Fauna Associated with American Alligator (Alligator mississippiensis) Nests in Coastal South Carolina, USA
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
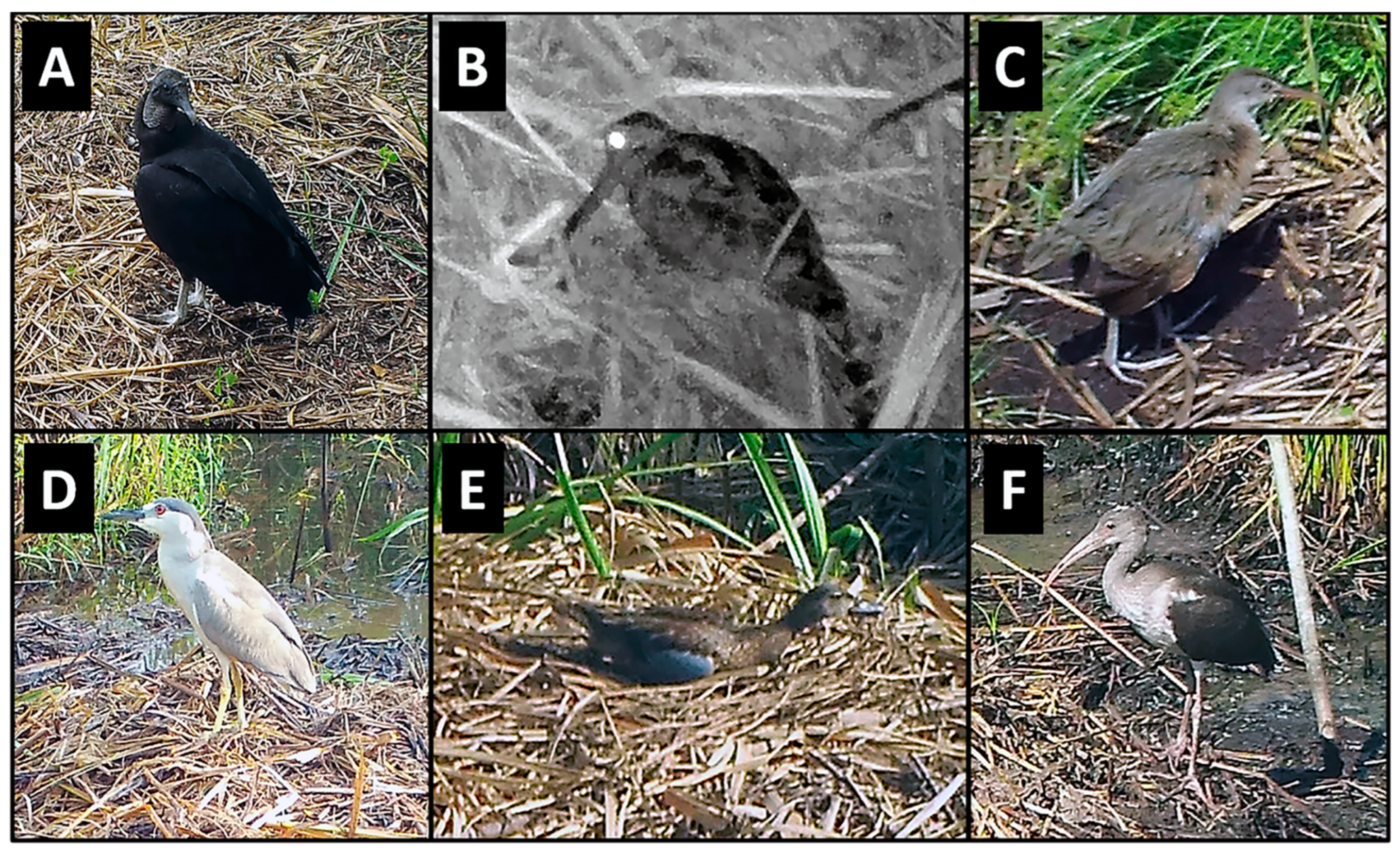
3.1. Birds
3.2. Mammals
3.3. Reptiles
3.4. Amphibians
3.5. Invertebrates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. In Ecosystem Management; Samson, F.B., Knopf, F.L., Eds.; Springer: New York, NY, USA, 1994; pp. 130–147. [Google Scholar]
- Somaweera, R.; Nifong, J.; Rosenblatt, A.; Brien, M.L.; Combrink, X.; Elsey, R.M.; Grigg, G.; Magnusson, W.E.; Mazzotti, F.J.; Pearcy, A.; et al. The ecological importance of crocodilians: Towards evidence-based justification for their conservation. Biol. Rev. 2020, 95, 936–959. [Google Scholar] [CrossRef]
- Craighead, F.C. The role of the alligator in shaping plant communities and maintaining wildlife in the southern Everglades. Florida Nat. 1968, 41, 67–74. [Google Scholar]
- Kushlan, J.A. An Ecological Study of an Alligator Pond in the Big Cypress Swamp of Southern Florida. Master’s Thesis, University of Miami, Coral Gables, FL, USA, 1972. [Google Scholar]
- Kushlan, J.A. Observations on the role of the American alligator (Alligator mississippiensis) in the southern Florida wetlands. Copeia 1974, 1974, 993–996. [Google Scholar] [CrossRef]
- Magnusson, W.E.; Taylor, J.A. Wallows of Crocodylus porosus as dry season refuges in swamps. Copeia 1982, 2, 478–480. [Google Scholar] [CrossRef]
- Wilkinson, P.M. Nesting Ecology of the American Alligator in Coastal South Carolina; Study Completion Report, August 1978–September 1983; South Carolina Wildlife and Marine Resources Department: Columbia, SC, USA, 1983. [Google Scholar]
- Campbell, M.R.; Mazzotti, F.J. Characterization of natural and artificial alligator holes. Southeast. Nat. 2004, 3, 583–594. [Google Scholar] [CrossRef]
- Palmer, M.L.; Mazzotti, F.J. Structure of everglades alligator holes. Wetlands 2004, 24, 115–122. [Google Scholar] [CrossRef]
- Merchant, M.; Murray, C.M.; Cooper, A. American alligator nests as microhabitat for a diversity of vertebrates. Herpetol. Rev. 2014, 45, 201–203. [Google Scholar]
- González-Desales, G.A.; Tello-Sahagún, L.A.; Cadena-Ramírez, C.P.; López-Luna, M.A.; Buenrostro-Silva, A.; García-Grajales, J.; González-Ramón, M.C.; Morales-Mavil, J.E.; Charruau, P.; Sigler, L.; et al. Egg predation and vertebrates associated with wild crocodilian nests in Mexico determined using camera traps. J. Nat. Hist. 2021, 54, 1813–1826. [Google Scholar] [CrossRef]
- Platt, S.G.; Rainwater, T.R.; McMurry, S.T. Fauna associated with the nests of Crocodylus moreletii and Crocodylus moreletii × acutus in Belize. J. Nat. Hist. 2021, 55, 133–149. [Google Scholar] [CrossRef]
- Joanen, T. Nesting ecology of alligator in Louisiana. Proc. Annu. Conf. Southeast. Assoc. Game Fish Comm. 1969, 24, 141–151. [Google Scholar]
- Woodward, A.R.; Elsey, R.M. American Alligator Alligator mississippiensis. In Crocodiles. Status Survey and Conservation Action Plan, 4th ed.; Manolis, S.C., Stevenson, C., Eds.; Crocodile Specialist Group: Darwin, Australia, 2019; pp. 1–6. [Google Scholar]
- McIlhenny, E.A. The Alligator’s Life History; Christopher Publishing House: Boston, MA, USA, 1935. [Google Scholar]
