The Role of Selenitetriglycerides in Enhancing Antioxidant Defense Mechanisms in Peripartum Holstein-Friesian Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Group Assignment
2.2. Blood Sampling, Biochemical Components, and Antioxidant Analyses
2.3. Liver Biopsy and RNA Extraction
2.4. cDNA Synthesis and RT-qPCR Analysis
2.5. Statistical Analyses
3. Results
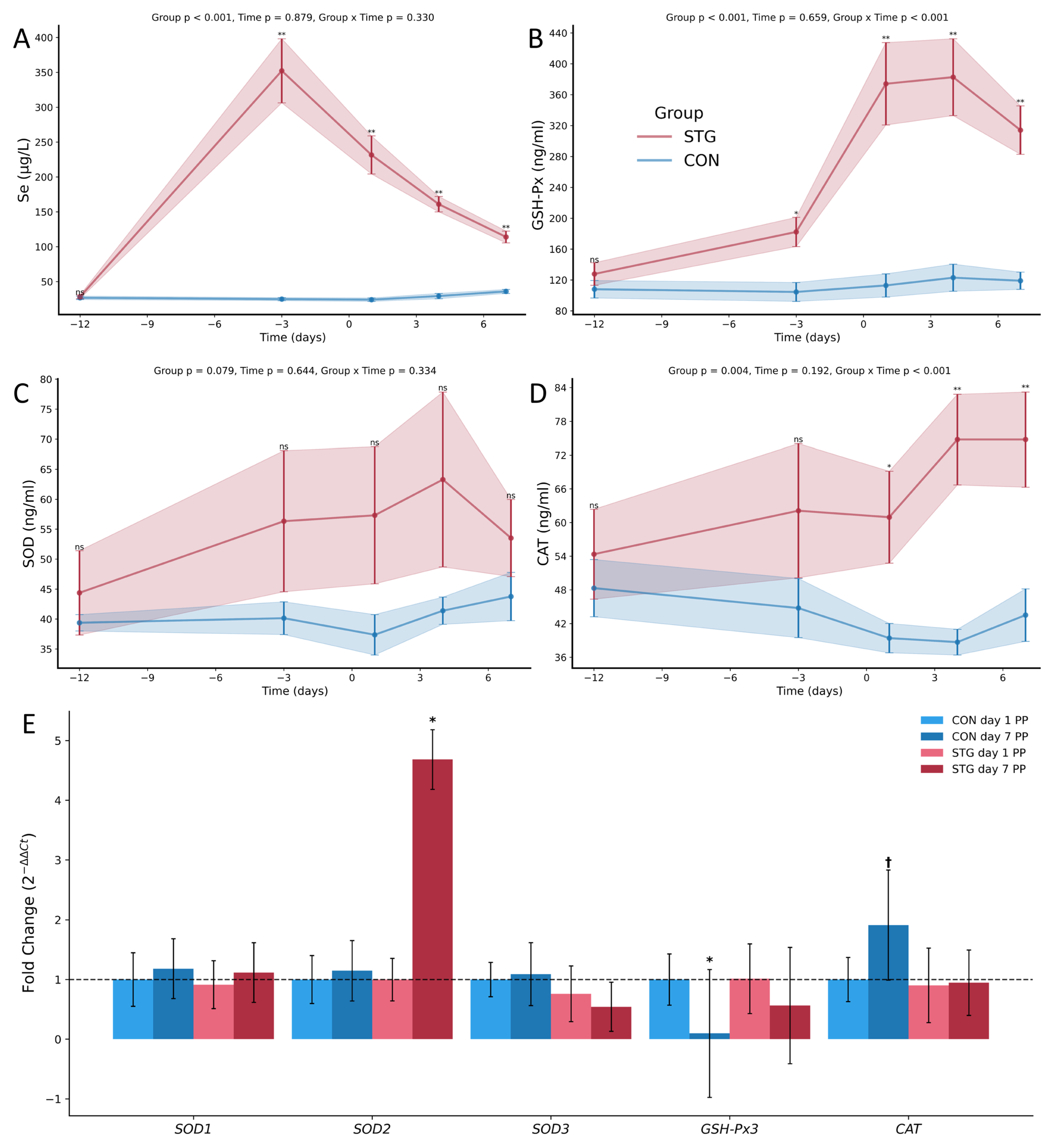
3.1. Biochemical Parameters
3.2. Antioxidant Parameters
3.3. Relative Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Żarczyńska, K.; Żarczyński, P.; Sobiech, P.; Snarska, A.; Stopyra, A.; Wieteska, M.; Płaczek, A. The effect of micronutrient deficiencies on the health status of transition cows. J. Elem. 2017, 22, 1223–1234. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.; Lauridsen, C.; Dunshea, F.R. The importance of dietary antioxidants on oxidative stress, meat and milk production, and their preservative aspects in farm animals: Antioxidant action, animal health, and product quality—Invited review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef] [PubMed]
- Jóźwik, A.; Krzyżewski, J.; Strzałkowska, N.; Bagnicka, E.; Poławska, E.; Horbańczuk, J. Oxidative stress in high yielding dairy cows during the transition period. Med. Weter. 2012, 68, 468–474. [Google Scholar]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef]
- Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The antioxidant properties of selenium and vitamin E; their role in periparturient dairy cattle health regulation. Antioxidants 2021, 10, 1555. [Google Scholar] [CrossRef] [PubMed]
- Shebis, Y.; Iluz, D.; Kinel-Tahan, Y.; Dubinsky, Z.; Yehoshua, Y. Natural antioxidants: Function and sources. Food Nutr. 2013, 4, 643–649. [Google Scholar] [CrossRef]
- Wroblewski, M.; Wroblewska, J.; Nuszkiewicz, J.; Pawlowska, M.; Wesolowski, R.; Wozniak, A. The Role of Selected Trace Elements in Oxidoreductive Homeostasis in Patients with Thyroid Diseases. Int. J. Mol. Sci. 2023, 24, 4840. [Google Scholar] [CrossRef] [PubMed]
- Żarczynska, K.; Sobiech, P.; Radwinska, J.; Rekawek, W. Effects of selenium on animal health. J. Elem. 2013, 18, 329–340. [Google Scholar] [CrossRef]
