Nature 2000, 403, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.C. How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshw. Res. 2014, 65, 936–941. [Google Scholar] [CrossRef]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the Curve of Global Freshwater Biodiversity Loss: An Emergency Recovery Plan. BioScience 2020, 70, 330–342. [Google Scholar] [CrossRef]
- Choudhury, B.C.; de Silva, A. Crocodylus palustris. In The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2013; p. e.T5667A3046723. [Google Scholar] [CrossRef]
- Behrouzi, B.; Abtin, E.; Mobaraki, A. Mugger crocodile habitat suitability study. Crocodile Spec. Group Newsl. 2010, 29, 8–10. [Google Scholar]
- Buenfil-Rojas, A.; Alvarez, T.; González, M.J.; Rendon von Osten, J.; Cedeño-Vázquez, J. Effectiveness of Morelet’s crocodile as a bioindicator of metal pollution and metallothionein response to spatial variations of metal exposure. Environ. Adv. 2022, 8, 100251. [Google Scholar] [CrossRef]
- Kommanee, J.; Preecharram, S.; Daduang, S.; Temsiripong, Y.; Dhiravisit, A.; Yamada, Y.; Thammasirirak, S. Antibacterial activity of plasma from crocodile (Crocodylus siamensis) against pathogenic bacteria. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Buenviaje, G. Studies on Skin Diseases of Crocodiles. Ph.D. Thesis, James Cook University, Townsville, Australia, 2000. [Google Scholar]
- Brown, G.P.; Shine, R. Do Microbiota in the Soil Affect Embryonic Development and Immunocompetence in Hatchling Reptiles? Front. Ecol. Evol. 2021, 9, 780456. [Google Scholar] [CrossRef]
- Vasudevan, K. Nesting ecology of mugger Crocodylus palustris in Amaravathi, southern India. Hamadryad 1998, 22, 107–110. [Google Scholar]
- de Silva, A. Crocodiles of Sri Lanka: Preliminary Assessment of Their Status and the Human Crocodile Conflict Situation; Report submitted after fulfillment of the project to Mohamed Bin Zayed Species Conservation Fund; Mohamed Bin Zayed Species Conservation Fund: Gampola, Sri Lanka, 2010. [Google Scholar]
- Choudhary, S.; Choudhury, B.C. Spatio-temporal partitioning between two sympatric crocodilians (Gavialis gangeticus & Crocodylus palustris) in Katarniaghat Wildlife Sanctuary, India. Aquat. Conserv. Mar. Freshw. 2018, 28, 10. [Google Scholar] [CrossRef]
- Hennessy, D.J.G. Green Aisles: A Story of the Jungle of Ceylon; Colombo Book Centre: Colombo, Sri Lanka, 1949. [Google Scholar]
- Somanader, S.V.O. The swamp crocodile of Ceylon. Loris 1941, 2, 227–229. [Google Scholar]
- Deraniyagala, P.E.P. Crocodiles of Ceylon. Spolia Zeylan. 1930, 16, 89–95. [Google Scholar]
- Nicholas, C.W. Administrative Report of the Warden; Department of Wild Life for 1955; The Government Press: Colombo, Sri Lanka, 1956. [Google Scholar]
- Whitaker, R.; Whitaker, Z. Sri Lanka crocodile survey. Loris 1977, 15, 239–241. [Google Scholar]
- Uragoda, C.G. Wildlife Conservation in Sri Lanka; Wildlife and Nature Protection Society: Colombo, Sri Lanka, 1994. [Google Scholar]
- Whitaker, R.; Daniel, R.C. The status of Asian crocodilians. Tiger Pap. 1978, 5, 12–17. [Google Scholar]
- Santiapillai, C.; de Silva, M.; Dissanayake, S.; Jayaratne, B.V.R.; Wijeyamohan, S. An ecological study of crocodiles in the Ruhuna National park, Sri Lanka. J. Bombay Nat. Hist. Soc. 2000, 97, 33–41. [Google Scholar]
- Madawala, M.B.; Amarasinghe, A.A.T.; Botejue, W.M.S.; Somaweera, R.; Karunarathna, D.M.S.; de Silva, A. The Muggers of Sri Lanka—Conflicts And Conservation. In Proceedings of the Association for Tropical Biology and Conservation 51st Annual Meeting, Cairns, Australia, 20–24 July 2014. [Google Scholar]
- de Silva, A.; Benjamin, L.; Weerawardhane, W.; Whitaker, R.; Riyas, A.; Ahamed, M.; Toshikazu, U. Invasion of Mugger (Crocodylus palustris) into recent man made habitats. Lyriocephalus J. Amphib. Reptile Res. Organ. Sri Lanka 2009, 7, 7–11. [Google Scholar]
- Kariyawasam, S.; Shanka, S. Infrastructure, Investment and Development Evaluation: A Complex Stakeholder Perception Mapping Approach for Improving Local and Regional Economic Development Outcomes. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2015. [Google Scholar]
- UNESCO (United Nations Educational, Scientific and Cultural Organization); MoAIMD (Ministry of Agriculture, Irrigation and Mahaweli Development). Sri Lanka Water Development Report; UNESCO Publishing: Paris, France, 2006. [Google Scholar]
- Paranavitana, S. The emperor of Ceylon at the time of the arrival of the Portuguese in 1505. Univ. Ceylon Rev. 1961, 19, 10–29. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Sri Lanka. Water Report; FAO: Rome, Italy, 2012. [Google Scholar]
- Werellagama, D.R.; Abeynayaka, A.; Manatunge, J. Collaborative program to prevent pollution of the upper reaches of Mahaweli River—Sri Lanka. In Proceedings of the 3rd Asia Pacific Association of Hydrology and Water Resources (APHW), Bangkok, Thailand, 16–18 October 2006. [Google Scholar]
- Department of Wildlife Conservation Sri Lanka. Wilpattu National Park Management Plan 2019–2024; Wilpattu National Park & Influence Zone Management Project: Colombo, Sri Lanka, 2018.
- Green, M.J.B. IUCN Directory of South Asian Protected Areas; IUCN-The World Conservation Union: Gland, Switzerland, 1990; pp. 242–246. ISBN 978-2-8317-0030-4. [Google Scholar]
- Whitaker, R.; Whitaker, Z. Status and conservation of the Asian crocodiles. In Crocodiles. Their Ecology, Managment and Conservation; IUCN: Gland, Switzerland, 1989; pp. 297–308. ISBN 2880329876. [Google Scholar]
- Ross, J.P. Crocodile Conservation Gains in Jeopardy. Crocodile Spec. Group Newsl. 1994, 13, 13. [Google Scholar]
- Ministry of Enviroment, Sri Lanka. National Drought Plan for Sri Lanka; Ministry of Environment: Battaramulla, Sri Lanka, 2020.
- de Klemm, C.; Navid, D. Crocodilians and the law. In Crocodiles. Their Ecology, Managment and Conservation; IUCN: Gland, Switzerland, 1989; pp. 80–100. ISBN 2880329876. [Google Scholar]
- Masum, K.; Malekur, R.; Zahed, M.; Alamgir, M.; Mamun, A.; Mamun, M.M.; Abdullah, A. Breeding Difficulty of Marsh Crocodile (Crocodylus palustris, Lesson, 1831) in Safari Park of Bangladesh. J. For. Environ. Sci. 2012, 28, 220–226. [Google Scholar] [CrossRef][Green Version]
- Bushra, N. Landscape Narrative of the Sundarban: Towards Collaborative Management by Bangladesh and India (English); World Bank Group: Washington, DC, USA, 2019. [Google Scholar]
- Cox, J.H.; Rahman, M.M. An assessment of crocodile resource potential in Bangladesh. In Proceedings of the 12th Working Meeting of the IUCN-SSC Crocodile Specialist Group, Pattaya, Thailand, 2–6 May 1994. [Google Scholar]
- Rao, R.J. Ecological studies of Indian crocodiles, an overview. In Proceedings of the 12th Working Meeting of the Crocodile Specialist Group, Pattaya, Thailand, 2–6 May 1994. [Google Scholar]
- Bhattacharya, P. Dhaka’s request for Indian marsh crocodiles is granted. Crocodile Spec. Group Newsl. 2005, 22, 10–13. [Google Scholar]
- Ahsan, M.M.; Aziz, N.; Morshed, H.M. Assessment of Management Effectiveness of Protected Areas of Bangladesh; SRCWP Project; Bangladesh Forest Department: Dhaka, Bangladesh, 2016. [Google Scholar]
- Charles, K.J.; Ong, L.A.; El Achi, N.; Ahmed, K.M.; Khan, M.R. Bangladesh MICS 2019: Water Quality Thematic Report; Bangladesh Bureau of Statistics Statistics Division, Ministry of Planning, Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, 2019.
