C6 Ceramide Inhibits Canine Mammary Cancer Growth and Metastasis by Targeting EGR3 through JAK1/STAT3 Signaling
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Statement of Ethics
2.2. Cell Lines and Cell Culture
2.3. Cell Viability Assay
2.4. Observations of Cell Morphology and Migration
2.5. Cell Migration Assay
2.6. Invasion Assay
2.7. Flow Cytometry
2.8. Mouse Xenografts
2.9. RNA Interference and Transfection
2.10. Reverse Transcription PCR (RT-PCR) and Quantitative Real-Time PCR (qPCR)
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
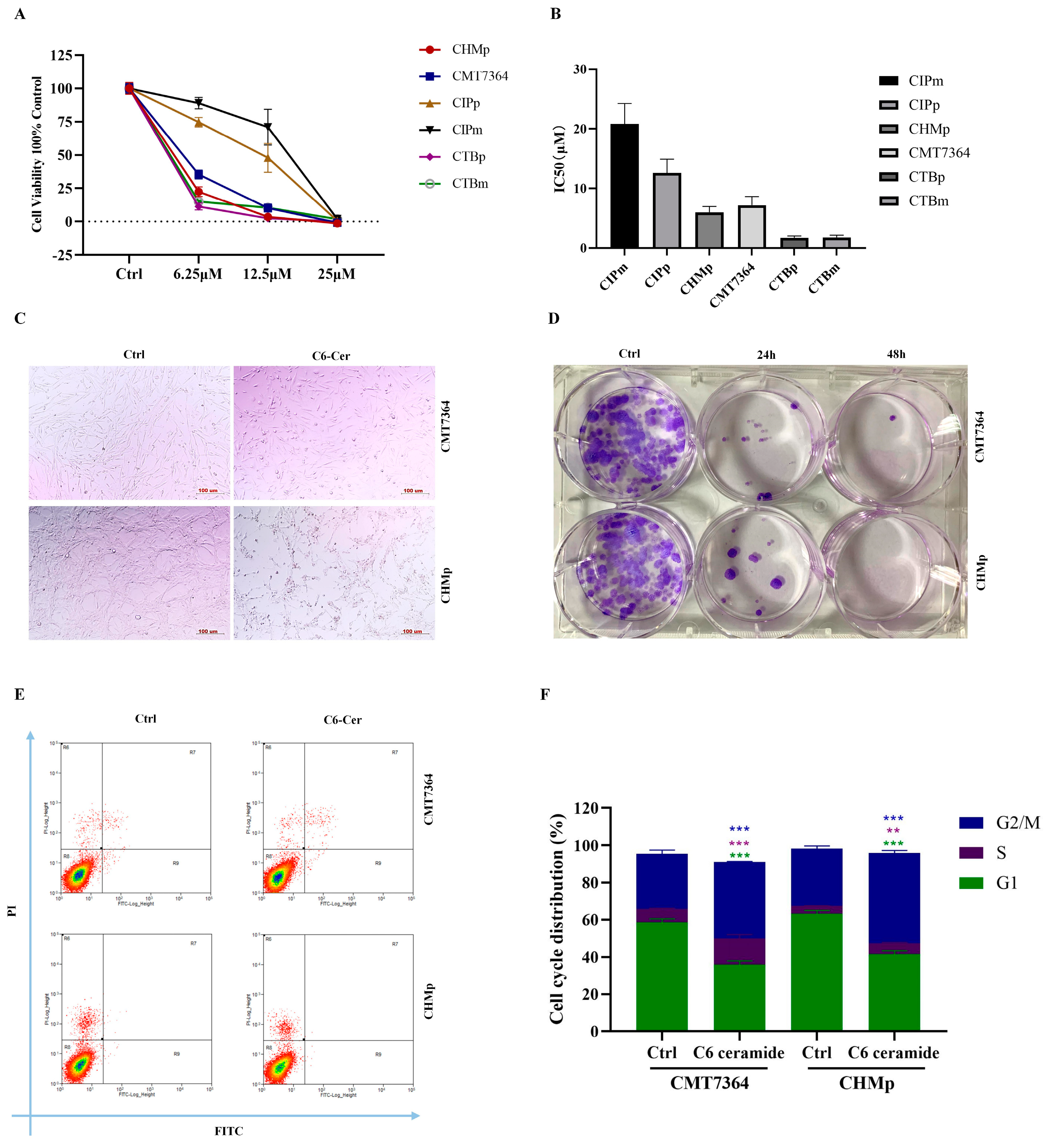
3.1. C6 Ceramide Inhibited Canine Cancer Cell Growth by Arresting the Cell Cycle at the S Phase
3.2. C6 Ceramide Inhibited Cell Migration and Invasion
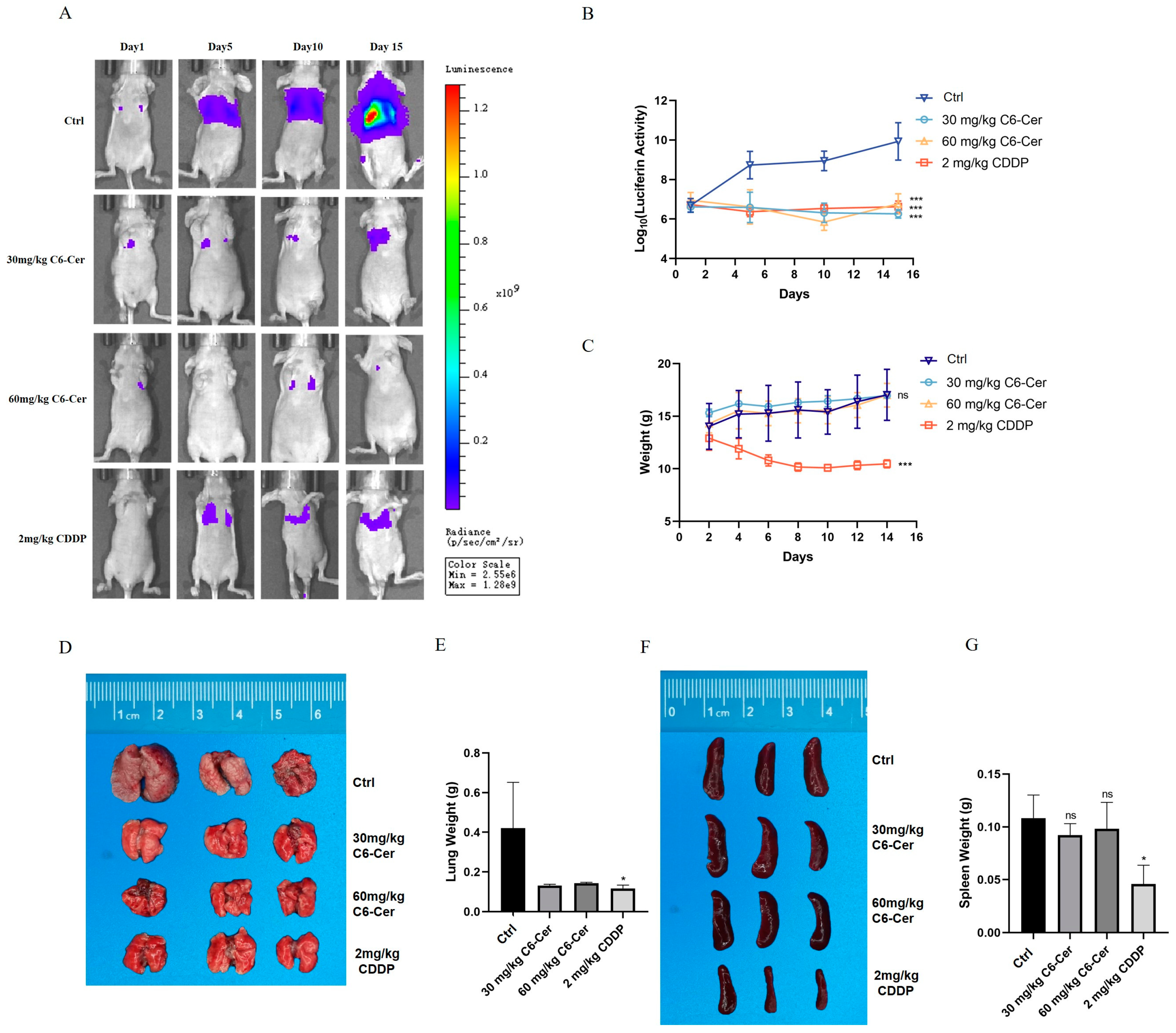
3.3. C6 Ceramide Suppresses CHMp Xenograft Tumor Growth In Vivo
3.4. Identification of EGR3 as a Target Gene for C6 Ceramide and EGR3 Promoted the Cell’s Abilities of Proliferation and Migration by Regulating the JAK1/STAT3 Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Vascellari, M.; Capello, K.; Carminato, A.; Zanardello, C.; Baioni, E.; Mutinelli, F. Incidence of mammary tumors in the canine population living in the Veneto region (Northeastern Italy): Risk factors and similarities to human breast cancer. Prev. Vet. Med. 2016, 126, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Benavente, M.A.; Bianchi, C.P.; Aba, M.A. Canine Mammary Tumors: Risk Factors, Prognosis and Treatments. J. Vet. Adv. 2016, 6, 1291–1300. [Google Scholar]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Król, M.; Jurka, P. Current biomarkers of canine mammary tumors. Acta Vet. Scand. 2018, 60, 66. [Google Scholar] [CrossRef]
- Stratmann, N.; Failing, K.; Richter, A.; Wehrend, A. Mammary tumor recurrence in bitches after regional mastectomy. Vet. Surg. 2008, 37, 82–86. [Google Scholar] [CrossRef]
- Sheridan, M.; Ogretmen, B. The Role of Ceramide Metabolism and Signaling in the Regulation of Mitophagy and Cancer Therapy. Cancers 2021, 13, 2475. [Google Scholar] [CrossRef]
- Fillet, M.; Bentires-Alj, M.; Deregowski, V.; Greimers, R.; Gielen, J.; Piette, J.; Bours, V.; Merville, M.-P. Mechanisms involved in exogenous C2- and C6-ceramide-induced cancer cell toxicity. Biochem. Pharmacol. 2003, 65, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Morad, S.A.F.; Ryan, T.E.; Neufer, P.D.; Zeczycki, T.N.; Davis, T.S.; MacDougall, M.R.; Fox, T.E.; Tan, S.-F.; Feith, D.J.; Loughran, T.P.; et al. Ceramide-tamoxifen regimen targets bioenergetic elements in acute myelogenous leukemia. J. Lipid Res. 2016, 57, 1231–1242. [Google Scholar] [CrossRef]