- Zhao, L.; Zong, W.; Zhang, H.; Liu, R. Kidney Toxicity and Response of Selenium Containing Protein-glutathione Peroxidase (Gpx3) to CdTe QDs on Different Levels. Toxicol. Sci. 2019, 168, 201–208. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Darke, A.K.; Penney, K.L.; Tangen, C.M.; Goodman, P.J.; Lee, G.-S.M.; Sun, T.; Peisch, S.; Tinianow, A.M.; Rae, J.M. Selenium-or vitamin E–related gene variants, interaction with supplementation, and risk of high-grade prostate cancer in SELECT. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1050–1058. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Malevu, T.D.; Sochor, J.; Baron, M.; Melcova, M.; Zidkova, J.; et al. A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species—A Critical Review. Int. J. Mol. Sci. 2017, 18, 2209. [Google Scholar] [CrossRef]
- Liu, L.; Wang, M.; Gong, N.; Tian, P.; Deng, H. Se improves GPX4 expression and SOD activity to alleviate heat-stress-induced ferroptosis-like death in goat mammary epithelial cells. Anim. Cells Syst. 2021, 25, 283–295. [Google Scholar] [CrossRef]
- Jastrzebski, Z.; Czyzewska-Szafran, H.; Remiszewska, M.; Fijalek, Z.; Fitak, B.A.; Suchocki, P. Pharmacokinetics of selol, a new agent containing selenium, in rats. Drugs Exp. Clin. Res. 1997, 23, 7–11. [Google Scholar] [PubMed]
- Koller, L.D.; Exon, J.H. The two faces of selenium-deficiency and toxicity--are similar in animals and man. Can. J. Vet. Res. 1986, 50, 297–306. [Google Scholar]
- Żarczynska, K.; Sobiech, P.; Mee, J.; Illek, J. The influence of short-term selenitetriglycerides supplementation on blood selenium, and hepatic, renal, metabolic and hematological parameters in dairy cows. Pol. J. Vet. Sci. 2020, 23, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Żarczynska, K.; Sobiech, P.; Tinson, A. Influence of selenitetriglyceride supplementation on selenium blood status and selected hematological and biochemical parameters in camels (Camelus dromedarius). J. Elem. 2020, 25, 1363–1373. [Google Scholar] [CrossRef]
- Żarczyńska, K.; Sobiech, P.; Tobolski, D.; Mee, J.F.; Illek, J. Effect of a single, oral administration of selenitetriglycerides, at two dose rates, on blood selenium status and haematological and biochemical parameters in Holstein-Friesian calves. Ir. Vet. J. 2021, 74, 11. [Google Scholar] [CrossRef]
- Świerczyński, G.; Tobolski, D.; Żarczynska, K. Effect of selenitetriglycerides supplementation in pregnant sows on hematological-biochemical profiles, Se concentration and transfer to offspring. J. Elem. 2024, 29, 21–35. [Google Scholar] [CrossRef]