- Islam, S.N. Sundarbans a Dynamic Ecosystem: An Overview of Opportunities, Threats and Tasks: Increasing Livelihood Security. In The Sundarbans: A Disaster-Prone Eco-Region; Sen, H.S., Ed.; Allied Fibers” Kim: Gujarat, India, 2019; pp. 29–58. ISBN 978-3-030-00679-2. [Google Scholar]
- Hossen, A.; Ahsan, M.F.; Kamruzzaman, M. Establishment of Dulahazra Safari Park towards the Protection and Conservation of Threatened Biota of Bangladesh on the Basis of Southasian Safari Park Concept Model. Jagannath Univ. J. Life Earth Sci. 2018, 4, 65–74. [Google Scholar]
- Whitaker, R.; Whitaker, Z. Reproductive biology of the mugger. J. Bombay Nat. Hist. Soc. 1984, 81, 297–316. [Google Scholar]
- Andrews, H.V. A note on the mugger breeding and nesting activities at the Croc Bank. Hamadryad India 1987, 12, 17–18. [Google Scholar]
- Bosch, M.; Bullock, S.; Kinunen, W. Crocodiles, Second Survey, Job Completion Report; Iran Fish and Game Department: Tehran, Iran, 1970; 26p, unpublished. [Google Scholar]
- Tuck, R. The crocodiles of Iran. In Natural History Museum; Department of the Environment: Canberra, Australia, 1975. [Google Scholar]
- Kami, H.G. Number of Crocodiles in Iran. Crocodile Spec. Group Newsl. 1994, 13, 7. [Google Scholar]
- Mobaraki, A. Mugger crocodile study in Iran. In Proceedings of the 16th Working Meeting of the Crocodile Specialist Group, Gainesville, FL, USA, 7–10 October 2002. [Google Scholar]
- Mobaraki, A.; Abtin, E.; Kami, H.G.; Kiabi, B.H. Reproductive biology of Mugger crocodile (Crocodylus palustris) in Iran. Zool. Midle East 2013, 59, 207–213. [Google Scholar] [CrossRef]
- Mobaraki, A. Sustainable Management and conservation of the and conservation of the Mugger Crocodile (Crocodylus palustris) in Iran. Master’s Thesis, International University of Andalusia, Seville, Spain, 2015. [Google Scholar]
- Naghizadeh, N.; Abbas, D.; Farvar, T. Recognising and Supporting Territories and Areas Conserved by Indigenous Peoples and Local Communities: Global Overview and National Case Studies; Technical Series No. 64; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2012. [Google Scholar]
- Scott, D.A. A Directory of Wetlands in the Middle East; IUCN: Gland, Switzerland; IWRB: Slimbridge, UK, 1995. [Google Scholar]
- Rafsanjani, A.K.; Karami, M. Eco-tourism necessity to preserve and maintain endangered species: A case study of mugger crocodile. Afr. J. GeoGraphy Reg. Plan. 2020, 7, 1–7. [Google Scholar]
- Food and Agriculture Organization of the United Nations. AQUASTAT Country Profile—Iran (Islamic Republic of); FAO: Rome, Italy, 2008. [Google Scholar]
- Zainudini, M.Z.; Sardarzaei, A. Flood Control and Flood Management of Sarbaz and Kajo Rivers in Makoran. Irrig. Drain. Syst. Eng. 2015, 4, 132. [Google Scholar]
- Varkouhi, S. Lead in Sarbaz River Basin Sediments, Sistan and Baluchestan, Iran. Integr. Environ. Assess. Manag. 2008, 5, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Gholamhosseini, A.; Banaee, M.; Soltanian, S.; Sakhaie, F. Heavy Metals in the Blood Serum and Feces of Mugger Crocodile (Crocodylus palustris) in Sistan and Baluchistan Province, Iran. Biol.Trace Elem. Res. 2022, 200, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, A.; McCaskill, L.; Schepp, U.; Masroor, R.; Pandhi, D.; Desai, B.; Muckerjee, S.; Rasheed, T.; Razzaque, S.A.; de Silva, A.; et al. Conservation status of the mugger (Crocodylus plaustris): Establishing a task force for a poster species of climate change. Crocodile Spec. Group Newsl. 2021, 40, 12–20. [Google Scholar]
- National Research Council. Water Conservation, Reuse, and Recycling: Proceedings of an Iranian-American Workshop; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Abtin, E. Second Fatal Mugger Crocodile Attack Reported in Iran. Crocodile Spec. Group Newsl. 2016, 35, 16–17. [Google Scholar]
- Yazdandoost, F. Dams, Droughts and Water Shortagein Today’s Iran. Iran. Stud. 2016, 49, 1017–1029. [Google Scholar] [CrossRef]
- Mobaraki, A.; Abtin, E. Movement Behaviour of Muggers: A Potential Threat. Crocodile Spec. Group Newsl. 2007, 26, 4–5. [Google Scholar]
- Abtin, E.; Mobaraki, A.; Hosseini, A.A. Trapped crocodiles released. Crocodile Spec. Group Newsl. 2007, 26, 9–10. [Google Scholar]
- de Silva, A.; Rashid, S.M.A.; Vyas, R.; Botheju, M. Crocodile Specialist Group Steering Committee Meeting; Universidad Nacional del Litoral: Santa Fe, Argentina, 2018. [Google Scholar]
- Rastegar-Pouyani, N.; Gholamifard, A.A.; Karamiani, R.; Bahmani, Z.; Mobaraki, A.; Abtin, E.; Faizi, H.; Heidari, N.; Takesh, M.; Sayyadi, F.; et al. Sustainable Management of the Herpetofauna of the Iranian Plateau and coastal Iran. Amphib. Reptile Conserv. 2015, 9, 1–15. [Google Scholar]
- Ahmed, A. The distribution and population of crocodiles in the province of Sindhh and Balochistan (Pakistan). J. Bombay Nat. Hist. Soc. 1986, 83, 220–223. [Google Scholar]
- Ghalib, S.A.; Rahman, H.; Iffat, F.; Hasnain, S.A. A checklist of the reptiles of Pakistan. Rec. Zool. Surv. Pak. 1981, 8, 37–59. [Google Scholar]
- Javed, H.I.; Rehman, H.; Fakhri, S. On the status of Marsh crocodile in Balochistan. Rec. Zool. Surv. Pak. 2005, 16, 40–45. [Google Scholar]
- Ahmed, A. Pakistan. Crocodile Spec. Group Newsl. 1990, 9, 15–16. [Google Scholar]
- Khan, A.A. Pakistan. Crocodile Spec. Group Newsl. 1989, 8, 5–6. [Google Scholar]
- Chang, M.S.; Gachal, G.S.; Qadri, A.H.; Sheikh, M. Bio-ecological status, Management and Conservation of Marsh Crocodiles (Crocodylus palustris) in Deh Akro 2, Sindhh–Pakistan. Sindhh Univ. Res. J. 2012, 44, 209–214. [Google Scholar]
- Chang, M.S.; Gachal, G.S.; Qadri, A.; Khowaja, Z.; Sheikh, M. Population and conservation status of Marsh Crocodiles, Crocodilus palustris in Karachi Zoological Garden, Samzu Park and Khar Center Karachi, Sindh-Pakistan. Sindh Univ. Res. J. 2013, 45, 534–541. [Google Scholar]
- Chang, M.S.; Gachal, G.S.; Qadri, A.; Memon, K.; Sheikh, M. Distribution, population status and threats of Marsh Crocodiles in Chotiari Wetland Complex Sanghar, Sindh-Pakistan. Biharean Biol. 2015, 9, 22–28. [Google Scholar]
- Khan, M.; Ahmed, S.; Zamir, A.; Saeed, S. First Record of Mugger Crocodile (Crocodylus palustris Lesson, 1831) from Haji Shahr, District Kachhi, Balochistan, Pakistan. Int. J. Biol. Biotech. 2022, 19, 549–552. [Google Scholar]
- Chaudhry, Q.U.Z. Climate Change Profile of Pakistan; Asian Development Bank: Manila, Philippines, 2017; ISBN 978-92-9257-721-6. [Google Scholar]
- Chang, M.S.; Gachal, G.S.; Quadri, A.H.; Jafri, S.I.H.; Khowaja, K.M.A.; Memon, K.H.; Sheikh, M.Y. Physicochemical assessment and its impacts on marsh crocodiles in Deh Akro II wildlife sanctuary. Int. J. Biosci. 2014, 4, 85–96. [Google Scholar]
- Chang, M.S. Ecological and Environmental Assessment of Nara Desert Wetland Complex (NDWC), Khairpur, Sindh—Pakistan. In Community and Global Ecology of Deserts; Hufnagel, L., Ed.; IntechOpen: London, UK, 2018; pp. 55–82. ISBN 978-1789238938. [Google Scholar]
- Rais, M.; Khan, M.Z.; Abbass, D.; Akber, G.; Nawaz, R.; Saeed-ul-Islam. A Qualitative Study on Wildlife of Chotiari Reservoir, Sanghar, Sindh, Pakistan. Pak. J. Zool. 2011, 43, 237–247. [Google Scholar]
- Mohammadpour, A.; Zarei, A.A.; Dehbandi, R.; Khaksefidi, R.; Shahasavani, E.; Rahimi, S.; Elshall, A.S.; Azhdarpoor, A. Comprehensive assessment of water quality and associated health risks in an arid region in south Iran. Regul. Toxicol. Pharmacol. 2022, 135, 105264. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Zafar, S.R.; Arif, S.; Hashmi, I. Quality of drinking water in Hub river catchment area, Sindh Pakistan. Int. J. Biol. Biotech. 2006, 3, 161–167. [Google Scholar]
- Khan, M.A.; Lang, M.; Shaukat, S.S.; Alamgir, A.; Baloch, T. Water Quality Assessment of Hingol River, Balochistan, Pakistan. Middle-East J. Sci. Res. 2014, 19, 306–313. [Google Scholar]
