Does Placental Efficiency and Vascularization Affect Puppy Health? A Study in Boxer and Dobermann Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reproductive Management
2.3. Birth Management and Data Collection
2.4. Puppy Management and Data Collection
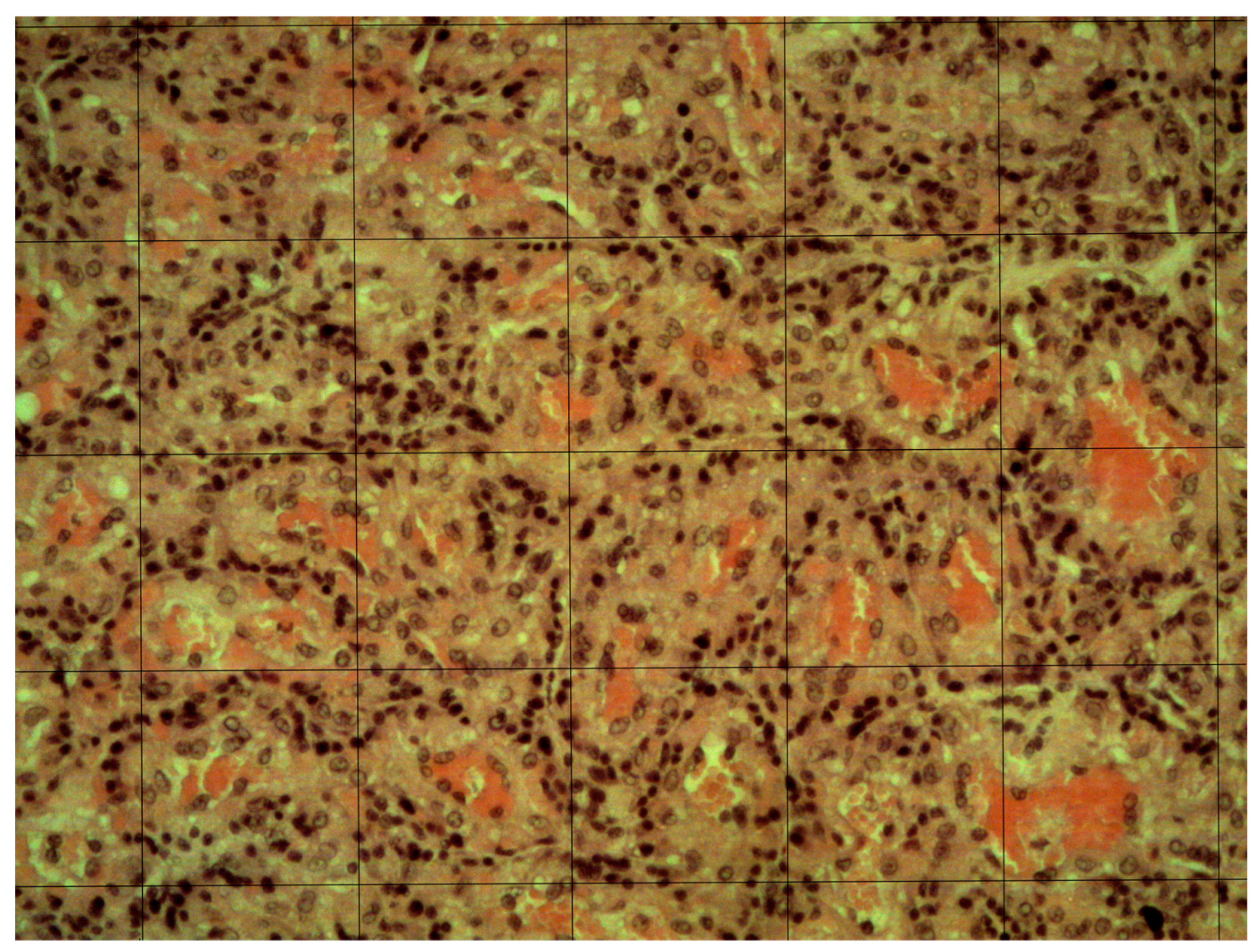
2.5. Placenta Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mossman, H.W. Comparative Morphogenesis of the Fetal Membranes and Accessory Uterine Structures. Placenta 1991, 12, 1–5. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. What Is the Placenta? Am. J. Obstet. Gynecol. 2015, 213, S6.e1–S6.e4. [Google Scholar] [CrossRef]
- Carter, A.M.; Enders, A.C. Placentation in Mammals: Definitive Placenta, Yolk Sac, and Paraplacenta. Theriogenology 2016, 86, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Aralla, M.; Groppetti, D.; Caldarini, L.; Cremonesi, F.; Arrighi, S. Morphological Evaluation of the Placenta and Fetal Membranes during Canine Pregnancy from Early Implantation to Term. Res. Vet. Sci. 2013, 95, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M.; Enders, A.C. Comparative Aspects of Trophoblast Development and Placentation. Reprod. Biol. Endocrinol. 2004, 2, 46. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.C.; Carter, A.M. The Evolving Placenta: Convergent Evolution of Variations in the Endotheliochorial Relationship. Placenta 2012, 33, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P.; Larsen, G.; Osmond, C.; Thornburg, K.L.; Kajantie, E.; Eriksson, J.G. The Placental Origins of Sudden Cardiac Death. Int. J. Epidemiol. 2012, 41, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P.; Thornburg, K.L. Placental Programming of Chronic Diseases, Cancer and Lifespan: A Review. Placenta 2013, 34, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Winder, N.R.; Krishnaveni, G.V.; Veena, S.R.; Hill, J.C.; Karat, C.L.S.; Thornburg, K.L.; Fall, C.H.D.; Barker, D.J.P. Mother’s Lifetime Nutrition and the Size, Shape and Efficiency of the Placenta. Placenta 2011, 32, 806–810. [Google Scholar] [CrossRef]
- Concannon, P.W. Reproductive Cycles of the Domestic Bitch. Anim. Reprod. Sci. 2011, 124, 200–210. [Google Scholar] [CrossRef]
- Mugnier, A.; Mila, H.; Guiraud, F.; Brévaux, J.; Lecarpentier, M.; Martinez, C.; Mariani, C.; Adib-Lesaux, A.; Chastant-Maillard, S.; Saegerman, C.; et al. Birth Weight as a Risk Factor for Neonatal Mortality: Breed-Specific Approach to Identify at-Risk Puppies. Prev. Vet. Med. 2019, 171, 104746. [Google Scholar] [CrossRef]
- Fowden, A.L.; Sferruzzi-Perri, A.N.; Coan, P.M.; Constancia, M.; Burton, G.J. Placental Efficiency and Adaptation: Endocrine Regulation. J. Physiol. 2009, 587, 3459–3472. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Ford, S.P. Comparative Aspects of Placental Efficiency. Reprod. Suppl. 2001, 58, 223–232. [Google Scholar] [CrossRef]
- Hayward, C.E.; Lean, S.; Sibley, C.P.; Jones, R.L.; Wareing, M.; Greenwood, S.L.; Dilworth, M.R. Placental Adaptation: What Can We Learn from Birthweight:Placental Weight Ratio? Front. Physiol. 2016, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Coan, P.M.; Angiolini, E.; Sandovici, I.; Burton, G.J.; Constância, M.; Fowden, A.L. Adaptations in Placental Nutrient Transfer Capacity to Meet Fetal Growth Demands Depend on Placental Size in Mice. J. Physiol. 2008, 586, 4567–4576. [Google Scholar] [CrossRef] [PubMed]