- Sochacka, M.; Giebultowicz, J.; Remiszewska, M.; Suchocki, P.; Wroczynski, P. Effects of Selol 5% supplementation on the activity or concentration of antioxidants and malondialdehyde level in the blood of healthy mice. Pharmacol. Rep. 2014, 66, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Dominiak, A.; Wilkaniec, A.; Jesko, H.; Czapski, G.A.; Lenkiewicz, A.M.; Kurek, E.; Wroczynski, P.; Adamczyk, A. Selol, an organic selenium donor, prevents lipopolysaccharide-induced oxidative stress and inflammatory reaction in the rat brain. Neurochem. Int. 2017, 108, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Pavlata, L.; Pechova, A.; Illek, J. Direct and indirect assessment of selenium status in cattle-a comparison. Acta Vet. Brno 2000, 69, 281–287. [Google Scholar] [CrossRef]
- Gong, J.; Xiao, M. Selenium and antioxidant status in dairy cows at different stages of lactation. Biol. Trace Elem. Res. 2016, 171, 89–93. [Google Scholar] [CrossRef]
- Stowe, H.D.; Herdt, T.H. Clinical assessment of selenium status of livestock. J. Anim. Sci. 1992, 70, 3928–3933. [Google Scholar] [CrossRef]
- Gerloff, B.J. Effect of selenium supplementation on dairy cattle. J. Anim. Sci. 1992, 70, 3934–3940. [Google Scholar] [CrossRef]
- Pavlata, L.; Illek, J.; Pechova, A.; Matějíček, M. Selenium status of cattle in the Czech Republic. Acta Vet. Brno 2002, 71, 3–8. [Google Scholar] [CrossRef]
- Li, Y.; Ding, H.; Wang, X.; Feng, S.; Li, X.; Wang, Z.; Liu, G.; Li, X. An association between the level of oxidative stress and the concentrations of NEFA and BHBA in the plasma of ketotic dairy cows. J. Anim. Physiol. Anim. Nutr. 2016, 100, 844–851. [Google Scholar] [CrossRef]
- Mikuła, R.; Pruszyńska-Oszmałek, E.; Pszczola, M.; Rząsińska, J.; Sassek, M.; Nowak, K.W.; Nogowski, L.; Kołodziejski, P.A. Changes in metabolic and hormonal profiles during transition period in dairy cattle—The role of spexin. BMC Vet. Res. 2021, 17, 359. [Google Scholar] [CrossRef] [PubMed]
- Sobiech, P.; Żarczyńska, K.; Rękawek, W.; Snarska, A.; Eleusizowa, A.; Kowalczyk, E.; Illek, J. Effect of parenteral supplementation of selenium and vitamin E on selected blood biochemical parameters in HF cows during the transition period. Med. Weter. 2015, 71, 683–689. [Google Scholar]
- Li, Z.; Tang, J.; Li, J.; Ling, D.; He, X.; Tang, Y.; Yi, P.; Yang, Y.; Khoo, H.E.; Liu, Y. Organic selenium supplementation increases serum selenium levels in healthy Xinjiang brown cattle fed selenised yeast. J. Anim. Feed. Sci. 2023. [Google Scholar] [CrossRef]