- Khan, M.Z.; Khan, I.S.; Ghalib, S.A.; Hussain, S.E.; Ahmed, W.; Siddiqui, S.; Yasmeen, G.; Zehra, A.; Hussain, B.; Kanwal, R.; et al. Assessment of Water Quality of Nagiopeer and Dangewari Wetlands and Status of the Wildlife of Nara Game Reserve, Sindh, Pakistan. Can. J. Pure Appl. Biosci. 2015, 9, 3503–3512. [Google Scholar]
- Chang, M.S.; Gachal, G.S.; Qadri, A.H.; Khowaja, M.; Sheikh, M.Y. Ecological status and threats of marsh crocodiles (Crocodylus palustris) in Manghopir Karachi. Int. J. Bioscences 2013, 3, 44–54. [Google Scholar]
- International Federation of Red Cross and Red Crescent Societies. Emergency Plan of Action (EPoA) Drought: Pakistan; Pakistan Red Crescent Society: Islamabad, Pakistan, 2019. [Google Scholar]
- Kunbhar, Z. Habitat Destruction at Sindh Sanctuary Threatens Pakistan’s Marsh Crocodiles. Crocodile Spec. Group News. 2021, 40, 22. [Google Scholar]
- Sideleau, B.M. A Brief Overview of Crocodilian Attacks Worldwide for the Decade. Crocodile Spec. Group Newsl. 2021, 40, 4–8. [Google Scholar]
- Whitaker, R.; Andrews, H.V. Pakistan Country Report. Crocodile Spec. Group Newsl. 1997, 16, 15. [Google Scholar]
- Brazaitis, P.; Watanabe, M.E. Pakistan Area Report. Crocodile Spec. Group Newsl. 1983, 2, 5. [Google Scholar]
- Khan, M.K. Pakistan Area Report. Crocodile Spec. Group Newsl. 1987, 6, 6. [Google Scholar]
- Lamichhane, S.; Bhattarai, D.; Karki, J.B.; Gautam, A.P.; Pandeya, P.; Tripathi, S.; Mahat, N. Population status, habitat occupancy and conservation threats to Mugger crocodile (Crocodylus palustris) in Ghodaghodi lake complex, Nepal. Glob. Ecol. Conserv. 2022, 33, e01977. [Google Scholar] [CrossRef]
- Andrews, H.V.; McEachern, P. Crocodile Conservation in Nepal; IUCN Nepal and USAID: Kathmandu, Nepal, 1994. [Google Scholar]
- Baral, H.S.; Shah, K.B. Status and Conservation of the Mugger Crocodile in Nepal. Crocodile Spec. Group Newsl. 2013, 32, 16–20. [Google Scholar]
- Khadka, B.B.; Maharjan, A.; Thapalia, B.P.; Lamichhane, B.R. Population Status of the Mugger in Chitwan National Park, Nepal. Crocodile Spec. Group Newsl. 2014, 33, 9–12. [Google Scholar]
- Khadka, B.B.; Acharya, P.M. Status of mugger crocodiles (Crocodylus palustris) in the Rapti and Narayani Rivers of Chitwan National Park, Nepal. Crocodile Spec. Group Newsl. 2019, 38, 11–15. [Google Scholar]
- Khadka, S.; Lamicchane. Population estimate for Mugger Crocodile (Crocodylus palustris) in Lowland Nepal. Crocodile Spec. Group Newsl. 2022, 41, 23–24. [Google Scholar]
- Rawat, Y.B.; Bhatarai, S.; Poudyal, L.P.; Subed, N. Herpetofauna of Shuklaphanta National Park, Nepal. J. Threat. Taxa 2020, 12, 15587–15611. [Google Scholar] [CrossRef]
- Ministry of Energy, Water Resources and Irrigation, Government of Nepal. National Water Plan—Nepal; Water and Energy Commission Water and Energy Commission Secretaria: Kathmandu, Nepal, 2005.
- Bagale, D.; Sigdel, M.; Aryal, D. Drought Monitoring over Nepal for the Last Four Decades and Its Connection with Southern Oscillation Index. Water 2021, 13, 3411. [Google Scholar] [CrossRef]
- Chitwan National Park Office. Management Plan for Chitwan National Park and It’s Buffer Zone 2013–2017; Department of National Parks and Wildlife Conservation, Ministry of Forests and Soil Conservation, Government of Nepal: Kathmandu, Nepal, 2015.
- Maskey, T.M. Movement and Survival of Captive Reared Gharial, Gavialis Gangeticus in the Narayani River, Nepal. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1989. [Google Scholar]
- Bhatt, H.P.; Saund, T.B.; Thapa, J.B. Status and Threats to Mugger Crocodile Crocodylus palustris Lesson, 1831 at Rani Tal, Shuklaphanta Wildlife Reserve, Nepal. Nepal J. Sci. Technol. 2012, 13, 125–131. [Google Scholar] [CrossRef]
- Bardiya National Park Office. Management Plan of Bardiya National Park and its Buffer Zone (2079/80-2083/84); Bardiya National Park Office: Bardiya, Nepal, 2022.
- Sharma, S.; Allen, M.; Oliver, S. Assessing water quality for ecosystem health of the Babai River in Royal Bardia National Park, Nepal. Katmandu Univ. J. Sci. Eng. Technol. 2005, 1, 7. [Google Scholar]
- Allendorf, T.D.; Smith, J.L.D.; Anderson, D.H. Residents’ perceptions of Royal Bardiya National Park, Nepal. Landsc Urban Plan 2007, 82, 33–40. [Google Scholar] [CrossRef]
- Chettri, N.; Chaudhary, S.; Kabir, U.; Bikash, S.; Pratikshya, K.K.; Maheshwar, N.D.; Eklabya, S. Biodiversity values of the Koshi Tappu Wildlife Reserve. In Connecting Flow and Ecology in Nepal: Current State of Knowledge for the Koshi Basin; Doody, T.M., Cuddy, S.M., Bhatta, L.D., Eds.; Sustainable Development Investment Portfolio (SDIP) Project: Barton, Australia, 2016; pp. 71–78. [Google Scholar]
- Adhikari, R.C. Anthropogenic impacts on Koshi Tappu Wetland, a Ramsar site of Nepal. J. Entomol. Zool. Stud. 2019, 7, 655–662. [Google Scholar]
- Chettri, N.; Uddin, K.; Chaudhary, K.; Sharma, E. Linking Spatio-Temporal Land Cover Change to Biodiversity Conservation in the Koshi Tappu Wildlife Reserve, Nepal. Diversity 2013, 5, 335–351. [Google Scholar] [CrossRef]
- Shrestha, R.M.; Alavalapati, J.R.R. Linking Conservation and Development: An Analysis of Local People’s Attitude towards Koshi Tappu Wildlife Reserve, Nepal. Environ. Dev. Sustain. 2006, 8, 69–84. [Google Scholar] [CrossRef]
- Koshi Tapu Wildlife Reserve. Wildlife Reserve and It’s Buffer Zone Management Plan (2074/75–2078/79); Koshi Tappu Wildlife Reserve Office: Pashchim Kushaha, Nepal, 2018. [Google Scholar]
- Diwakar, J.; Bajracharya, S.; Yadav, U.R. Ecological study of Ghodaghodi Lake. Banko Janakari 2009, 19, 18–23. [Google Scholar] [CrossRef][Green Version]
- Kumar, R.B.K. Livelihood in the Wetland of Ghodaghodi Lake, Kailali. Master’s Thesis, Tribhuvan University, Kirtipur, Nepal, 2008. [Google Scholar]
- The World Bank. Nepal Development Update; International Bank for Reconstruction and Development: Washington, DC, USA, 2022. [Google Scholar]
- Pradhan, N.M.B.; Khatri, T.B.; Pandey, M. Mainstreaming the Conservation and Sustainable Use of Wetlands and their Resources into Key National Planning Processes in Nepal; Ramsar Convention Secretariat: Gland, Switzerland, 2018. [Google Scholar]
- de Vos, A. Crocodile Conservation in India. Biol. Conserv. 1984, 29, 183–189. [Google Scholar] [CrossRef]
- Rao, R.J.; Choudhury, B.C. Sympatric distribution of gharial Gavialis gangeticus and mugger crocodile Crocodylus palustris in India. J. Bombay Nat. Hist. Soc. 1992, 89, 312–318. [Google Scholar]
- Jacobson, C. Reintroduction of the mugger crocodile Crocodylus palustris in India. Restor. Reclam. Rev. 1999, 4, 7. [Google Scholar]
- Andrews, H. Preliminary survey of Crocodylus palustris in the Kabini river. Crocodile Spec. Group Newsl. 1995, 14, 11. [Google Scholar]
- Jayson, E.A.; Chandrakasan, S.; Padmanabhan, P. Review of the reintroduction programme of the Mugger crocodile Crocodylus palustris in Neyyar Reservoir, India. Herpetol. J. 2006, 16, 69–76. [Google Scholar]
- Gour, R.; Whitaker, N.; Kartik, A. Status and ditribution of Mugger crocodile Crocodylus palustris in the southern stretch of river Cauvery in Melagiris, India. J. Threat. Taxa 2022, 14, 20733–20739. [Google Scholar] [CrossRef]
- Narasimmarajah, K.; Gopal, A.; Palanivel, S.; Mathai, M.T. Status of Mugger Crocodiles (Crocodylus palustris) in River Moyar, Southern India. Cobra 2018, 12, 1–9. [Google Scholar]
- Samson, A.; Santhosh, P.; Princy, J.; Ramakrishtan, B. Population status of mugger crocodile (Crocodylus palustris) in moyar river, Tamil Nadu, Southern India. Int. J. Pure Appl. Zool. 2021, 9, 1–3. [Google Scholar]
- Kerala Forest Department. Tiger Conservation Plan for Parambikulam Tiger Reserve; Kerala Forest Department: Thiruvananthapuram, India, 2011.