- Buresova, M.; Zidek, V.; Musilova, A.; Simakova, M.; Fucikova, A.; Bila, V.; Kren, V.; Kazdova, L.; Di Nicolantonio, R.; Pravenec, M. Genetic Relationship between Placental and Fetal Weights and Markers of the Metabolic Syndrome in Rat Recombinant Inbred Strains. Physiol. Genom. 2006, 26, 226–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurz, H.; Zechner, U.; Orth, A.; Fundele, R. Lack of Correlation between Placenta and Offspring Size in Mouse Interspecific Crosses. Anat. Embryol. 1999, 200, 335–343. [Google Scholar] [CrossRef]
- Sarli, G.; Castagnetti, C.; Bianco, C.; Ballotta, G.; Tura, G.; Caporaletti, M.; Cunto, M.; Avallone, G.; Benazzi, C.; Ostanello, F.; et al. Canine Placenta Histological Findings and Microvascular Density: The Histological Basis of a Negative Neonatal Outcome? Animals 2021, 11, 1418. [Google Scholar] [CrossRef]
- Tesi, M.; Miragliotta, V.; Scala, L.; Aronica, E.; Lazzarini, G.; Fanelli, D.; Abramo, F.; Rota, A. Relationship between Placental Characteristics and Puppies’ Birth Weight in Toy and Small Sized Dog Breeds. Theriogenology 2020, 141, 1–8. [Google Scholar] [CrossRef]
- Tahir, M.Z.; Thoumire, S.; Raffaelli, M.; Grimard, B.; Reynaud, K.; Chastant-Maillard, S. Effect of Blood Handling Conditions on Progesterone Assay Results Obtained by Chemiluminescence in the Bitch. Domest. Anim. Endocrinol. 2013, 45, 141–144. [Google Scholar] [CrossRef]
- Chastant-Maillard, S.; Viaris de Lesegno, C.; Chebrout, M.; Thoumire, S.; Meylheuc, T.; Fontbonne, A.; Chodkiewicz, M.; Saint-Dizier, M.; Reynaud, K. The Canine Oocyte: Uncommon Features of in Vivo and in Vitro Maturation. Reprod. Fertil. Dev. 2011, 23, 391–402. [Google Scholar] [CrossRef]
- Concannon, P.W.; McCann, J.P.; Temple, M. Biology and Endocrinology of Ovulation, Pregnancy and Parturition in the Dog. J. Reprod. Fertil. Suppl. 1989, 39, 3–25. [Google Scholar]
- Freeman, L.; Becvarova, I.; Cave, N.; Mackay, C.; Nguyen, P.; Rama, B.; Takashima, G.; Tiffin, R.; Tsjimoto, H.; van Beukelen, P. WSAVA Nutritional Assessment Guidelines. J. Small Anim. Pract. 2011, 52, 385–396. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Panzani, S.; Faustini, M.; Rota, A. An Apgar Scoring System for Routine Assessment of Newborn Puppy Viability and Short-Term Survival Prognosis. Theriogenology 2009, 72, 401–407. [Google Scholar] [CrossRef]
- Groppetti, D.; Pecile, A.; Palestrini, C.; Marelli, S.P.; Boracchi, P. A National Census of Birthweight in Purebred Dogs in Italy. Animals 2017, 7, 43. [Google Scholar] [CrossRef]
- Moon-Massat, P.F.; Erb, H.N. Perioperative Factors Associated with Puppy Vigor after Delivery by Cesarean Section. J. Am. Anim. Hosp. Assoc. 2002, 38, 90–96. [Google Scholar] [CrossRef]