- Gong, J.; Xiao, M. Increasing selenium supply during the close-up dry period improves nutrient metabolism and attenuates inflammatory response after calving in dairy cows. Anim. Sci. J. 2021, 92, e13551. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Chamani, M.; Amanlou, H.; Nikkhah, A.; Sadeghi, A.A. Effects of different sources of selenium supplementation on antioxidant indices, biochemical parameters, thyroid hormones and Se status in transition cows. Acta Sci. Anim. Sci. 2019, 41, e44392. [Google Scholar] [CrossRef]
- Djoković, R.; Šamanc, H.; Jovanović, M.; Nikolić, Z. Blood concentrations of thyroid hormones and lipids and content of lipids in the liver of dairy cows in transitional period. Acta Vet. Brno 2007, 76, 525–532. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free. Radic. Biol. Med. 2008, 45, 231–241. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernandez, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef]
- Ren, Z.H.; Bai, L.P.; Shen, L.H.; Luo, Z.Z.; Zhou, Z.H.; Zuo, Z.C.; Ma, X.P.; Deng, J.L.; Wang, Y.; Xu, S.Y.; et al. Comparative iTRAQ Proteomics Reveals Multiple Effects of Selenium Yeast on Dairy Cows in Parturition. Biol. Trace Elem. Res. 2020, 197, 464–474. [Google Scholar] [CrossRef]
- Hall, J.A.; Bobe, G.; Vorachek, W.R.; Estill, C.T.; Mosher, W.D.; Pirelli, G.J.; Gamroth, M. Effect of supranutritional maternal and colostral selenium supplementation on passive absorption of immunoglobulin G in selenium-replete dairy calves. J. Dairy. Sci. 2014, 97, 4379–4391. [Google Scholar] [CrossRef]
- Duffield, T.F.; Lissemore, K.D.; McBride, B.W.; Leslie, K.E. Impact of hyperketonemia in early lactation dairy cows on health and production. J. Dairy. Sci. 2009, 92, 571–580. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy. Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Vovk, M.A.; Batalova, A.A.; Jenkins, R.O.; Goncharov, N.V. The Universal Soldier: Enzymatic and Non-Enzymatic Antioxidant Functions of Serum Albumin. Antioxidants 2020, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Pregel, P.; Bollo, E.; Cannizzo, F.T.; Biolatti, B.; Contato, E.; Biolatti, P.G. Antioxidant capacity as a reliable marker of stress in dairy calves transported by road. Vet. Rec. 2005, 156, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Celi, P. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 2011, 33, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kupczyński, R.; Chudoba-Drozdowska, B. Values of selected biochemical parameters of cows’ blood during their drying-off and the beginning of lactation. Electron. J. Pol. Agric. Univ. Ser. Vet. Med. 2002, 5, 1–10. [Google Scholar]