- Vyas, R. Recent scenario of Mugger (Crocodylus plustris) population in three district of Gujarat State, India. World Crocodile Conference. In Proceedings of the 22nd Working Meeting of the IUCN-SSC Crocodile Specialist Group, Negombo, Sri Lanka, 21–23 May 2013; IUCN: Gland, Switzerland, 2013; pp. 220–226. [Google Scholar]
- Vasava, A.; Patel, D.; Vyas, R.; Mistry, V.; Patel, M. Crocs of Charotar: Status, Distribution and Conservation of Mugger Crocodiles in Charotar Region, Gujarat, India; Voluntary Nature Conservancy: Anand, India, 2015. [Google Scholar] [CrossRef]
- Dave, U.; Bhatt, N. Basking Behavior of Marsh Crocodiles (Crocodylus palustris) in Pond Deva, Anand District, Gujarat, India. Reptiles Amphib. 2021, 28, 26–29. [Google Scholar] [CrossRef]
- Dhakad, M.; Khandal, D.; Katdare, S.; Lang, J. Gharial and Mugger in Upstream Tributaries of the Chambal River, North India. Crocodile Spec. Group Newsl. 2017, 36, 11–16. [Google Scholar]
- Das, A.; Rathore, H.; Pandav, B. Mugger Crocodile population from Similipal Tiger Reserve, Odisha. Herpetol. Bull. 2021, 156, 28–30. [Google Scholar]
- Singh, H.; Behera, S. Status of the Important Bioresources of Girwa River with Special Reference to Ganges River Dolphin (Platanista gangetica gangetica) in Katerniaghat Wildlife Sanctuary, Uttar Pradesh, India. In Biological Resources of Water; Ray, S., Ed.; IntechOpen: London, UK, 2018; pp. 285–296. ISBN 978-1-78923-081-9. [Google Scholar]
- Belal, S.; Dhakate, P.; Jeet, R. Status and distribution of Marsh Crocodile Crocodylus palustris in Terai Arc Landscape, Uttarakhand, India. Ela J. For. Wildl. 2021, 10, 855–886. [Google Scholar]
- Central Pollution Control Board, Ministry of Enviroment, Forests and Climate Change. River Stretches for Restoration of Water Quality. MINARS/37 /2014-15; Central Pollution Control Board: Delhi, India, 2015.
- Whitaker, N.; Srinivasan, M. Human crocodile conflict on the Cauvery river delta region, Tamil Nadu, south India. Int. J. Fish. Aquat. Stud. 2020, 8, 1–5. [Google Scholar]
- Inland Waterways Authority of India. Consultancy Services for Preparation of Second Stage Dpr of Cluster-6: (Karnataka)—River Kabini- Nw 51, Detailed Project Report-Kabini River (23.171km)-(nw-51) Volume-i Main Report Document No. P.010256-W-10305-003; Government of India: New Delhi, India, 2021.
- Hejabi, A.; Basavarajappa, H.T.; Qaid, A.; Saeed, A. Heavy Metal Pollution in Kabini River Sediments. Int. J. Environ. Res. 2010, 4, 629–636. [Google Scholar] [CrossRef]
- Karthik, P.; Joseph, S.V.; Mathew, J.C.; Satheendran, S. Habitat Mapping of Mugger Crocodiles Along Kabini River Basin of Wayanad District, Kerala Using Geospatial Technology. JETIR 2019, 6, 325–337. [Google Scholar]
- Karnataka State Pollution Control Board. Action Plan for Rejuvenation of River Kabini; Karnataka State Pollution Control Board: Bangalore, India, 2019.
- Ambedkar, G.; Muniyan, M. Bioaccumulation of some Heavy Metals in the selected five freshwater fish from Kollidam River, Tamilnadu, India. Adv. Appl. Sci. Res. 2011, 2, 221–225. [Google Scholar]
- Thirumalai, P.; Anand, P.H.; Sudha, D.; Manivel, P.; Sentikhumal, R. Sand Quarry Locations on the Segment of Kollidam River and its Impact on Rural Environment Using Spatial Information Technology. Int. J. Recent Sci. Res. 2015, 6, 3239–3244. [Google Scholar]
- Central Ground Water Board, Ministry of Water Resources, River Development and Ganga Rejuvenation, Government of India. Report on Aquifer Mapping and Ground Water Management; Bhavani River Basin: Tamil Nadu; Central Ground Report Board: Bhujal Bhawan, India, 2017.
- Kulandaivel, A.R.K.; Kumar, P.E.; Perumal, V.; Magudeswaran, P.N. Water quality index of river Bhavani at erode region, Tamil Nadu, India. Nat. Environ. Pollut. Technol. 2009, 8, 551–554. [Google Scholar]
- Gowshikan, D.; Balamurugan, P.; Rajesh, R. Assessment of Bhavani River Water Pollution Using Geospatial Technology. IJSTM 2021, 10, 128–142. [Google Scholar]
- Office of the Chief Engineer, Coimbatore Region, Water Resources Department Government of Tamil Nadu. Dam Rehabilitation and Improvement Project (Drip) Phase II; Environmental and Social Due Diligence Report; Office of the Cief Engineer: Coimbatore, India, 2022. [Google Scholar]
- Tamil Nadu Pollution Control Board. Action Plan on Rejuvenation of River Bhavani Sirumugai to Kalingarayan Stretch (Priority-IV); Tamil Nadu Pollution Control Board: Chennai, India, 2019.
- Central Ground Water Board, Ministry of Water Resources, River Development and Ganga Rejuvenation, Government of India. Report on Aquifer Mapping and Ground Water Management; Amaravathi Basin: Tamil Nadu; Central Ground Report Board: Bhujal Bhawan, India, 2017.
- Ahamed, A.J.; Loganathan, K. Water quality concern in the Amaravathi River Basin of Karur district: A view at heavy metal concentration and their interrelationships using geostatistical and multivariate analysis. Geol. Ecol. Lands. 2017, 1, 19–36. [Google Scholar] [CrossRef]
- Karnataka State Pollution Control Board. Action Plan for Rejuvenation of River Bhadra; Karnataka State Pollution Control Board: Bangalore, India, 2019.
- Karnataka State Pollution Control Board. Action Plan for Rejuvenation of River Kali; Karnataka State Pollution Control Board: Bangalore, India, 2019.
- Badusha, M.; Santhosh, S. Assessment of Water Quality of Neyyar River, Kerala, India. J. Aquat. Biol. Fish. 2017, 5, 79–86. [Google Scholar]
- Babu, D.; Nandakumar, V.; John, B.; Jayaprasad, B.; Pramod, S. Siltation analysis in the Neyyar reservoir and forest degradation in its catchment: A study from Kerala state, India. Environ. Geol. 2000, 39, 390–397. [Google Scholar] [CrossRef]
- Jayson, E.A.; Padmanabhan, P. Evaluation of the Problems of Captive/Natural Population of Crocodiles in Neyyar Wildlife Sanctuary and Suggestions for Their Management with Special Emphasis on Reduction of Human—Animal Conflict; Report No. 237; Kerala Forest Research Institute: Kerala, India, 2002. [Google Scholar]
- Vijayasoorya, A.; Smitha, A.V.; Reghunath, R. Land Use Change Analysis of Neyyar Wildlife Sanctuary, Kerala Using GIS and Remote Sensing Methods. Emergent Life Sci. Res. 2016, 2, 59–62. [Google Scholar]
- Bhangaonkar, P.D.; Patel, J.S. Water quality zoning of Vishwamitri River to access environmental flow requirements through aggregation of water quality index. Int. J. Hum. Cap. Urban Manag. 2017, 2, 281–292. [Google Scholar] [CrossRef]
- Vyas, R. The Muggers (Crocodylus palustris) of Vishwamitri River: Past and Present; Herpetology & Environmental Research Project (HERP): Vadodara, India, 2010. [Google Scholar]
- Vyas, R. Results of the 2015 Mugger Crocodile (Crocodylus palustris) count at Vadodara, Gujarat, India. Reptiles Amphib. 2018, 25, 20–25. [Google Scholar] [CrossRef]
- Kerala Forest Department. Neyyar Wildlife Sanctuary Managment Plan; Kerala Fprest Department: Thiruvananthapuram, India, 2012.
- Silawat, R.; Chauhan, R. Analysis of Water Quality of River Kaliyasot Bhopal, (M.P.) India. J. Sci. Technol. Res. 2021, 3, 33–36. [Google Scholar] [CrossRef]
- Silawat, R. Examination of Physico-Chemical Traits and Eutrophication Status of Kaliyasot River in Bhopal, Madhya Pradesh. Int. J. Res. Publ. Rev. 2022, 3, 130–139. [Google Scholar]
- Dahake, S. Taming Godavari River: Navigating through religious, developmental, and environmental narratives. Wiley Interdiscip. Rev. Water 2018, 5, 12. [Google Scholar] [CrossRef]
- Central Water Comission. Krishna Basin Report; Ministry of Water Resources, Government of India: New Delhi, India, 2014.
- Gophane, B.N. Environmental Degradation of River Krishna in Maharashtra—A Geographical Study. The Project Submitted Under Minor Research Scheme in GeoGraphy to University Grants Commission (W. Zone); University Grant Comission: New Delhi, India, 2013. [Google Scholar]
- Atigre, R. Crocodiles of river Krishna: Impact on agriculture, economy, and the sociology of human population in Sangli, Maharashtra, India. J. Threat. Taxa 2018, 10, 12571–12576. [Google Scholar] [CrossRef]
- Mass Initiative for Truth Research & Action (MITRA). Comprehensive Study Report on Krishna River Stretch (Dhom dam to Rathare weir, Satara, Rethare wier to Rajaram Bandhara, Shirol, Sangli); MITRA: Delhi, India, 2014. [Google Scholar]
- Bahali, D.D.; Agrawala, D.K.; Chowdhery, H.J. 2018. Similipal Tiger Reserve. In Floristic Diversity of Tiger Reserves in India; Sanjappa, M., Sing, P., Eds.; Botanical Survey of India: Kolkata, India, 2018; pp. 418–441. [Google Scholar]
- Dash, M.; Behera, B. Management of Similipal Biosphere Reserve Forest Issues and Challenges. Adv. For. Lett. 2012, 1, 7–15. [Google Scholar]
- Vasundhara, B.D.; Mishra, T.; Vasundhara, M.D. Recognition of Community Forest Rigths Under the Forest Rights Act: Experiences from Similipal Tiger Reserve; Vasundhara: Bhubaneswar, India, 2016. [Google Scholar] [CrossRef]
- Inland Waterways Authority of India. Final Feasibility Report on Detailed HydroGraphic Survey in Chambal River (NW-24), from Chakarpura to Awari; Government of India: New Delhi, India, 2017.