- Grigg, G.; Kirshner, D. Biology and Evolution of Crocodylians. Cornell University Press: Ithaca, NY, USA, 2015. [Google Scholar]
- Joanen, T.; McNease, L.L. Ecology and physiology of nesting and early development of the American Alligator. Am. Zool. 1989, 29, 987–998. [Google Scholar] [CrossRef]
- Hunt, R.H.; Ogden, J.J. Selected aspects of the nesting ecology of American alligators in the Okefenokee Swamp. J. Herpetol. 1991, 25, 448–453. [Google Scholar] [CrossRef]
- Platt, S.G.; Hastings, R.W.; Brantley, C.G. Nesting ecology of American alligators in southeastern Louisiana. Proc. Annu. Conf. Southeast. Assoc. Fish Wildl. Agencies 1995, 49, 629–639. [Google Scholar]
- Liu, Z.; Brandt, L.A.; Ogurcak, D.E.; Mazzotti, F.J. Morphometric and hydrologic characteristics of alligator holes in Everglades National Park, Florida from 1994 to 2007. Ecohydrology 2013, 6, 275–286. [Google Scholar] [CrossRef]
- Platt, S.G.; Resetar, A.; Stuart, B.L. Maximum clutch size of the American alligator. Florida Field Nat. 2004, 32, 102–106. [Google Scholar]
- Eversole, C.B.; Henke, S.E. Effects of red imported fire ants (Solenopsis invicta) presence on success and depredation of American alligator (Alligator mississippiensis) nests: Potential value of a non-native invasive species. Herpetol. Rev. 2018, 49, 22–25. [Google Scholar]
- Gunzburger, M.S. Evaluation of the hatching trigger and larval ecology of the salamander Amphiuma means. Herpetologica 2003, 59, 459–468. [Google Scholar] [CrossRef]
- Thompson, L.M.; Wood, A.C.; Howard, H.J. Amphiuma means (Two-toed Amphiuma). Reproduction. Herpetol. Rev. 2020, 51, 289–290. [Google Scholar]
- Kushlan, J.A.; Kushlan, M.S. Everglades alligator nests: Nesting sites for marsh reptiles. Copeia 1980, 1980, 930–932. [Google Scholar] [CrossRef]
- Elsey, R.M.; Selman, W.; King, R.; Miller, M.; Platt, S.G. Alligator mississippiensis (American alligator). Nests used by other reptiles in coastal Louisiana. Herpetol. Rev. 2013, 44, 659–660. [Google Scholar]
- Elsey, R.M.; Miller, M.; LeJeune, D.; Selman, W. Commensal nesting of Scincella lateralis (Little Brown Skink) in Alligator mississippiensis (American Alligator) nests and Ondatra zibethicus (Muskrat) houses in southwestern Louisiana. Southeast. Nat. 2016, 15, 653–668. [Google Scholar] [CrossRef]
- Rainwater, T.R.; Singh, R.; Tuten, C.A.; Parker, S.L.; Nelson, A.A.; Hart, M.P.; Grosse, A.M.; Treptow, J.; McAlister, M.; Gibbons, P.W.; et al. Alligator mississippiensis (American Alligator). Novel nest associate. Herpetol. Rev. 2023, in press. [Google Scholar]
- Hall, P.M.; Meier, A.J. Reproduction and behavior of western mud snakes (Farancia abacura reinwardtii) in American alligator nests. Copeia 1993, 1993, 219–222. [Google Scholar] [CrossRef]
- Goodwin, T.M.; Marion, W.R. Occurrence of Florida red-bellied turtle eggs in northcentral Florida alligator nests. Florida Sci. 1977, 40, 237–238. [Google Scholar]
- Deitz, D.C.; Jackson, D.R. Use of American alligator nests by nesting turtles. J. Herpetol. 1979, 13, 510–512. [Google Scholar] [CrossRef]
- Deitz, D.C.; Hines, T.C. Alligator nesting in north-central Florida. Copeia 1980, 1980, 249–258. [Google Scholar] [CrossRef]
- Enge, K.M.; Percival, H.F.; Rice, K.G.; Jennings, M.L.; Masson, G.R.; Woodward, A.R. Summer nesting of turtles in alligator nests in Florida. J. Herpetol. 2000, 34, 497–503. [Google Scholar] [CrossRef]