- Companioni, O.; Mir, C.; Garcia-Mayea, Y.; Lleonart, M.E. Targeting Sphingolipids for Cancer Therapy. Front. Oncol. 2021, 11, 745092. [Google Scholar] [CrossRef]
- Gray, M.; Meehan, J.; Martinez-Perez, C.; Kay, C.; Turnbull, A.K.; Morrison, L.R.; Pang, L.Y.; Argyle, D. Naturally-Occurring Canine Mammary Tumors as a Translational Model for Human Breast Cancer. Front. Oncol. 2020, 10, 617. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Moskwa, N.; Kang, W.; Fan, T.M.; Lee, C. Canine as a Comparative and Translational Model for Human Mammary Tumor. J. Breast Cancer 2023, 26, 1–13. [Google Scholar] [CrossRef]
- Li, X.-Z.; Tu, Y.-J.; Zhou, T.; Zhang, J.-B.; Xiao, R.-W.; Yang, D.-W.; Zhang, P.-F.; You, P.-T.; Zheng, X.-H. MicroRNA-483-5p Predicts Poor Prognosis and Promotes Cancer Metastasis by Targeting EGR3 in Nasopharyngeal Carcinoma. Front. Oncol. 2021, 11, 720835. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Kim, I.; Lee, J.E.; Lee, M.; Park, J.W. Loss of EGR3 is an independent risk factor for metastatic progression in prostate cancer. Oncogene 2020, 39, 5839–5854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xia, C.; Xu, C.; Liu, J.; Zhu, H.; Yang, Y.; Xu, F.; Zhao, J.; Chang, Y.; Zhao, Q. Early growth response 3 inhibits growth of hepatocellular. Int. J. Oncol. 2017, 50, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Jia, X.-C. WTAP-mediated N6-methyladenosine modification on EGR3 in different types of epithelial ovarian cancer. J. Biol. Reg. Homeos Ag. 2020, 34, 1505–1512. [Google Scholar]
- Wang, Z.-D.; Qu, F.-Y.; Chen, Y.-Y.; Ran, Z.-S.; Liu, H.-Y.; Zhang, H.-D. Involvement of microRNA-718, a new regulator of EGR3, in regulation of malignant phenotype of HCC cells. J. Zhejiang Univ.-Sci. B 2017, 18, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Massague, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef]
- Knudsen, A.M.; Eilertsen, I.; Kielland, S.; Pedersen, M.W.; Sørensen, M.D.; Dahlrot, R.H.; Boldt, H.B.; Munthe, S.; Poulsen, F.R.; Kristensen, B.W. Expression and prognostic value of the transcription factors EGR1 and EGR3 in gliomas. Sci. Rep. 2020, 10, 9285. [Google Scholar] [CrossRef]