- Yadav, N.S.; Sharma, M.P.; Kumar, A.; Pani, S. Water Quality Assessment of Chambal River in National Chambal Sanctuary of Madhya Pradesh. In Environmental Sustainability: Concepts, Principles, Evidences and Innovations; Mishra, G.C., Ed.; Excellent Publishing House: Delhi, India, 2014; pp. 24–35. ISBN 978-93-83083-75-6. [Google Scholar]
- Singh, N.; Sharma, M.P.; Kumar, A. Ecological Health Assessment of Chambal River, India. J. Mater. Environ. Sci. 2015, 6, 613–618. [Google Scholar]
- Ground Water Department, Rajasthan. Hydrogeological Atlas of Rajasthan Banas River Basin; Rolta India Limited: Mumbai, India, 2013.
- Singh, B.P.; Tandon, P.K. Effect of water pollution on Pistia stratiotes in river Suheli of Dudhwa National Park and river Gomti of Lucknow city. Res. Enviromental Life Sci. 2009, 2, 173–178. [Google Scholar]
- Shrestha, A.K.; Shah, R.D.T. River Health Assessment of Mohana Watershed, Nepal: Implication for River Conservation. Appl. Ecol. Environ. Sci. 2019, 7, 153–161. [Google Scholar]
- Vasistha, G.; Lang, J.W.; Dhakate, P.M.; Kothamasi, D. Sand addition promotes gharial nesting in a regulated river-reservoir habitat. Ecol. Solut. Evid. 2021, 2021, e12068. [Google Scholar] [CrossRef]
- Mathur, P.K.; Midha, N. Mapping of National Parks and Wildlife Sanctuaries, Dudhwa Tiger Reserve, WII-NNRMS-MoEF Project, Final Technical Report; Wildlife Institute of India: Dehradun, India, 2008. [Google Scholar]
- Gir Welfare Fund. Gir National Park & Sanctuary; Gir Welfare Fund: Mumbai, India, 2020. [Google Scholar]
- Hertog, S.; Gerlan, P.; Wilmoth, J. India Overtakes China as the World’s Most Populous Country; Future of the world policy brief no. 153; Department of Economic and Social Affairs, United Nations: New York, NY, USA, 2023. [Google Scholar]
- Singh, J.; Mahajan, V. India’s Water Crisis: Challenges, Solutions and Barriers; Working paper; Rajiv Gandhi Institute for Contemporary Studies: New Delhi, India, 2019. [Google Scholar]
- Taenzler, D.; Ruettinger, L.; Ziegenhagen, K.; Murthy, G.G. Water, Crisis and Climate Change in India: A Policy Brief; The Initiative for Peacebuilding—Early Warning Analysis to Action (IfP-EW): Brussels, Belgium, 2011. [Google Scholar]
- Venkatasubramanian, P.; Selvam, K.P.; Jain, A. Access To Clean Drinking Water for All in India—A Matter of Sustainability of Technological and Other Interventions; Clean Water Initiative of the Global Wellness Institute: Miami, FL, USA, 2021. [Google Scholar]
- Bandyopadhyay, S. Sustainable Access to Treated Drinking Water in Rural India. In Rural Water Systems for Multiple Uses and Livelihood Security; Kumar, M.D., James, A.J., Kabir, Y., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 203–227. ISBN 978-0-12-804132-1. [Google Scholar]
- Tripathi, P. Flood Disaster in India: An Analysis of trend and Preparedness. Interdiscip. J. Contemp. Res. 2015, 2, 91–98. [Google Scholar]
- Administration of National Parks and Wildlife Sanctuaries in Karnataka. Encroachment/Occupation of Forest Land and Rehabilitation of Villagers; in Report No. 6; Administration of National Parks and Wildlife Sanctuaries in Karnataka: Bangalore, India, 2017; pp. 43–53.
- Springer, J.; Almeida, F. Protected Areas and the Land Rights of Indigenous Peoples and Local Communities; Current Issues and Future Agenda; Rights and Resources Initiative: Washington, DC, USA, 2015. [Google Scholar]
- Leberg, P.L.; Firmin, B. Role of inbreeding depression and purging in captive breeding and restoration programmes. Mol. Ecol. 2008, 17, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.; Bravo, I.; Wang, J. Inbreeding load and purging: Implications for the short-term survival and the conservation management of small populations. Heredity 2017, 118, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Effective population size/adult population size ratios in wildlife: A review. Genet. Res. 1995, 66, 95–107. [Google Scholar] [CrossRef]
- Reed, D.H.; Frankham, R. Correlation between Fitness and Genetic Diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Vyas, R.; Mistry, V.; Vagasiya, P.; Chauhan, D. Review of mugger Crocodylus palustris Lesson, 1831 mortality by vehicle collisions in Gujarat state, India. J. Anim. Divers. 2023, 5, 80–91. [Google Scholar]
- Vyas, R.; Stevenson, C. Review and analysis of human and Mugger Crocodile conflict in Gujarat, India from 1960 to 2013. J. Threat. Taxa 2017, 9, 11016–11024. [Google Scholar] [CrossRef]
- Department of Fishieries, Handbook on Fishieries Statistics; Ministry of Fishieries, Animal Husbandry and Dairying, Government of India: New Delhi, India, 2020.
- Whitaker, R. Summary report from the Western Asia region. Crocodile Spec. Group Newsl. 1990, 9, 6. [Google Scholar]
- Andrews, H. Summary status report of the Madras Crocodile Bank Trust. Crocodile Spec. Group Newsl. 1990, 9, 6–7. [Google Scholar]
- Manan, S. India. Crocodile Spec. Group Newsl. 1988, 7, 4–5. [Google Scholar]
- Kar, S.K. Crocodile Research and Managment in Orissa. Crocodile Spec. Group Newsl. 1991, 10, 19–20. [Google Scholar]
- Kumar, V.V. Home range of introduced muggers. Crocodile Spec. Group Newsl. 1992, 11, 9. [Google Scholar]
- Singh, J.A.K. Mugger management in Similipal Tiger Reserve, Orissa. Crocodile Spec. Group Newsl. 1993, 12, 4. [Google Scholar]
- Kumar, V.V. Mugger crocodile conservation in Gujarat. Crocodile Spec. Group Newsl. 1994, 13, 6–7. [Google Scholar]
- Prasad, K.K.; Srinivasulu, C.; Srinivasulu, A.; Rao, G.R.K.; Shivaiah, C. Reassessment of Status and Spatial Analysis of the Distribution of Crocodylus palustris in Manjeera Wildlife Sanctuary, Telangana State, India. Herpetol. Conserv. Biol. 2018, 13, 569–575. [Google Scholar]
- Karanth, K.K.; Gupta, S.; Vanamamalai, A. Compensation payments, procedures and policies towards human-wildlife conflict management: Insights from India. Biol. Conserv. 2018, 227, 383–389. [Google Scholar] [CrossRef]




| Habitat | Legal Status | Elevation | Surface Water Availability | Water Quality | Nesting and Basking Site Availability | Interactions with Humans | Notes | Suitability |
|---|---|---|---|---|---|---|---|---|
| Mahaweli Ganga | Flows partly through the Flood Plains National Park [33] | >60 masl | Perennial river | Contaminated with household and industrial waste, siltation | Riverbanks partially altered by sand mining and transport [34] | N/A | Large dams along the river | Moderate |
| Willpatu National Park | Protected Area | >240 masl | Numerous flood plains, swamps, mangroves, estuaries, villus, perennial and seasonal rivers [35] | Good to moderate | N/A | Encroachment, illegal timber extraction [36] | Good | |
| Yala Protected Area Complex | Protected Area | >125 masl | Streams, tanks, water holes, rock pools and lagoons, some drying during the dry season | Good to moderate | N/A | Encroachment, poaching and free-range livestock [37] | Good |
| Habitat | Legal Status | Elevation | Surface Water Availability | Water Quality | Nesting and Basking Site Availability | Interactions with Humans | Notes | Suitability |
|---|---|---|---|---|---|---|---|---|
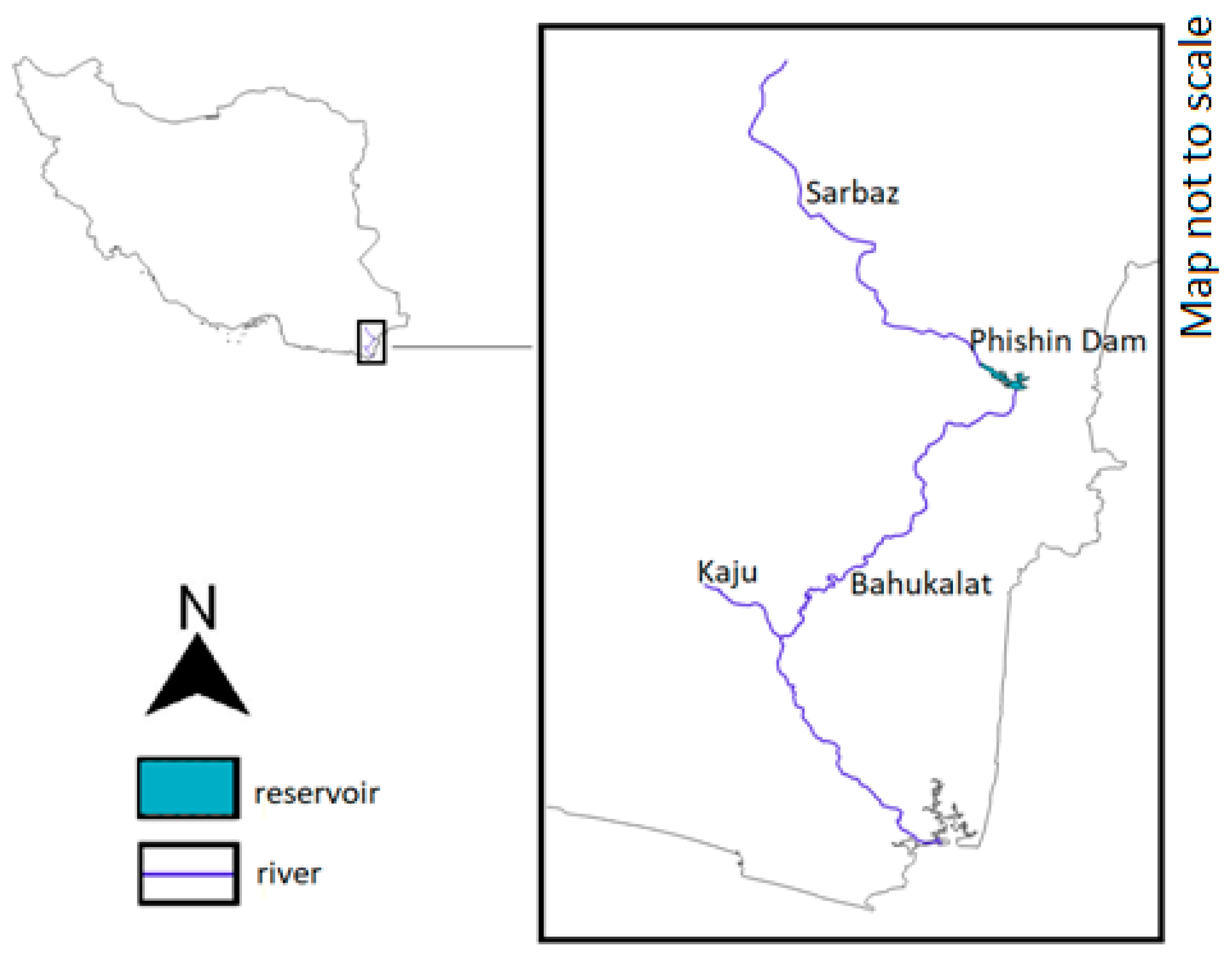
| Kaju River | Gando Protected Area | N/A | Susceptible to droughts and overflows [63] | N/A | Inadequate nesting sites along big dams [58] | Loss of livestock to crocodiles | Ziridian Dam on the river serves as a refuge during prolonged droughts [58] | Moderate |
| Sarbaz River | Gando Protected Area | N/A | Susceptible to drought and overflows [63] | High concentration of lead in sediments [64] | Inadequate nesting sites along big dams [58] | Loss of livestock to crocodiles | Phishin Dam on the river holding one-third of the estimated Iranian mugger population [58] | Moderate |