- Fleming, D.M.; Palmisano, A.W.; Joanen, T. Food habits of coastal marsh raccoons with observations of alligator nest predation. Proc. Annu. Conf. Southeast. Assoc. Fish Wildl. Agencies 1976, 30, 357–378. [Google Scholar]
- Elsey, R.M.; Mouton, E.C.; Kinler, N. Effects of feral swine (Sus scrofa) on alligator (Alligator mississippiensis) nests in Louisiana. Southeast. Nat. 2012, 11, 205–218. [Google Scholar] [CrossRef]
- Metzen, W.D. Nesting ecology of alligators on the Okefenokee National Wildlife Refuge. Proc. Annu. Conf. Southeast. Assoc. Fish Wildl. Agencies 1977, 31, 29–32. [Google Scholar]
- Mazzotti, F.J.; McEachern, M.; Rochford, M.; Reed, R.N.; Eckles, J.E.; Vinci, J.; Edwards, J.; Wasilewski, J. Tupinambis merianae as nest predators of crocodiles and turtles in Florida, USA. Biol. Invasions 2015, 17, 47–50. [Google Scholar] [CrossRef]
- Allen, C.R.; Rice, K.G.; Wojcik, D.P.; Percival, H.F. Effect of red imported fire ant envenomation on neonatal American alligators. J. Herpetol. 1997, 31, 318–321. [Google Scholar] [CrossRef]
- Reagan, S.R.; Ertel, J.M.; Wright, V.L. David and Goliath retold. fire ants and alligators. J. Herpetol. 2000, 34, 475–478. [Google Scholar] [CrossRef]
- Wilkinson, P.M.; Rainwater, T.R.; Woodward, A.R.; Leone, E.H.; Carter, C. Determinate growth and reproductive lifespan in the American alligator (Alligator mississippiensis): Evidence from long-term recaptures. Copeia 2016, 104, 843–852. [Google Scholar] [CrossRef]
- Mace, M.M.; Kimball, M.E.; Haffey, E.R. Recruitment and habitat use of early life stage tarpon (Megalops atlanticus) in South Carolina estuaries. Estuaries Coasts 2017, 41, 841–854. [Google Scholar] [CrossRef]
- McCoy, J.A.; Parrott, B.B.; Rainwater, T.R.; Wilkinson, P.M.; Guillette, L.J., Jr. Incubation prior to the canonical thermosensitive period determines sex in the American alligator. Reproduction 2015, 150, 279–287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hobbs, M.T.; Brehme, C.S. An improved camera trap for amphibians, reptiles, small mammals, and large invertebrates. PLoS ONE 2017, 12, e0185026. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.G.; Kinnaird, M.F.; Wibisono, H.T. Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim. Conserv. 2003, 6, 131–139. [Google Scholar] [CrossRef]
- Mohd-Azlan, J.; Engkamat, L. Camera trapping and conservation in Lanjak Entimau Wildlife Sanctuary, Sarawak, Borneo. Raffles Bull. Zool. 2013, 61, 397–405. [Google Scholar]
- Hamilton, W.J. Habits of the swamp rat, Oryzomys palustris palustris (Harlan). Am. Midl. Nat. 1946, 36, 730–736. [Google Scholar] [CrossRef]
- Cameron, G.N.; Kruchek, B.L. Use of coastal wetlands by hispid cotton rats (Sigmodon hispidus). Southwest. Nat. 2005, 50, 397–402. [Google Scholar] [CrossRef]
- Donnelly, T.M.; Bergen, I.; Ihrig, M. Biology and diseases of other rodents. In Laboratory Animal Medicine, 3rd ed.; Fox, J.G., Anderson, L.C., Otto, G.M., Pritchett-Corning, K.R., Whary, M.T., Eds.; Academic Press: New York, NY, USA, 2015; pp. 285–349. [Google Scholar]
- Lamb, T. The influence of sex and breeding condition on microhabitat selection and diet in the pig frog Rana grylio. Am. Midl. Nat. 1984, 111, 311–318. [Google Scholar] [CrossRef]