- Tóthová, C.; Nagy, O.; Seidel, H.; Konvičná, J.; Farkašová, Z.; Kováč, G. Acute phase proteins and variables of protein metabolism in dairy cows during the pre- and postpartal period. Acta Vet. Brno 2008, 77, 51–57. [Google Scholar] [CrossRef]
- Piccione, G.; Messina, V.; Marafioti, S.; Casella, S.; Giannetto, C.; Fazio, F. Changes of some haematochemical parameters in dairy cows during late gestation, post partum, lactation and dry periods. Vet. Med. Zoot 2012, 58, 59–64. [Google Scholar]
- Huang, M.J.; Zhao, J.Y.; Xu, J.J.; Li, J.; Zhuang, Y.F.; Zhang, X.L. lncRNA ADAMTS9-AS2 Controls Human Mesenchymal Stem Cell Chondrogenic Differentiation and Functions as a ceRNA. Mol. Ther. Nucleic Acids 2019, 18, 533–545. [Google Scholar] [CrossRef]
- Kehm, R.; Baldensperger, T.; Raupbach, J.; Hohn, A. Protein oxidation—Formation mechanisms, detection and relevance as biomarkers in human diseases. Redox Biol. 2021, 42, 101901. [Google Scholar] [CrossRef]
- Kolagal, V.; Karanam, S.; Dharmavarapu, P.; D’Souza, R.; Upadhya, S.; Kumar, V.; Kedage, V.; Muttigi; Shetty, J.K.; Prakash, M. Determination of oxidative stress markers and their importance in early diagnosis of uremia-related complications. Indian J. Nephrol. 2009, 19, 8–12. [Google Scholar]
- Juniper, D.T.; Phipps, R.H.; Givens, D.I.; Jones, A.K.; Green, C.; Bertin, G. Tolerance of ruminant animals to high dose in-feed administration of a selenium-enriched yeast. J. Anim. Sci. 2008, 86, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zagrodzki, P.; Bik, D.; Fitak, B.A.; Suchocki, P.; Niemczuk, K. Selenoenzymes in animal tissues after supplementation with SELOL. Bull. Vet. Inst. Pulawy 2000, 44, 215–220. [Google Scholar]
- Phillippo, M.; Arthur, J.R.; Price, J.; Halliday, G.J. The effects of selenium, housing and management on the incidence of pneumonia in housed calves. Vet. Rec. 1987, 121, 509–512. [Google Scholar] [CrossRef]
- Arthur, J.R. The glutathione peroxidases. Cell Mol. Life Sci. 2000, 57, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Pilarczyk, B.; Jankowiak, D.; Tomza-Marciniak, A.; Pilarczyk, R.; Sablik, P.; Drozd, R.; Tylkowska, A.; Skólmowska, M. Selenium Concentration and Glutathione Peroxidase (GSH-Px) Activity in Serum of Cows at Different Stages of Lactation. Biol. Trace Elem. Res. 2011, 147, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.L.; Karcher, E.L.; Rezamand, P.; Gandy, J.C.; VandeHaar, M.J.; Capuco, A.V.; Sordillo, L.M. Evaluation