| Bahukalat River | Gando Protected Area | N/A | Perennial river | High concentration of iron, mercury and lead in blood serum of resident crocodiles [65] | N/A | Loss of livestock to crocodiles | Pollution caused by discharge of industrial and municipal effluents and mine drainage | Moderate |
| Habitat | Legal Status | Elevation | Surface Water Availability | Water Quality | Nesting and Basking Site Availability | Interactions with Humans | Notes | Suitability |
|---|---|---|---|---|---|---|---|---|
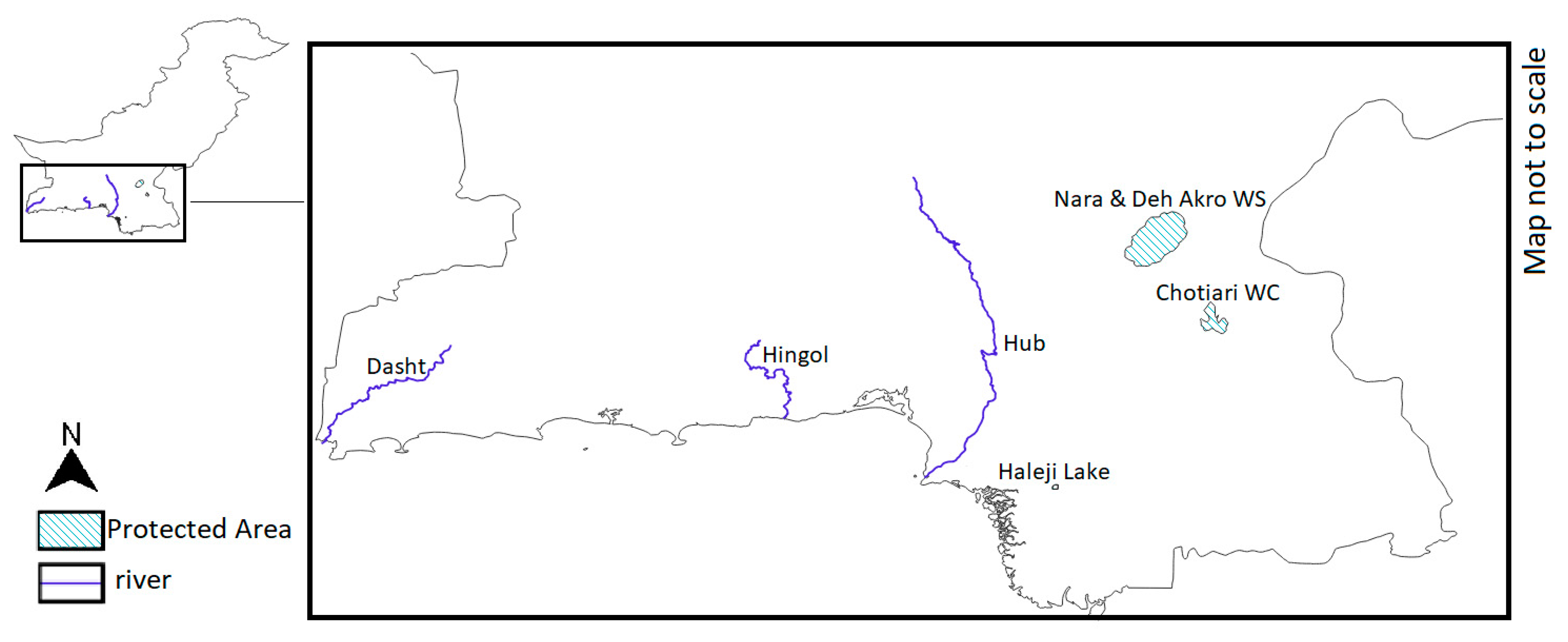
| Deh Akro II Wildlife Sanctuary | Protected Area | 60–80 masl | 36 lakes varying in size (40 to 750 ha) and depth (2 to 15 m) | High levels of chloride, sulphate, calcium, bicarbonate and carbon trioxide, high salinity and TDS factor, eutrophication [84] | Suitable nesting materials | Drownings in fishnets, noise and light disturbance [79] | Siltation is mitigated by annual desiltation of the Nara and Jamrau canals carried out by the government. Despite this, due to water shortage, climate change and eutrophication surface water availability and quality steadily decreases. | Moderate/bad |
| Nara Desert Wildlife Sanctuary | Protected Area under risk of overexploitation | 60–80 masl | More than 200 small lakes, irrigation canals in the northern part [85] | Moderate, varies greatly across lakes, should be monitored [85] | Suitable nesting materials | Gas exploration, road construction, wood cutting, waste dumping [85] | As of 2012, crocodiles were present in at least 19 lakes [80]. Surface water availability has steadily decreased. | Moderate |
| Chotiari Wetland Complex | Protected Area | 60 masl | Wetland comprises many freshwater and brackish lakes [86] | Elevated levels of chloride, calcium, magnesium, sodium, potassium, sulphate, bicarbonate, high salinity, eutrophication [80] | Limited nesting sites [80] | Drownings in fishnets, human disturbance on potential nesting sites [80] | As of 2015, presence of crocodiles was confirmed in 16 lakes [80]. Water pollutants seem to be introduced mostly by agricultural runoff [80]. Surface water availability and quality have steadily decreased. | Bad |
| Dasht River | Not under legal protection | Approximately 78 masl | Seasonal river | Elevated levels of fluoride [87] | N/A | Livestock losses to crocodiles, preventative killings [4] | Moderate | |
| Hub River | Hub Dam Wildlife Sanctuary | 90–141 masl | Perennial river (under normal conditions) | Contaminated with fecal matter and heavy metals [88] | N/A | N/A | Moderate | |
| Hingol River | Partially flowing through the Hingol National Park | N/A | Ephemeral river [89] | Contaminated with fecal matter and lead [89] | N/A | N/A | Data deficient | |
| Haleji Lake Wildlife Sanctuary | Protected Area | N/A | Lake covering 6.58 km2 | Good to moderate depending on the season [81] | N/A | Drownings in fishnets, seven injured and three killed by crocodiles, pointing to significant human–crocodile conflict [80] | Moderate | |
| Mangho Pir | Shrine | Approximately 8 masl | Lake 5–6 m deep, fed from a nearby stream [80,90] | Water quality moderate, actively maintained [80,90] | N/A | Human disturbance (tourist spot) | Lake protected by a brick wall. Crocodile density became an issue, as crocodiles are often observed fighting over space and food [91]. | Moderate |
| Habitat | Legal Status | Elevation | Surface Water Availability | Water Quality | Nesting and Basking Site Availability | Interactions with Humans | Notes | Suitability |
|---|---|---|---|---|---|---|---|---|
| Chitwan National Park | Protected Area under risk of overexploitation | 110–850 masl | Three main rivers draining the park, 58 lakes and wetlands, rivers experience severe flooding [101,107,108] | Siltation | Flooding of nesting sites | Poaching, intensive fishing, preventative killings, (approximately five muggers annually are killed) [101,107] | Invasive plant species and siltation cause wetlands to steadily shrink. Maskey proposed that flooding is the main factor limiting crocodile population in the Naryani river [108]. Rivers are inhabited by the gharial and river dolphin [107]. | Moderate |
| Shuklaphatna National Park | Protected Area under risk of overexploitation | 174–1386 masl | Three major rivers flow through the park | The water quality parameters (dissolved oxygen, total hardness, free carbon dioxide, orthophosphate, biological oxygen demand and ammonia) of Rani Tal wetlands in Shuklaphanta exceeded the normal range to support the mugger [109] | N/A | Poaching, preventive killings, feral animals, habitat degradation and pesticide use in the buffer zone [104] | Moderate | |
| Bardiya National Park | Protected Area | 152–1564 masl | Two major rivers drain the area; due to water scarcity in dry season, 50 man-made ponds have been provided to wildlife, though in monsoon season the region experiences flash floods [110] | Pollutants were detected in Babai river flowing through the Bardiya National Park [111] | Overexploitation of river bank materials [110] | Human–wildlife conflict is a major issue, but local communities are more focused on crop loss to elephants and rhinos and tiger attacks than crocodiles [112] | The park is also inhabited by the gharial. | Moderate |
| Koshi Tappu Wildlife Reserve | Protected Area under risk of overexploitation | 85–90 masl | Perennial river Koshi Tappu, overflowing in the monsoon season | N/A | N/A | Thousands of locals enter Koshi Tappu WR daily to collect firewood, grasses, timber and fish, causing a significant human–wildlife conflict [113,114]. Human–crocodile conflict in the area stems mostly from depredation of fish in private ponds [114] | Study on land cover changes between 1976 and 2010 show a decline in river and swamp cover of about 17% [115]. There is a considerable conflict of interest between local communities and Wildlife Reserve personnel and Royal Nepalese Army, tasked with preventing encroachment [116]. Planned construction of a high dam on the Koshi river is a major concern, since the dam would drastically change the relatively undisturbed river basin [117]. As of this writing, the project remains in planning stage. The reserve is also inhabited by the gharial and the Ganges river dolphin [113] | Moderate |
| Lake Ghodaghodi | Lake Ghodaghodi Complex Ramsar site | 205 masl | Lake Ghosaghodi covers an area of 150 ha; 14 other lakes in vicinity form the Ramsar site. The area faces water shortage in dry seasons and severe floods during monsoon season [118] | Ghodaghodi lake was characterized as hypertrophic (due to high phosphate levels), polluted by high nutrient deposition from decaying aquatic flora [118] | N/A | Wetlands are threatened by poaching, sedimentation, settlement development, invasive species and drainage/reclamation of land for agricultural purposes [119]. It is also a holy site for indigenous Tharu community, celebrating festivals (Agan Panchami) by entering the lake [119] | Threat assessment carried out by Lamichhane et al. listed illegal fishing and habitat modification as most prevalent threats to mugger [98]. They reported human–crocodile conflict to be on a manageable level. | Moderate/bad |
| Habitat | Legal Status | Elevation | Surface Water Availability | Water Quality | Nesting and Basking Site Availability | Interactions with Humans | Notes | Suitability |
|---|---|---|---|---|---|---|---|---|
| Kaveri River (Tamil Nadu, Karnataka, Kerala). | Part of the Kaweri flows in the boundaries of Cauvery Wildlife Sanctuary and Ranganathittu Bird Sanctuary. | 41.88% of the basin lies below 400 masl. | Perennial river, multiple water bodies are located in the basin: over 42,000 lakes, ponds and reservoirs. | 200 km stretch of river non-complying to the Water Quality Criteria [138]. | The basin faces a serious issue of river bank erosion, with high slopes along most of its run. | There were 20 reported attacks on humans by muggers from 2009 to 2019 in Kaweri’s delta, seven of those being fatal [139]. | There are 96 dams of varied size along the Kaweri. Parts of the river are located in regions with high human density; the human population growth was estimated at 17.25% in the region. There are a number of industries in the basin, including the textile industry, cement factories and metal plants. | Moderate. |