- Somaweera, R.; Brien, M.; Shine, R. The role of predation in shaping crocodilian natural history. Herpetologica 2013, 27, 23–51. [Google Scholar] [CrossRef]
- Platt, S.G.; Charruau, P.; Rainwater, T.R. Scavenging of crocodile eggs by vultures (Cathartes Aura and Coragyps atratus) in Quintana Roo, Mexico. Bull. Texas Ornithol. Soc. 2014, 47, 37–40. [Google Scholar]
- Sherman, A.R. Additional evidence against the house wren. Wilson Bull. 1925, 37, 129–132. [Google Scholar]
- Ruckel, S.W.; Steele, G.W. Alligator nesting ecology in two habitats in Southern Georgia. Proc. Annu. Conf. Southeast. Assoc. Fish Wildl. Agencies 1984, 38, 212–221. [Google Scholar]
- Hayes-Odum, L.A.; Valdez, D.; Lowe, M.; Weiss, L.; Reiff, P.H.; Jones, D. American alligator (Alligator mississippiensis) nesting at an inland Texas site. Texas J. Sci. 1993, 45, 51–61. [Google Scholar]
- Saalfeld, D.T.; Conway, W.C.; Calkins, G.E. Nest success and hatchling survival of American alligators within inland wetlands of east Texas. J. Wildl. Manag. 2012, 76, 1568–1575. [Google Scholar] [CrossRef]
- Merchant, M.; Savage, D.; Cooper, A.; Slaughter, M.; Perkin, J.S.; Murray, C.M. Nest attendance patterns in the American alligator (Alligator mississippiensis). Copeia 2018, 106, 421–426. [Google Scholar] [CrossRef]
- Torralvo, K.; Botero-Arias, R.; Magnusson, W.E. Temporal variation in black-caiman-nest predation in the varzea of central Brazilian amazonia. PLoS ONE 2017, 12, e0183476. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Galván, A.H.; Elsey, R.M.; McCann, F.; Cupul-Magaña, F.G.; López-Luna, M.A. Putting eggs in one big basket: Communal egg-laying between long-lived reptiles. North-West. J. Zool. 2019, 15, 96–100. [Google Scholar]
- Dugan, B.A.; Rand, A.S.; Burghardt, G.M.; Bock, B.C. Interactions between nesting crocodiles and iguanas. J. Herpetol. 1981, 15, 409–414. [Google Scholar] [CrossRef]
- Platt, S.G.; Rainwater, T.R.; Thorbjarnarson, J.B.; Hekkala, E.R. Iguana iguana (Green Iguana). Nesting. Herpetol. Rev. 2010, 41, 493–494. [Google Scholar]
- Maffei, F.; Da Silveira, R. First record of multiple nests of yellow-spotted river turtle (Podocnemis unifilis) in a nest of black caiman (Melanosuchus niger). Bol Mu. Para. Emílio Goeldi Ciênc. Nat. 2013, 8, 461–465. [Google Scholar] [CrossRef]
- Magnusson, W.E.; Lima, A.P.; Sampaio, R.M. Sources of heat for nests of Paleosuchus trigonatus and a review of crocodilian nest temperatures. J. Herpetol. 1985, 19, 199–207. [Google Scholar] [CrossRef]
- López-Luna, M.A.; Hidalo-Mihart, M.G.; Aguirre-León, G.; González-Ramón, M.d.C.; Rangel-Mendoza, J.A. Effects of nesting environment on incubation temperature and hatching success of Morelet’s crocodile (Crocodylus moreletii) in an urban lake of southeastern Mexico. J. Therm. Biol. 2015, 50, 66–73. [Google Scholar] [CrossRef]
- Pooley, A.C. Preliminary studies on the breeding of the Nile Crocodile Crocodylus niloticus, in Zululand. Lammergeyer 1969, 3, 22–45. [Google Scholar]
- Staton, M.A.; Dixon, J.R. Breeding Biology of the Spectacled Caiman, Caiman Crocodilus Crocodilus, in the Venezuelan Llanos; Research Report, No. 5; United States Fish and Wildlife Service: Washington, DC, USA, 1977; p. 23.