of antioxidant and proinflammatory gene expression in bovine mammary tissue during the periparturient period. J. Dairy. Sci. 2009, 92, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Xiao, M. Effect of Organic Selenium Supplementation on Selenium Status, Oxidative Stress, and Antioxidant Status in Selenium-Adequate Dairy Cows During the Periparturient Period. Biol. Trace Elem. Res. 2018, 186, 430–440. [Google Scholar] [CrossRef]
- Konvičná, J.; Vargová, M.; Paulíková, I.; Kováč, G.; Kostecká, Z. Oxidative stress and antioxidant status in dairy cows during prepartal and postpartal periods. Acta Vet. Brno 2015, 84, 133–140. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, N.K.; Singh, O.P.; Pandey, V.K.; Verma, P.K. Oxidative Stress and Antioxidant Status during Transition Period in Dairy Cows. Asian-Australas. J. Anim. Sci. 2011, 24, 479–484. [Google Scholar] [CrossRef]
- Aggarwal, A.; Ashutosh; Chandra, G.; Singh, A.K. Heat shock protein 70, oxidative stress, and antioxidant status in periparturient crossbred cows supplemented with alpha-tocopherol acetate. Trop. Anim. Health Prod. 2013, 45, 239–245. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ozai, R.; Sugino, T.; Kawashima, K.; Kushibiki, S.; Kim, Y.H.; Sato, S. Changes in peripheral blood oxidative stress markers and hepatic gene expression related to oxidative stress in Holstein cows with and without subacute ruminal acidosis during the periparturient period. J. Vet. Med. Sci. 2020, 82, 1529–1536. [Google Scholar] [CrossRef]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Shilo, S.; Pardo, M.; Aharoni-Simon, M.; Glibter, S.; Tirosh, O. Selenium supplementation increases liver MnSOD expression: Molecular mechanism for hepato-protection. J. Inorg. Biochem. 2008, 102, 110–118. [Google Scholar] [CrossRef]
- Elgendey, F.; Al Wakeel, R.A.; Hemeda, S.A.; Elshwash, A.M.; Fadl, S.E.; Abdelazim, A.M.; Alhujaily, M.; Khalifa, O.A. Selenium and/or vitamin E upregulate the antioxidant gene expression and parameters in broilers. BMC Vet. Res. 2022, 18, 310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, L.; Guo, K.; Zheng, L.; Liu, B.; Yu, W.; Guo, C.; Liu, Z.; Chen, Y.; Tang, Z. Effects of different selenium levels on gene expression of a subset of selenoproteins and antioxidative capacity in mice. Biol. Trace Elem. Res. 2013, 154, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Pang, K.; Fu, T.; Phillips, C.J.C.; Gao, T. Nano-selenium Supplementation Increases Selenoprotein (Sel) Gene Expression Profiles and Milk Selenium Concentration in Lactating Dairy Cows. Biol. Trace Elem. Res. 2021, 199, 113–119. [Google Scholar] [CrossRef] [PubMed]