- Batista, M.; Moreno, C.; Vilar, J.; Golding, M.; Brito, C.; Santana, M.; Alamo, D. Neonatal Viability Evaluation by Apgar Score in Puppies Delivered by Cesarean Section in Two Brachycephalic Breeds (English and French Bulldog). Anim. Reprod. Sci. 2014, 146, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Vilar, J.M.; Batista, M.; Pérez, R.; Zagorskaia, A.; Jouanisson, E.; Díaz-Bertrana, L.; Rosales, S. Comparison of 3 Anesthetic Protocols for the Elective Cesarean-Section in the Dog: Effects on the Bitch and the Newborn Puppies. Anim. Reprod. Sci. 2018, 190, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J.; Ellerbrock, R.E.; Wallace, M.L.; Schmiedt, C.W.; Sutherland, B.J.; Grimes, J.A. Risk Factors for Neonatal Mortality Prior to Hospital Discharge in Brachycephalic and Nonbrachycephalic Dogs Undergoing Cesarean Section. Vet. Surg. 2022, 51, 1052–1060. [Google Scholar] [CrossRef]
- Nielen, A.L.; van der Gaag, I.; Knol, B.W.; Schukken, Y.H. Investigation of Mortality and Pathological Changes in a 14-Month Birth Cohort of Boxer Puppies. Vet. Rec. 1998, 142, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Nobre Pacifico Pereira, K.H.; Cruz dos Santos Correia, L.E.; Ritir Oliveira, E.L.; Bernardo, R.B.; Nagib Jorge, M.L.; Mezzena Gobato, M.L.; Ferreira de Souza, F.; Rocha, N.S.; Chiacchio, S.B.; Gomes Lourenço, M.L. Incidence of Congenital Malformations and Impact on the Mortality of Neonatal Canines. Theriogenology 2019, 140, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Doebeli, A.; Michel, E.; Bettschart, R.; Hartnack, S.; Reichler, I.M. Apgar Score after Induction of Anesthesia for Canine Cesarean Section with Alfaxalone versus Propofol. Theriogenology 2013, 80, 850–854. [Google Scholar] [CrossRef]
- Hyndman, T.H.; Fretwell, S.; Bowden, R.S.; Coaicetto, F.; Irons, P.C.; Aleri, J.W.; Kordzakhia, N.; Page, S.W.; Musk, G.C.; Tuke, S.J.; et al. The Effect of Doxapram on Survival and APGAR Score in Newborn Puppies Delivered by Elective Caesarean: A Randomized Controlled Trial. J. Vet. Pharmacol. Ther. 2023, 46, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, C.L.; Persson, G. A Survey of Dystocia in the Boxer Breed. Acta Vet. Scand. 2007, 49, 8. [Google Scholar] [CrossRef]
- Baur, R. Morphometry of the Placental Exchange Area. In Advances in Anatomy, Embryology and Cell Biology; Springer: Berlin, Germany, 1977; Volume 53, pp. 3–65. [Google Scholar] [CrossRef]
- Wooding, P.; Burton, G. Comparative Placentation: Structures, Functions and Evolution; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-78796-9. [Google Scholar]
- Hemachandra, A.H.; Klebanoff, M.A.; Duggan, A.K.; Hardy, J.B.; Furth, S.L. The Association between Intrauterine Growth Restriction in the Full-Term Infant and High Blood Pressure at Age 7 Years: Results from the Collaborative Perinatal Project. Int. J. Epidemiol. 2006, 35, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Bull, A.R.; Osmond, C.; Simmonds, S.J. Fetal and Placental Size and Risk of Hypertension in Adult Life. BMJ 1990, 301, 259–262. [Google Scholar] [CrossRef]
- Risnes, K.R.; Romundstad, P.R.; Nilsen, T.I.L.; Eskild, A.; Vatten, L.J. Placental Weight Relative to Birth Weight and Long-Term Cardiovascular Mortality: Findings from a Cohort of 31,307 Men and Women. Am. J. Epidemiol. 2009, 170, 622–631. [Google Scholar] [CrossRef]