- Webb, G.J.W.; Messel, H.; Magnusson, W.E. The nesting of Crocodylus porosus in Arnhem Land, Northern Australia. Copeia 1977, 1977, 238–249. [Google Scholar] [CrossRef]
- Marco, M.V.P.; Larriera, A.; Piña, C.I. Red fire ant (Solenopsis invicta) effects on broad-snouted Caiman (Caiman latirostris) nest success. J. Herpetol. 2015, 49, 70–74. [Google Scholar] [CrossRef]
- Christy, J.H. Burrow structure and use in the sand fiddler crab, Uca pugilator (Bosc). Anim. Behav. 1982, 30, 687–694. [Google Scholar] [CrossRef]
- Greenspan, B.N. Semi-monthly reproductive cycles in male and female fiddler crabs, Uca pugnax. Anim. Behav. 1982, 30, 1084–1092. [Google Scholar] [CrossRef]
- Michaels, R.E.; Zieman, J.C. Fiddler crab (Uca spp.) burrows have little effect on surrounding sediment oxygen concentrations. J. Exp. Mar. Biol. Ecol. 2013, 448, 104–113. [Google Scholar] [CrossRef]
- Bock, S.L.; Loera, Y.; Johnson, J.M.; Smaga, C.R.; Haskins, D.L.; Tuberville, T.D.; Singh, R.; Rainwater, T.R.; Wilkinson, P.M.; Parrott, B.M. Differential early-life survival underlies the adaptive significance of temperature-dependent sex determination in a long-lived reptile. Funct. Ecol. 2023, 37, 2895–2909. [Google Scholar] [CrossRef]













| Animal Group | Common Name | Scientific Name | Number Recorded | # Nests Where Recorded | Behavior |
|---|---|---|---|---|---|
| Vertebrates | |||||
| Birds | |||||
| Passerines | |||||
| Blue-gray Gnatcatcher | Polioptila caerulea | 5 | 4 (5%) | Feeding/Foraging, Loafing | |
| Blue Grosbeak | Passerina caerulea | 3 | 3 (3%) | Loafing | |
| Blue Jay | Cyanocitta cristata | 2 | 2 (2%) | Loafing | |
| Boat-tailed Grackle | Quiscalus major | 6 | 4 (5%) | Feeding/Foraging, Loafing | |
| Brown-headed Cowbird | Molothrus ater | 1 | 1 (1%) | Feeding/Foraging | |
| Brown Thrasher | Toxostoma rufum | 10 | 7 (8%) | Feeding/Foraging, Loafing | |
| Carolina Wren | Thryothorus ludovicianus | 48 | 18 (23%) | Feeding/Foraging, Traveling, Loafing | |
| Common Grackle | Quiscalus quiscula | 18 | 5 (6%) | Feeding/Foraging, Loafing | |
| Common Yellowthroat | Geothlypis trichas | 45 | 15 (19%) | Feeding/Foraging, Loafing | |
| Eastern Kingbird | Tyrannus tyrannus | 67 | 2 (2%) | Feeding/Foraging, Loafing | |
| Eastern Towhee | Pipilo erythrophthalmus | 4 | 3 (3%) | Feeding/Foraging, Loafing | |
| Gray Catbird | Dumatella carolinensis | 3 | 1 (1%) | Feeding/Foraging | |
| Great Crested Flycatcher | Myiarchus crinitus | 7 | 5 (6%) | Feeding/Foraging, Loafing | |
| Northern Cardinal | Cardinalis cardinalis | 165 | 21 (26%) | Feeding/Foraging, Loafing, Traveling | |
| Northern Mockingbird | Mimus polyglottos | 27 | 4 (5%) | Feeding/Foraging, Loafing | |
| Northern Waterthrush | Parkesia noveboracensis | 10 | 6 (7%) | Feeding/Foraging, Loafing | |
| Orchard Oriole | Icterus spurirus | 4 | 2 (2%) | Feeding/Foraging, Loafing | |
| Painted Bunting | Passerina ciris | 58 | 15 (19%) | Feeding/Foraging, Loafing | |
| Palm Warbler | Setophaga palmarum | 2 | 2 (2%) | Feeding/Foraging, Loafing | |
| Prairie Warbler | Setophaga discolor | 1 | 1 (1%) | Loafing | |
| Red-winged Blackbird | Agelaius phoeniceus | 318 | 36 (46%) | Feeding/Foraging, Traveling, Loafing | |
| White-eyed Vireo | Vireo griseus | 1 | 1 (1%) | Loafing | |
| Unidentified Passerine 1 | Unknown | 169 | 34 (43%) | Feeding/Foraging, Traveling, Loafing | |
| Unidentified Waterthrush 1 | Parkesia sp. | 6 | 5 (6%) | Feeding/Foraging, Loafing | |
| Wading birds | |||||
| American Bittern | Botaurus lentiginosus | 1 | 1 (1%) | Feeding/Foraging | |
| American Woodcock | Scolopax minor | 10 | 3 (3%) | Feeding/Foraging | |
| Black-crowned Night Heron | Nycticorax nycticorax | 24 | 5 (6%) | Feeding/Foraging, Loafing | |
| Clapper Rail | Rallus crepitans | 125 | 16 (20%) | Feeding/Foraging, Traveling, Loafing | |
| Common Gallinule | Gallinula galeata | 46 | 6 (7%) | Feeding/Foraging, Traveling, Loafing | |
| Great Blue Heron | Ardea herodias | 4 | 2 (2%) | Feeding/Foraging, Traveling, Loafing | |