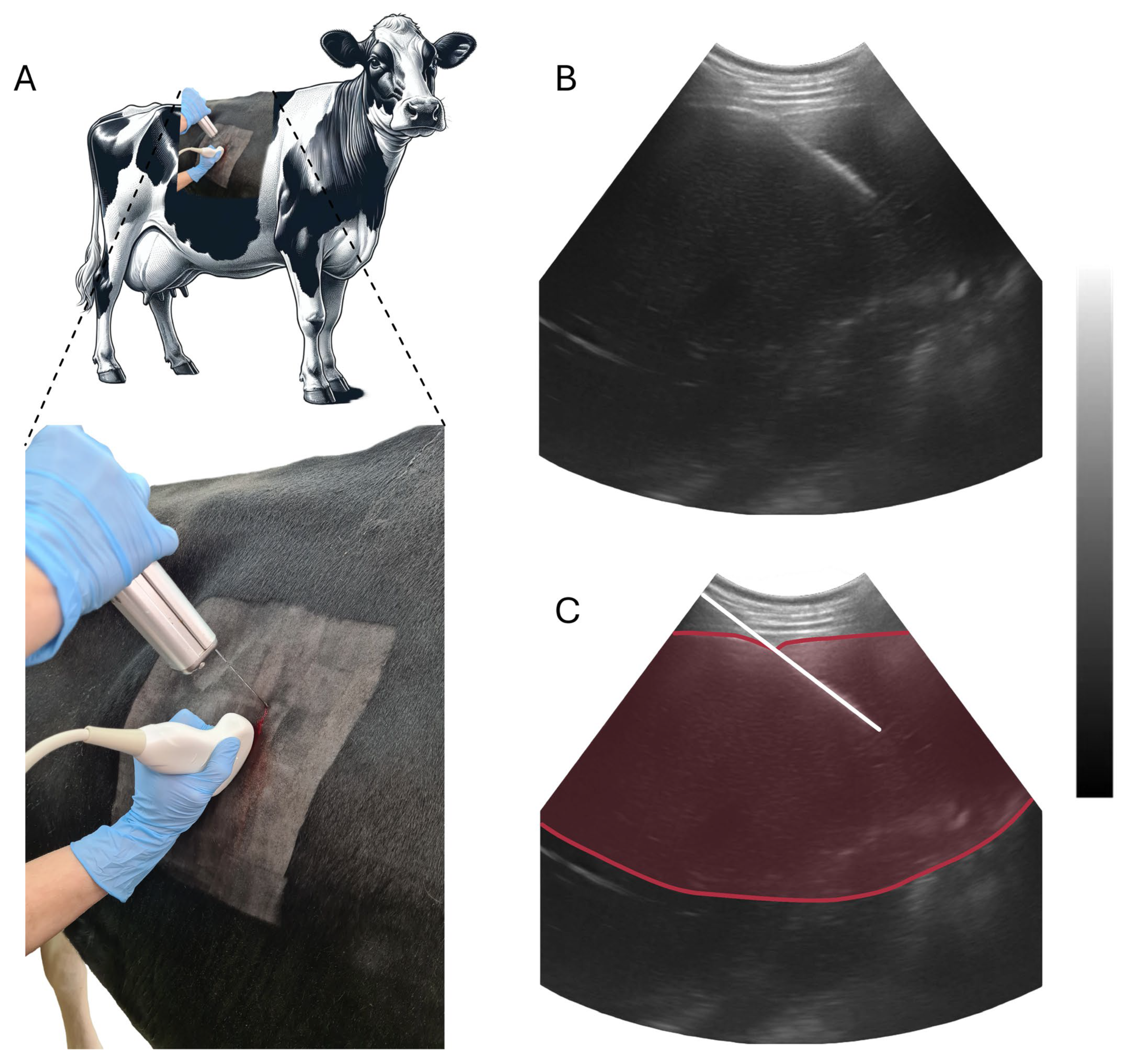
| Ingredient | Weight/Cow | DM 1 | Final DMI 1 |
|---|---|---|---|
| (kg) | (%) | (kg) | |
| Corn Silage | 17.00 | 31.00 | 5.27 |
| Grass Straw | 11.00 | 23.00 | 2.53 |
| Straw | 1.00 | 84.00 | 0.84 |
| Soybean Meal | 1.80 | 88.00 | 1.58 |
| Rapeseed Meal | 1.30 | 88.00 | 1.14 |
| Vitamin-mineral preparation | 0.40 | 88.00 | 0.35 |
| Total | 32.50 | 11.72 |
| Ingredient | Weight/Cow | DM 1 | Final DMI 1 |
|---|---|---|---|
| (kg) | (%) | (kg) | |
| Corn Silage | 29.00 | 31.00 | 8.99 |
| High Starch Concentrate | 3.00 | 88.00 | 2.64 |
| Concentrate | 3.00 | 88.00 | 2.64 |
| Alfalfa Silage | 10.00 | 41.00 | 4.10 |
| Haylage | 2.50 | 47.00 | 1.18 |
| Straw | 0.30 | 84.00 | 0.25 |
| Beet Molasses | 0.50 | 75.00 | 0.38 |
| Brewer’s spent grain | 5.00 | 21.00 | 1.05 |
| Soybean Meal | 1.80 | 88.00 | 1.58 |
| Rapeseed Meal | 1.30 | 88.00 | 1.14 |
| Sodium Bicarbonate | 0.25 | 99.00 | 0.25 |
| Propylene Glycol | 0.13 | 95.00 | 0.12 |
| Rumen-Protected Fat | 0.20 | 97.00 | 0.19 |
| Vitamin-mineral preparation | 0.66 | 88.00 | 0.58 |
| Total | 57.64 | 25.10 |
| Gene Symbol | Primer Sequences | Annealing Temp. | Tm | Amplicon Size | E | Error |
|---|---|---|---|---|---|---|
| (F—Forward/R—Reverse) (5′-3′) | (°C) | (°C) | bp | 10−1/slope | MSE | |
| SOD1 | F—TGTTGCCATTCGTGGATATTGTAG R—CCCAAGTCATCTGGTTTTTCATG a | 60 | 80.2 | 103 | 1.98 | 0.012 |
| SOD2 | F—CGCTGGAGAAGGGTGATGTT R—GATTTGTCCAGAAGATGCTGTGAT | 60 | 82.5 | 99 | 1.99 | 0.006 |
| SOD3 | F—GCAGCAGATGGGCTCCAA R—GCATCATCTCCTGCCAGATCTC | 58 | 85.3 | 80 | 2.06 | 0.011 |
| GSH-Px3 | F—GTCAACGTGGCCAGCTACTGA R—CAGAATGACCAGACCAAATGGTT | 60 | 83.9 | 93 | 1.99 | 0.019 |
| CAT | F—GGAAACGCCTGTGTGAGAAC R—CTGCGTTCTTAGGTTTCTCCTC | 58 | 81.7 | 159 | 1.95 | 0.005 |
| GAPDH reference gene | F—GTCTTCACTACCATGGAGAAGG R—TCATGGATGACCTTGGCCAG | 60 | 86.1 | 197 | 2.03 | 0.011 |
| RPL32 reference gene | F—AAAGAGGACCAAGAAGTTCATTAG R—CGCCAGTTCCGCTTGATTT | 60 | 78.1 | 66 | 1.98 | 0.013 |
| Day Relative to Parturition | ||||||
|---|---|---|---|---|---|---|
| Group | −12 | −3 | 1 | 4 | 7 | |
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | ||
| AST (U/L) | CON | 75.00 ± 12.40 | 70.00 ± 6.90 | 82.67 ± 13.95 | 84.16 ± 9.31 | 78.50 ± 14.80 |