- Suzuki, T.; Inoue, A.; Miki, Y.; Moriya, T.; Akahira, J.-I.; Ishida, T.; Hirakawa, H.; Yamaguchi, Y.; Hayashi, S.-I.; Sasano, H. Early growth responsive gene 3 in human breast carcinoma: A regulator of estrogen-meditated invasion and a potent prognostic factor. Endocr. Relat. Cancer 2007, 14, 279–292. [Google Scholar] [CrossRef]
- Zhao, F.; Li, X.; Liu, J.; Zhang, D.; Diao, H.; Lin, D. Establishment of stable expression of firefly luciferase and EGFP in a canine inflammatory mammary carcinoma cell line and tumor-bearing model in nude mice. Front. Vet. Sci. 2022, 9, 935005. [Google Scholar] [CrossRef]
- Qi, X.; Wu, F.; Kim, S.H.; Kaifi, J.T.; Kimchi, E.T.; Snyder, H.; Illendula, A.; Fox, T.; Kester, M.; Staveley-O’Carroll, K.F.; et al. Nanoliposome C6-Ceramide in combination with anti-CTLA4 antibody improves anti-tumor immunity in hepatocellular cancer. FASEB J. 2022, 36, e22250. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, R.; Eckes, T.; Imre, G.; Kippenberger, S.; Meissner, M.; Thomas, D.; Trautmann, S.; Merlio, J.-P.; Chevret, E.; Kaufmann, R. C6 Ceramide (d18:1/6:0) as a Novel Treatment of Cutaneous T Cell Lymphoma. Cancers 2021, 13, 270. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, B.; Zhang, J.; Zhang, R.; Zhang, Q. Short-chain C6 ceramide sensitizes AT406-induced anti-pancreatic cancer cell activity. Biochem. Biophys. Res. Commun. 2016, 479, 166–172. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C.; Zhou, Y.; Ma, N.; Zhou, J. C6 ceramide motivates the anticancer sensibility induced by PKC412 in preclinical head and neck squamous cell carcinoma models. J. Cell Physiol. 2018, 233, 9437–9446. [Google Scholar] [CrossRef]
- Zhang, P.; Fu, C.; Hu, Y.; Dong, C.; Song, Y.; Song, E. C6-ceramide nanoliposome suppresses tumor metastasis by eliciting PI3K and PKCζ tumor-suppressive activities and regulating integrin affinity modulation. Sci. Rep. 2015, 5, 9275. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, D.; Kimchi, E.T.; Kaifi, J.T.; Qi, X.; Manjunath, Y.; Liu, X.; Deering, T.; Avella, D.M.; Fox, T.; et al. Nanoliposome C6-Ceramide Increases the Anti-tumor Immune Response and Slows Growth of Liver Tumors in Mice. Gastroenterology 2018, 154, 1024–1036. [Google Scholar] [CrossRef]