| Great Egret | Ardea alba | 4 | 4 (5%) | Feeding/Foraging, Traveling, Loafing | |
| Green Heron | Butorides virescens | 88 | 16 (20%) | Feeding/Foraging, Traveling, Loafing | |
| King Rail | Rallus elegans | 11 | 3 (3%) | Feeding/Foraging, Loafing | |
| Least Bittern | Ixobrychus exilis | 45 | 13 (16%) | Feeding/Foraging, Traveling, Loafing | |
| Little Blue Heron | Egretta caerulea | 28 | 3 (3%) | Feeding/Foraging, Traveling, Loafing | |
| Snowy Egret | Egretta thula | 7 | 5 (6%) | Feeding/Foraging, Traveling | |
| Sora | Porzana carolina | 4 | 1 (1%) | Feeding/Foraging | |
| Tricolored Heron | Egretta tricolor | 48 | 5 (6%) | Feeding/Foraging, Traveling, Loafing | |
| White Ibis | Eudocimus albus | 40 | 5 (6%) | Feeding/Foraging, Loafing | |
| Yellow-crowned Night Heron | Nyctanassa violacea | 13 | 3 (3%) | Feeding/Foraging, Traveling, Loafing | |
| Unidentified Wading Bird 1 | Unknown | 32 | 13 (16%) | Feeding/Foraging, Traveling, Loafing | |
| Raptors | |||||
| Black Vulture | Coragyps atratus | 5 | 2 (2%) | Feeding/Foraging, Loafing | |
| Cooper’s Hawk | Accipiter cooperii | 1 | 1 (1%) | Feeding/Foraging | |
| Eastern Screech Owl | Megascops asio | 69 | 14 (17%) | Feeding/Foraging, Loafing | |
| Red-shouldered Hawk | Buteo lineatus | 1 | 1 (1%) | Feeding/Foraging | |
| Non-passerine land birds | |||||
| Common Ground Dove | Columbina passerina | 2 | 1 (1%) | Feeding/Foraging | |
| Downy Woodpecker | Picoides pubescens | 1 | 1 (1%) | Feeding/Foraging | |
| Mourning Dove | Zenaida macroura | 25 | 2 (2%) | Feeding/Foraging, Loafing | |
| Northern Flicker | Colaptes auratus | 1 | 1 (1%) | Feeding/Foraging | |
| Waterfowl | |||||
| Black-bellied Whistling Duck | Dendrocygna autumnalis | 14 | 1 (1%) | Loafing | |
| Blue-winged Teal | Anas discors | 4 | 2 (2%) | Traveling, Loafing | |
| Other unidentified birds 1 | |||||
| Unknown | Unknown | 6 | 4 (5%) | Feeding/Foraging, Traveling, Loafing | |
| Total birds | 1639 | ||||
| Mammals | Bobcat | Lynx rufus | 17 | 7 (8%) | Feeding/Foraging, Traveling, Loafing |
| Eastern Gray Squirrel | Sciurus carolinensis | 1 | 1 (1%) | Traveling | |
| Eastern Woodrat | Neotoma floridana | 1 | 1 (1%) | Feeding/Foraging | |
| Marsh Rabbit | Sylvilagus palustris | 422 | 50 (64%) | Feeding/Foraging, Traveling, Loafing | |
| Marsh Rice Rat | Oryzomys palustris | 183 | 31 (39%) | Feeding/Foraging, Burrowing/Shelter, Traveling | |
| Raccoon | Procyon lotor | 648 | 50 (64%) | Feeding/Foraging, Traveling, Loafing | |
| River Otter | Lontra canadensis | 4 | 2 (2%) | Traveling | |
| Virginia Opossum | Didelphis virginiana | 152 | 24 (30%) | Feeding/Foraging, Traveling | |
| White-tailed Deer | Odocoileus virginianus | 66 | 26 (33%) | Feeding/Foraging, Traveling, Loafing | |
| Unidentified Rat 1 | Unknown | 214 | 32 (41%) | Feeding/Foraging, Traveling | |
| Total mammals | 1708 | ||||
| Reptiles | |||||
| Crocodilians | |||||
| American Alligator 2 | Alligator mississippiensis | 229 | 29 (37%) | Feeding/Foraging, Basking, Traveling | |
| Snakes | |||||
| Banded Watersnake | Nerodia fasciata | 25 | 16 (20%) | Feeding/Foraging, Basking, Traveling, Burrowing/Shelter | |
| Canebrake Rattlesnake | Crotalus horridus | 1 | 1 (1%) | Traveling | |
| Copperhead | Agkistrodon contortrix | 2 | 2 (2%) | Traveling | |
| Corn Snake | Pantherophis guttatus | 3 | 3 (3%) | Traveling | |
| Eastern Black Racer | Coluber constrictor | 188 | 37 (47%) | Basking, Burrowing/Shelter, Traveling | |
| Eastern Cottonmouth | Agkistrodon piscivorus | 60 | 22 (28%) | Traveling | |
| Eastern Kingsnake | Lampropeltis getula | 5 | 3 (3%) | Traveling | |
| Eastern Rat Snake | Pantherophis alleghaniensis | 13 | 8 (10%) | Feeding/Foraging, Traveling | |
| Ribbon Snake | Thamnophis sauritus | 65 | 24 (30%) | Feeding/Foraging, Basking, Burrowing/Shelter, Traveling | |
| Ringneck Snake | Diadophis punctatus | 1 | 1 (1%) | Traveling | |
| Rough Green Snake | Opheodrys aestivus | 3 | 3 (3%) | Traveling | |
| Scarlet Snake | Cemophora coccinea | 2 | 1 (1%) | Traveling | |