| STG | 66.33 ± 10.76 | 68.83 ± 7.65 | 85.33 ± 13.39 | 87.83 ± 22.15 | 88.17 ± 16.75 | |
| GGT (U/L) | CON | 28.17 ± 2.48 | 27.33 ± 3.83 | 31.67 ± 8.36 | 28.33 ± 4.41 | 28.16 ± 5.91 |
| STG | 26.00 ± 2.53 | 26.17 ± 3.37 | 29.12 ± 10.00 | 27.83 ± 3.37 | 29.67 ± 3.44 | |
| GLU (mmol/L) | CON | 3.26 ± 0.23 | 3.45 ± 0.40 | 3.63 ± 0.81 | 3.37 ± 0.50 | 3.59 ± 0.49 |
| STG | 3.19 ± 0.39 | 4.13 ± 0.84 | 3.15 ± 0.65 | 3.26 ± 0.45 | 3.22 ± 0.43 | |
| TG (mmol/L) | CON | 0.19 ± 0.09 | 0.22 ± 0.06 | 0.13 ± 0.09 | 0.09 ± 0.03 | 0.11 ± 0.05 |
| STG | 0.15 ± 0.10 | 0.19 ± 0.13 | 0.10 ± 0.04 | 0.07 ± 0.03 | 0.08 ± 0.05 | |
| CHOL (mmol/L) | CON | 2.92 ± 0.70 | 2.83 ± 0.67 | 2.37 ± 0.37 | 2.27 ± 0.50 | 2.36 ± 0.52 |
| STG | 2.55 ± 0.58 | 2.71 ± 0.68 | 2.19 ± 0.50 | 2.05 ± 0.26 | 2.14 ± 0.33 | |
| NEFA (mmol/L) | CON | 0.44 ± 0.22 | 0.41 ± 0.18 | 0.56 ± 0.30 | 0.55 ± 0.31 a | 0.58 ± 0.27 a |
| STG | 0.39 ± 0.12 | 0.39 ± 0.15 | 0.51 ± 0.15 | 0.41 ± 0.08 b | 0.36 ± 0.17 b | |
| BHB (mmol/L) | CON | 0.69 ± 0.14 | 0.66 ± 0.13 a | 0.64 ± 0.16 | 0.70 ± 0.11 | 0.72 ± 0.23 |
| STG | 0.61 ± 0.20 | 0.85 ± 0.15 b | 0.80 ± 0.14 | 0.83 ± 0.15 | 0.82 ± 0.20 | |
| ALB (g/L) | CON | 39.70 ± 1.91 | 39.13 ± 0.95 | 38.46 ± 1.38 | 37.40 ± 1.75 | 38.18 ± 2.66 |
| STG | 39.26 ± 2.41 | 38.96 ± 1.62 | 38.21 ± 3.84 | 36.65 ± 3.56 | 36.91 ± 3.49 | |
| TP (g/L) | CON | 68.88 ± 2.95 | 67.22 ± 3.76 a | 66.27 ± 3.59 | 63.70 ± 4.33 a | 64.25 ± 3.56 a |
| STG | 72.68 ± 5.35 | 72.95 ± 4.22 b | 68.53 ± 2.37 | 67.87 ± 3.28 b | 68.37 ± 2.31 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żarczyńska, K.; Brym, P.; Tobolski, D. The Role of Selenitetriglycerides in Enhancing Antioxidant Defense Mechanisms in Peripartum Holstein-Friesian Cows. Animals 2024, 14, 610. https://doi.org/10.3390/ani14040610
Żarczyńska K, Brym P, Tobolski D. The Role of Selenitetriglycerides in Enhancing Antioxidant Defense Mechanisms in Peripartum Holstein-Friesian Cows. Animals. 2024; 14(4):610. https://doi.org/10.3390/ani14040610
Chicago/Turabian StyleŻarczyńska, Katarzyna, Paweł Brym, and Dawid Tobolski. 2024. "The Role of Selenitetriglycerides in Enhancing Antioxidant Defense Mechanisms in Peripartum Holstein-Friesian Cows" Animals 14, no. 4: 610. https://doi.org/10.3390/ani14040610
APA StyleŻarczyńska, K., Brym, P., & Tobolski, D. (2024). The Role of Selenitetriglycerides in Enhancing Antioxidant Defense Mechanisms in Peripartum Holstein-Friesian Cows. Animals, 14(4), 610. https://doi.org/10.3390/ani14040610