- Adiseshaiah, P.P.; Clogston, J.D.; McLeland, C.B.; Rodriguez, J.; Potter, T.M.; Neun, B.W.; Skoczen, S.L.; Shanmugavelandy, S.S.; Kester, M.; Stern, S.T.; et al. Synergistic combination therapy with nanoliposomal C6-ceramide and vinblastine is associated with autophagy dysfunction in hepatocarcinoma and colorectal cancer models. Cancer Lett. 2013, 337, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Stover, T.C.; Sharma, A.; Robertson, G.P.; Kester, M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin. Cancer Res. 2005, 11, 3465–3474. [Google Scholar] [CrossRef]
- Liu, L.; Ye, Q.; Liu, L.; Bihl, J.C.; Chen, Y.; Liu, J.; Cheng, Q. C6-ceramide treatment inhibits the proangiogenic activity of multiple myeloma exosomes via the miR-29b/Akt pathway. J. Transl. Med. 2020, 18, 298. [Google Scholar] [CrossRef]
- Symonds, A.L.J.; Miao, T.; Busharat, Z.; Li, S.; Wang, P. Egr2 and 3 maintain anti-tumour responses of exhausted tumour infiltrating CD8+ T cells. Cancer Immunol. Immunother. 2023, 72, 1139–1151. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, M.; Kim, Y.; Jeong, M.S.; Jung, H.S.; Jeoung, D. EGR3-HDAC6-IL-27 Axis Mediates Allergic Inflammation and Is Necessary for Tumorigenic Potential of Cancer Cells Enhanced by Allergic Inflammation-Promoted Cellular Interactions. Front. Immunol. 2021, 12, 2989–2998. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Evans, I.; Britton, G.; Zachary, I. The zinc-finger transcription factor, early growth response 3, mediates VEGF-induced angiogenesis. Oncogene 2007, 27, 2989–2998. [Google Scholar] [CrossRef]
- Mellman, I.; Chen, D.S.; Powles, T.; Turley, S.J. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity 2023, 56, 2188–2205. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Miao, T.; Symonds, A.L.J.; Omodho, B.; Li, S.; Wang, P. Egr2 and 3 Inhibit T-bet–Mediated IFN-γ Production in T Cells. J. Immunol. 2017, 198, 4394–4402. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Symonds, A.L.J.; Singh, R.; Symonds, J.D.; Ogbe, A.; Omodho, B.; Zhu, B.; Li, S.; Wang, P. Egr2 and 3 control adaptive immune responses by temporally uncoupling expansion from T cell differentiation. J. Exp. Med. 2017, 214, 1787–1808. [Google Scholar] [CrossRef]
- Uyama, R.; Nakagawa, T.; Hong, S.H.; Mochizuki, M.; Nishimura, R.; Sasaki, N. Establishment of four pairs of canine mammary tumour cell lines derived from primary and metastatic origin and their E-cadherin expression. Vet. Comp. Oncol. 2006, 4, 104–113. [Google Scholar] [CrossRef]