| Kabini River (Kerala, Karnataka). | Not under legal protection. | N/A | Perennial river. | 5 km polluted stretch of the river [140]. Heavy metal pollution in sediment samples, namely manganese, copper and zinc [141]. | Siltation is observed around the river banks [142]. | The river is mainly utilized as water source for crops and livestock, with intense fishing in Kittur village only [142]. | There is one large dam—Kabini dam—creating a vast reservoir. Currently, the Kabini river is a candidate for developing an Inland Water Transport route, which would require human interference into river banks [142]. The main pollution sources are sewage discharge and municipal solid waste in Nanjanagud [143]. | Moderate. |
| Kollidam Canal (Tamil Nadu). | Not under legal protection. | N/A | Perennial canal, high risk of flooding due to sediment deposition from sand mining operations | Heavy metal contamination (copper and cadmium) in estuarine sediments and five species of freshwater fish [144]. | Severe river bank erosion due to both legal and illegal sand mining [145]. | There were 20 reported attacks on humans by muggers from 2009 to 2019 in Kaweri’s delta, seven of those being fatal [139]. | Bad. | |
| Bhavani River (Kerala, Tamil Nadu). | Not under legal protection. | 200–3000 masl. | Perennial river, threatened by decreasing groundwater levels [146]. | Heavy metal contamination, fluoride concentration exceeding permissible limit on a 60 km stretch of Bhavani [146]. | N/A | N/A | Contamination results from considerable industrialization of the region, with 400 units of paper, dyeing, sugar and bleaching industries, that both use Bhavani’s water and expel waste into the river [147,148]. Tamil Nadu Pollution Control Board recognizes discharge of untreated domestic sewage as the main pollution source [149]. There are two dams on Bhavani, out of which Bhavani Sagar is known to house crocodiles. It is one of the largest earthen dams in the world and creates the second largest reservoir in Tamil Nadu, with capacity of 928,000,000 m3 [148,150]. | Moderate. |
| Amaravathi River (Tamil Nadu). | Not under legal protection. | 40–500 masl in the plains [151]. | Perennial river. | Heavily polluted; Ahamed and Loganathan detected lead, cadmium and nickel that exceeded permitted levels for drinking water, and categorized gathered water samples as semi-critical in water quality [152]. | N/A | N/A | Pollution is the major issue, due to textile and bleaching industry units located along the river [151,152]. Amaravathi Dam is believed to house one of the largest populations of muggers in India [18]. | Moderate. |
| Moyar River (Karnataka, Tamil Nadu). | Partially flows through Mudumalai Tiger reserve, Sathyamangalam Tiger Reserve and Nilgiri North and South Divisions. | 250–2054. | Perennial river. | Stretches of river are under considerable eutrophication. | N/A | Agricultural runoff, hydroelectric projects, unrestricted fishing activities (including occasional use of dynamite), pesticides and spilling of motor oil [128]. | Moderate. | |
| Bhadra River (Karnataka) | Falls under Bhadra Wildlife Sanctuary and Bhadra Tiger Reserve. | N/A | Perennial river. | There is an identified 10 km stretch of polluted water starting at Holehunnur and ending at Bhadravathi [140,153]. | N/A | N/A | Data deficient. | |
| Kali River (Karnataka). | Not under legal protection. | N/A | Perennial river. | Identified as polluted on a 10 km stretch of Dandeli, due to sewage discharge [154]. | N/A | The river is utilized for tourism and recreational purposes and fishing. There are five reports of mugger attacks on humans in Kali’s vicinity in the last decade. Rising frequency of attacks on humans is likely caused by construction on the river in Dandeli. | Moderate. | |
| Neyyar Wildlife Sanctuary (Kerala). | Protected Area. | 100–1868 masl. | Reservoir. | Moderate [155]. | Erosion of reservoir banks [156]. | 36 muggers were reintroduced into Neyyar reservoir in 1983, which resulted in considerable fueling of human–crocodile conflict, due to 30 reported attacks from 1983 to 2001 [126,157]. Locals also reported frequent attacks on livestock [126]. Local attitude towards crocodiles was reported as hostile in 2001 [126]. | Vijayasoorya et al. reported degradation of forest cover in the sanctuary, which, according to landscape analyses, declined by 10% between 2011 and 2015 [158]. | Moderate. |
| Parambikulam Tiger Reserve (Kerala). | Protected Area. | 300–1438 masl. | Apart from Parambikulam, there are two other man-made reservoirs and two rivers flowing through the reserve. | N/A | N/A | N/A | Data deficient. | |
| Vishwamitri River. | Not under legal protection. | N/A | Seasonal river, susceptible to flooding. | The river is heavily polluted due to sewer and industrial waste disposal and solid waste dump sites [159]. | Suitable nesting sites available [160]. | Numerous attacks on livestock and domestic animals suggest dependency of crocodiles on livestock as food source [131]. They are also observed scavenging on dumping sites and on carcasses, possibly illegally dumped into the river by hospitals [131]. From the period of 2014–2015 alone, 24 attacks on humans were reported, 12 being fatal [161]. | The river is surrounded by urban, rural and industrial landscape, the historic river being converted into a sewer [159]. | Moderate. |
| Narmada River (Madhya Pradesh, Gujarat). | Not under legal protection. | N/A | Perennial river. | 160 km stretch of polluted river in the boundaries of Madhya Pradesh [100]. | N/A | Indian media report five attacks on humans in last decade. | There are 21 major dams on the river [162]. | Moderate. |
| Kaliyasot Dam (Madhya Pradesh). | Not under legal protection. | N/A | Reservoir. | Moderate, high turbidity and alkalinity, high nitrate and sulphate levels [163,164]. | Suitable nesting sites. | Human disturbance and frequent encroachment. | Kaliyasot dam is located within Bhopal, a city with a population of two million. According to Silawat and Chauhan, the reservoir is under high environmental stress due to human encroachment, siltation, high macrophytic growth and sewage discharge [163]. | Moderate/bad. |
| Godawari River (Maharashtra, Telangana, Andhra Pradesh, Chhattisagarh, Odisha). | Not under legal protection. | N/A | Perennial river. | N/A | N/A | N/A | The river is under environmental stress due to rapid urbanization, building of dams, destruction of riparian vegetation, unregulated construction along river banks a sewer discharge [165]. | Data deficient. |
| Krishna River (Karnataka, Maharashtra, Andhra Pradesh). | Not under legal protection. | 300–600 masl on the plateau [166]. | Perennial river. | Central Pollution Control Board deemed that more than half of the river (750 km) should be considered polluted [140]. | River banks susceptible to land sliding [167]. | Atigre reports 16 attacks on humans and 62 attacks on cattle from 2003 to 2017 in Sangli district alone [168]. | As of 2014, total of 660 dams were built on the Krishna [166]. In 2014, there were 11 894 industries in the basin, including sugar factories and sand mining operations [169]. MITRA recognizes the major sources of pollution to be disposal of untreated sewage, industrial effluent, agricultural runoff, religious waste, disposal of municipal solid waste, biomedical waste, hazardous waste and sand mining [169]. | Moderate/bad. |