| Unidentified Snake 1 | Unknown | 72 | 25 (32%) | Basking, Traveling, Burrowing/Shelter | |
| Lizards | |||||
| Broad-headed Skink | Plestiodon laticeps | 38 | 15 (19%) | Basking, Traveling | |
| Eastern Glass Lizard | Ophisaurus ventralis | 50 | 19 (24%) | Feeding/Foraging, Basking, Burrowing/Shelter, Traveling | |
| Green Anole | Anolis carolinensis | 27 | 11 (14%) | Basking, Traveling | |
| Ground Skink | Scincella lateralis | 1 | 1 (1%) | Basking | |
| Unidentified Skink 1 | Unknown | 108 | 26 (33%) | Traveling | |
| Unidentified Lizard 1 | Unknown | 21 | 11 (14%) | Basking, Traveling | |
| Turtles | |||||
| Eastern Mud Turtle | Kinosternon subrubrum | 13 | 9 (11%) | Burrowing/Shelter (Nesting?), Traveling | |
| Yellow-bellied Slider | Trachemys scripta | 2 | 2 (2%) | Basking, Traveling, Nesting? | |
| Total reptiles | 929 | ||||
| Amphibians | American Bullfrog/Pig Frog | Lithobates catesbeiana/Lithobates grylio | 29 | 9 (11%) | Feeding/Foraging, Traveling, Breeding? |
| Green Tree Frog | Hyla cinerea | 31 | 7 (8%) | Feeding/Foraging, Traveling, Breeding? | |
| Southern Leopard Frog | Lithobates sphenocephala | 254 | 32 (41%) | Feeding/Foraging, Burrowing/Shelter, Traveling, Breeding | |
| Unidentified Frog 1 | Unknown | 1194 | 55 (70%) | Feeding/Foraging, Burrowing/Shelter, Traveling, Breeding? | |
| Total amphibians | 1508 | ||||
| Invertebrates | |||||
| Malacostracans | Red-jointed Fiddler Crab | Minuca minax | 7 | 1 [1%] | Traveling, Burrowing/Shelter |
| Unidentified Fiddler Crab 1 | Minuca or Leptuca spp. | 247 | 17 [21%] | Traveling, Burrowing/Shelter | |
| Insects | Fire Ant | Solenopsis invicta | Not quantified | Not quantified | Nesting |
| Total Invertebrates | 254 |
| Behavior | ||||||
|---|---|---|---|---|---|---|
| Animal Group/ Common Name | Feeding/ Foraging | Basking | Nesting | Burrowing/ Shelter | Traveling | Loafing |
| Birds | ||||||
| Passerines | ||||||
| Blue-gray Gnatcatcher | 2 (40%) | 4 (80%) | ||||
| Blue Grosbeak | – | – | – | – | – | 3 (100%) |
| Blue Jay | – | – | – | – | – | 2 (100%) |
| Boat-tailed Grackle | 2 (33%) | – | – | – | – | 4 (66%) |
| Brown-headed Cowbird | 1 (100%) | – | – | – | – | – |
| Brown Thrasher | 6 (60%) | – | – | – | – | 5 (50%) |
| Carolina Wren | 16 (33%) | – | – | – | 1 (2%) | 35 (72%) |
| Common Grackle | 10 (55%) | – | – | – | – | 8 (44%) |
| Common Yellowthroat | 14 (31%) | – | – | – | – | 36 (80%) |
| Eastern Kingbird | 19 (28%) | – | – | – | – | 63 (94%) |
| Eastern Towhee | 1 (25%) | – | – | – | – | 4 (100%) |
| Gray Catbird | 3 (100%) | – | – | – | – | – |
| Great Crested Flycatcher | 2 (28%) | – | – | – | 6 (85%) | |
| Northern Cardinal | 40 (24%) | – | – | – | 1 (0.6%) | 137 (83%) |
| Northern Mockingbird | 9 (33%) | – | – | – | – | 19 (70%) |
| Northern Waterthrush | 5 (50%) | – | – | – | – | 5 (50%) |
| Orchard Oriole | 2 (50%) | – | – | – | – | 2 (50%) |
| Painted Bunting | 10 (17%) | – | – | – | – | 50 (86%) |
| Palm Warbler | 1 (50%) | – | – | – | – | 2 (100%) |
| Prairie Warbler | – | – | – | – | – | 1 (100%) |
| Red-winged Blackbird | 120 (37%) | – | – | – | 5 (1%) | 209 (65%) |
| White-eyed Vireo | – | – | – | – | – | 1 (100%) |
| Wading birds | ||||||
| American Bittern | 1 (100%) | – | – | – | – | – |
| American Woodcock | 10 (100%) | – | – | – | – | – |
| Black-crowned Night Heron | 19 (79%) | – | – | – | – | 10 (41%) |
| Clapper Rail | 99 (79%) | – | – | – | 36 (28%) | 16 (12%) |
| Common Gallinule | 33 (71%) | – | – | – | 11 (23%) | 3 (6%) |
| Great Blue Heron | 2 (50%) | – | – | – | 2 (50%) | 2 (50%) |
| Great Egret | 1 (25%) | – | – | – | 2 (50%) | 1 (25%) |
| Green Heron | 78 (88%) | – | – | – | 6 (6%) | 29 (32%) |
| King Rail | 11 (100%) | – | – | – | – | 2 (18%) |
| Least Bittern | 35 (77%) | – | – | – | 7 (15%) | 8 (17%) |
| Little Blue Heron | 28 (100%) | – | – | – | 1 (3%) | 9 (32%) |
| Snowy Egret | 6 (85%) | – | – | – | 1 (14%) | – |
| Sora | 4 (100%) | – | – | – | – | – |
| Tricolored Heron | 23 (47%) | – | – | – | 4 (8%) | 26 (54%) |
| White Ibis | 38 (95%) | – | – | – | – | 11 (27%) |