- Guo, C.; Gao, Y.-Y.; Ju, Q.-Q.; Zhang, C.-X.; Gong, M.; Li, Z.-L. HELQ and EGR3 expression correlate with IGHV mutation status and prognosis in chronic lymphocytic leukemia. J. Transl. Med. 2021, 19, 42. [Google Scholar] [CrossRef]
- Ott, N.; Faletti, L.; Heeg, M.; Andreani, V.; Grimbacher, B. JAKs and STATs from a Clinical Perspective: Loss-of-Function Mutations, Gain-of-Function Mutations, and Their Multidimensional Consequences. J. Clin. Immunol. 2023, 43, 1326–1359. [Google Scholar] [CrossRef]
- Jin, W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial-Mesenchymal Transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef]
- Park, J.H.; van Wyk, H.; McMillan, D.C.; Quinn, J.; Clark, J.; Roxburgh, C.S.; Horgan, P.G.; Edwards, J. Signal Transduction and Activator of Transcription-3 (STAT3) in Patients with Colorectal Cancer: Associations with the Phenotypic Features of the Tumor and Host. Clin. Cancer Res. 2017, 23, 1698–1709. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, Q.; Chen, Y.; Shen, N.; Zhang, N.; Li, A.; Li, L.; Li, J. PRMT6 methylation of STAT3 regulates tumor metastasis in breast cancer. Cell Death Dis. 2023, 14, 655. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.; Wang, T.C. Inflammation and Cancer: IL-6 and STAT3 Complete the Link. Cancer Cell 2009, 15, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhao, F.; Zhang, Y.; Lin, Z.; Chen, J.-L.; Diao, H. C6 Ceramide Inhibits Canine Mammary Cancer Growth and Metastasis by Targeting EGR3 through JAK1/STAT3 Signaling. Animals 2024, 14, 422. https://doi.org/10.3390/ani14030422
Liu J, Zhao F, Zhang Y, Lin Z, Chen J-L, Diao H. C6 Ceramide Inhibits Canine Mammary Cancer Growth and Metastasis by Targeting EGR3 through JAK1/STAT3 Signaling. Animals. 2024; 14(3):422. https://doi.org/10.3390/ani14030422
Chicago/Turabian StyleLiu, Jiayue, Fangying Zhao, Yan Zhang, Zhaoyan Lin, Ji-Long Chen, and Hongxiu Diao. 2024. "C6 Ceramide Inhibits Canine Mammary Cancer Growth and Metastasis by Targeting EGR3 through JAK1/STAT3 Signaling" Animals 14, no. 3: 422. https://doi.org/10.3390/ani14030422
APA StyleLiu, J., Zhao, F., Zhang, Y., Lin, Z., Chen, J.-L., & Diao, H. (2024). C6 Ceramide Inhibits Canine Mammary Cancer Growth and Metastasis by Targeting EGR3 through JAK1/STAT3 Signaling. Animals, 14(3), 422. https://doi.org/10.3390/ani14030422