| Silimpal Tiger Reserve (Odisha). | 500–600 masl. | Numerous perennial streams forming three main river systems [170]. | N/A | N/A | Native tribes live in the vicinity of the reserve, with 65 villages falling into its boundaries, highly dependent on resources provided by the forests [171,172]. Poaching has become a big problem, especially during Akhand Shikar, a ritual mass hunting event. Losses in livestock to predators are an issue in the area. | Moderate. | ||
| Chambal River (Madhya Pradesh, Rajasthan, Uttar Pradesh). | Partially flowing through National Chambal Sanctuary. | 111–843 masl. | Perennial river. | Good [173,174,175]. | Suitable nesting sites. | Overfishing, drownings of crocodilians in fishing nets and illegal sand mining. Limited human–crocodile conflict in the area, mostly due to depredation of livestock. | Good. | |
| Banas River (Rajasthan). | Not under legal protection. | 176–1291 masl. | Seasonal river. | There is a 60 km long patch of the river polluted with chloride, nitrate and fluoride [176]. | N/A | N/A | The Banas River has dried out since the Bisalpur Dam was completed in 1999, with restricted flow outside of monsoon season [134]. A proportion of 90% of Rajasthan was experiencing water stress as of 2014. There are occasional reports of gharial sightings in Banas [134]. | Moderate. |
| Dudhwa Tiger Reserve (Uttar Pradesh). | Protected Area. | 110–185 masl. | Three major rivers susceptible to flooding, seasonal streams. | Suheli and Mohana rivers are moderately polluted with sewer discharge, detergents and fertilizers [177,178]. | After a channel shift due to a flood in 2010, sandy open banks of the river became covered in woody vegetation, limiting nesting spot availability for both muggers and gharials [179]. To remedy this transition, in 2020, a project meant to build additional sand banks was carried out [179]. Muggers and gharials alike swiftly adopted these sand banks, although the authors of the project warn that this solution is only temporary [179]. | The three major Protected Areas forming Dudhwa Tiger Reserve are separated by privately owned land and 125 villages within a 5 km boundary (as of 2001), further causing encroachment into the forests and difficulties in maintaining the reserve’s role as an ecological corridor [180]. | Moderate. | |
| Gir National Park (Uttar Pradesh). | Protected Area. | N/A | Seven major perennial rivers, reservoirs, smaller rivers and streams and 388 artificial water points [181]. | N/A | N/A | There is conflict between the park and local communities, as Maldharis settled in Gir forest caused major damage to the park by overgrazing livestock. Eventually, they were relocated outside of the Protected Area in 1972, but encroachment and overgrazing remain issues [181]. | Mugger crocodiles inhabit major reservoirs in Gir forest and several rivers [181]. | Data deficient. |
| Location | No of Individuals | State | Breeding Centre of Origin |
|---|---|---|---|
| Coringa Wildlife Sanctuary | 3 | Andhra Pradesh | - * |
| Nagarjunsagar-Srisailam Tiger Reserve | 136 | Andhra Pradesh | - |
| Manjira Wildlife Sanctuary | 212 | Telangana | Nehru Zoological Park, Manjira Wildlife Sanctuary Crocodile Breeding Center |
| Pakhal Wildlife Sanctuary | 15 | Telangana | - |
| Kinnersani Wildlife Sanctuary | 33 | Telangana | - |
| Kaweri South Wildlife Sanctuary | 130 | Tamil Nadu | Madras Crocodile Bank Trust |
| Hoggenakal | 48 | Tamil Nadu | - |
| Mundanthurai Wildlife Sanctuary | 25 | Tamil Nadu | - |
| Shivpuri National Park | 25 | Madhya Pradesh | - |
| Neyyar Wildlife Sanctuary | 36 | Kerala | |
| Similipal Tiger Reserve | 390 | Odisha | Nandanakanan, Ramatirtha |
| Mahanadi | 112 | Odisha | Nandanakanan, Ramatirtha |
| Gir National Park | 857 | Gujarat | Sasan, Gandhinagar |
| Country | Change from Historic Range | Population Size Trend | Habitat Suitability | Status of Captive Populations | Major Threats | Chance of Survival | Recommendations |
|---|---|---|---|---|---|---|---|
| Sri Lanka | Insufficient data | Stable | Moderate | No captive populations | 1. Habitat degradation (land conversion, draining of wetlands). 2. Human–crocodile conflict (poaching, “preventative” killings, deaths in fishnets). | Moderate | 1. Monitoring current range and status of muggers in Sri Lanka. 2. Creation of an intense education program on safe coexistence practices and muggers’ role in the ecosystem. 3. Identifying migration corridors and maintaining their permeability. |
| Bangladesh | Extinct in the wild | Extinct in the wild | Bad | 29 muggers (lack of breeding success) | 1. Lack of breeding success in the captive population. 2. High possibility of developing human–crocodile conflict after reintroduction. | Low | 1. Identifying causes of lack of breeding success in the captive population, including genetic testing. 2. In case of future reintroduction, launching an educational program on safe coexistence prior to reintroduction. |
| Iran | No change | Stable | Moderate | Approximately 120 muggers (as of 2018) in three centers. | 1. Climate change (droughts, floods). 2. Habitat degradation (water pollution). | Moderate | 1. Prioritizing water security and combating climate change. 2. Maintaining captive crocodiles for gene preservation. 3. Monitoring water quality in known mugger habitats. |
| Pakistan | Insufficient data | Declining | Bad | Approximately 140 non-hatchlings (overstocked) | 1. Climate change (drought, flooding). 2. Habitat degradation (water shortage, water pollution). 3. Human–crocodile conflict (“preventative” killings, killings for perceived competition, killings for sport, deaths in fishnets). | Low | 1. Prioritizing water security and combating climate change. 2. Monitoring current range and status of muggers in Pakistan. 3. Creation of an intense education program on safe coexistence practices and mugger’s role in the ecosystem. |
| Nepal | Disappeared from Gaidahawa Tal, Jagadishpur Reservoir and Reu river system | Rising | Moderate | Breeding captive population in Gharial Breeding Center in Kasaran | 1. Habitat degradation (siltation, eutrophication, invasive plant species, dam and barrage construction). 2. Climate change (flooding). 3. Human–crocodile conflict “preventative” killings, killings for perceived competition, deaths in fishnets). | Moderate | 1. Monitoring current range and status of mugger in Nepal. 2. Egg collection and hatchling rearing to reduce loss of breeding success to flooding. 3. Creating an education program with emphasis on integrating local communities into crocodile conservation. |
| India | Insufficient data (likely shrinking) | Rising | Moderate | 1968 individuals held in nine captive centers (as of 2021). | 1. Habitat degradation (encroachment, water pollution, dam and barrage construction. 2. Climate change (water shortage). 3. Human–crocodile conflict (deaths in fishnets, opposition from local communities to conservation efforts, lack of safe access to water in rural areas). | High | 1. Prioritizing water safety by better management of sewage and industrial discharge. 2. Creating an education program with emphasis on safe coexistence practices and integrating local communities into crocodile conservation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bors, M.S.; Gowri Shankar, P.; Gruszczyńska, J. Current State of Mugger Populations. Animals 2024, 14, 691. https://doi.org/10.3390/ani14050691
Bors MS, Gowri Shankar P, Gruszczyńska J. Current State of Mugger Populations. Animals. 2024; 14(5):691. https://doi.org/10.3390/ani14050691
Chicago/Turabian StyleBors, Milena Sylwia, Pogiri Gowri Shankar, and Joanna Gruszczyńska. 2024. "Current State of Mugger Populations" Animals 14, no. 5: 691. https://doi.org/10.3390/ani14050691
APA StyleBors, M. S., Gowri Shankar, P., & Gruszczyńska, J. (2024). Current State of Mugger Populations. Animals, 14(5), 691. https://doi.org/10.3390/ani14050691