| Yellow-crowned Night Heron | 12 (92%) | – | – | – | 1 (7%) | 2 (15%) |
| Raptors | ||||||
| Black Vulture | 2 (40%) | – | – | – | – | 5 (100%) |
| Cooper’s Hawk | 1 (100%) | – | – | – | – | – |
| Eastern Screech Owl | 60 (86%) | – | – | – | – | 13 (18%) |
| Red-shouldered Hawk | 1 (100%) | – | – | – | – | – |
| Non-passerine land birds | ||||||
| Common Ground Dove | 2 (100%) | – | – | – | – | – |
| Downy Woodpecker | 1 (100%) | – | – | – | – | – |
| Mourning Dove | 15 (60%) | – | – | – | 14 (56%) | |
| Northern Flicker | 1 (100%) | – | – | – | – | – |
| Waterfowl | ||||||
| Black-bellied Whistling Duck | – | – | – | – | – | 14 (100%) |
| Blue-winged Teal | – | – | – | – | 3 (75%) | 1 (25%) |
| Mammals | ||||||
| Bobcat | 4 (23%) | – | – | – | 7 (41%) | 6 (35%) |
| Eastern Grey Squirrel | – | – | – | – | 1 (100%) | – |
| Eastern Woodrat | 1 (100%) | – | – | – | – | – |
| Marsh Rabbit | 335 (79%) | – | – | – | 120 (28%) | 9 (2%) |
| Marsh Rice Rat | 97 (53%) | – | – | 4 (2%) | 98 (53%) | – |
| Raccoon | 522 (80%) | – | – | – | 172 (26%) | 9 (1%) |
| River Otter | – | – | – | – | 4 (100%) | – |
| Virginia Opossum | 67 (44%) | 94 (61%) | ||||
| White-tailed Deer | 29 (43%) | – | – | – | 37 (56%) | 6 (9%) |
| Reptiles | ||||||
| Crocodilians | ||||||
| American Alligator | 6 (2%) | 112 (48%) | – | – | 123 (53%) | – |
| Snakes | ||||||
| Banded Watersnake | 4 (16%) | 5 (20%) | 1 (4%) | 17 (68%) | – | |
| Canebrake Rattlesnake | – | – | – | – | 1 (100%) | – |
| Copperhead | – | – | – | – | 2 (100%) | – |
| Corn Snake | – | – | – | – | 3 (100%) | – |
| Eastern Black Racer | – | 91 (48%) | – | 1 (0.5%) | 103 (54%) | – |
| Eastern Cottonmouth | – | – | – | – | 60 (100%) | – |
| Eastern Kingsnake | – | – | – | – | 5 (100%) | – |
| Eastern Rat Snake | 2 (15%) | – | – | – | 11 (84%) | – |
| Ribbon Snake | 4 (6%) | 20 (30%) | – | 1 (1%) | 45 (69%) | – |
| Ringneck Snake | – | – | – | – | 1 (100%) | – |
| Rough Green Snake | – | – | – | – | 3 (100%) | – |
| Scarlet Snake | – | – | – | – | 2 (100%) | – |
| Lizards | ||||||
| Broad-headed Skink | – | 38 (71%) | – | – | 12 (31%) | – |
| Eastern Glass Lizard | 4 (8%) | 2 (4%) | – | 5 (10%) | 44 (88%) | – |
| Green Anole | – | 23 (85%) | – | – | 5 (18%) | – |
| Ground Skink | – | 1 (100%) | – | – | – | – |
| Turtles | ||||||
| Eastern Mud Turtle | – | – | ? | 2 (15%) | 11 (84%) | – |
| Yellow-bellied Slider | – | 1 (50%) | ? | – | 1 (50%) | – |
| Amphibians | ||||||
| American Bullfrog/Pig Frog | 19 (65%) | – | – | – | 11 (37%) | – |
| Green Tree Frog | 23 (74%) | – | – | – | 10 (32%) | – |
| Southern Leopard Frog | 196 (77%) | – | – | 15 (5%) | 75 (29%) | – |
| Malacostracans | ||||||
| Red-jointed Fiddler Crab | – | – | – | 7 (100%) | 7 (100%) | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rainwater, T.R.; Singh, R.; Tuten, C.A.; Given, A.M.; Gibbons, P.W.; Song, B.; Platt, S.G.; Wilkinson, P.M.; Bodinof Jachowski, C.M. Fauna Associated with American Alligator (Alligator mississippiensis) Nests in Coastal South Carolina, USA. Animals 2024, 14, 620. https://doi.org/10.3390/ani14040620
Rainwater TR, Singh R, Tuten CA, Given AM, Gibbons PW, Song B, Platt SG, Wilkinson PM, Bodinof Jachowski CM. Fauna Associated with American Alligator (Alligator mississippiensis) Nests in Coastal South Carolina, USA. Animals. 2024; 14(4):620. https://doi.org/10.3390/ani14040620
Chicago/Turabian StyleRainwater, Thomas R., Randeep Singh, Clarissa A. Tuten, Aaron M. Given, Parker W. Gibbons, Bo Song, Steven G. Platt, Philip M. Wilkinson, and Catherine M. Bodinof Jachowski. 2024. "Fauna Associated with American Alligator (Alligator mississippiensis) Nests in Coastal South Carolina, USA" Animals 14, no. 4: 620. https://doi.org/10.3390/ani14040620
APA StyleRainwater, T. R., Singh, R., Tuten, C. A., Given, A. M., Gibbons, P. W., Song, B., Platt, S. G., Wilkinson, P. M., & Bodinof Jachowski, C. M. (2024). Fauna Associated with American Alligator (Alligator mississippiensis) Nests in Coastal South Carolina, USA. Animals, 14(4), 620. https://doi.org/10.3390/ani14040620