- Sandovici, I.; Hoelle, K.; Angiolini, E.; Constância, M. Placental Adaptations to the Maternal-Fetal Environment: Implications for Fetal Growth and Developmental Programming. Reprod. Biomed. Online 2012, 25, 68–89. [Google Scholar] [CrossRef]
- Salafia, C.M.; Zhang, J.; Miller, R.K.; Charles, A.K.; Shrout, P.; Sun, W. Placental Growth Patterns Affect Birth Weight for given Placental Weight. Birth Defects Res. Part A Clin. Mol. Teratol. 2007, 79, 281–288. [Google Scholar] [CrossRef]
- Bacon, B.J.; Gilbert, R.D.; Kaufmann, P.; Smith, A.D.; Trevino, F.T.; Longo, L.D. Placental Anatomy and Diffusing Capacity in Guinea Pigs Following Long-Term Maternal Hypoxia. Placenta 1984, 5, 475–487. [Google Scholar] [CrossRef]
- Gilbert, R.D.; Cummings, L.A.; Juchau, M.R.; Longo, L.D. Placental Diffusing Capacity and Fetal Development in Exercising or Hypoxic Guinea Pigs. J. Appl. Physiol. 1979, 46, 828–834. [Google Scholar] [CrossRef]
- Lueder, F.L.; Kim, S.B.; Buroker, C.A.; Bangalore, S.A.; Ogata, E.S. Chronic Maternal Hypoxia Retards Fetal Growth and Increases Glucose Utilization of Select Fetal Tissues in the Rat. Metabolism 1995, 44, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Leturque, A. Fetal Weight and Its Relationship to Placental Blood Flow and Placental Weight in Experimental Intrauterine Growth Retardation in the Rat. J. Dev. Physiol. 1982, 4, 237–246. [Google Scholar] [PubMed]
- Reid, G.J.; Lane, R.H.; Flozak, A.S.; Simmons, R.A. Placental Expression of Glucose Transporter Proteins 1 and 3 in Growth-Restricted Fetal Rats. Am. J. Obs. Gynecol. 1999, 180, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Wlodek, M.E.; Westcott, K.T.; O’Dowd, R.; Serruto, A.; Wassef, L.; Moritz, K.M.; Moseley, J.M. Uteroplacental Restriction in the Rat Impairs Fetal Growth in Association with Alterations in Placental Growth Factors Including PTHrP. Am. J. Physiol. Integr. Comp. Physiol. 2005, 288, R1620–R1627. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.N.; Woollett, L.A.; Barbour, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. High-Fat Diet before and during Pregnancy Causes Marked up-Regulation of Placental Nutrient Transport and Fetal Overgrowth in C57/BL6 Mice. FASEB J. 2009, 23, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Woodall, S.M.; Breier, B.H.; Johnston, B.M.; Gluckman, P.D. A Model of Intrauterine Growth Retardation Caused by Chronic Maternal Undernutrition in the Rat: Effects on the Somatotrophic Axis and Postnatal Growth. J. Endocrinol. 1996, 150, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Vonnahme, K.A.; Wilson, M.E.; Ford, S.P. Relationship between Placental Vascular Endothelial Growth Factor Expression and Placental/Endometrial Vascularity in the Pig. Biol. Reprod. 2001, 64, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Regnault, T.R.H.; de Vrijer, B.; Galan, H.L.; Davidsen, M.L.; Trembler, K.A.; Battaglia, F.C.; Wilkening, R.B.; Anthony, R.V. The Relationship between Transplacental O2 Diffusion and Placental Expression of PlGF, VEGF and Their Receptors in a Placental Insufficiency Model of Fetal Growth Restriction. J. Physiol. 2003, 550, 641–656. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Caton, J.S.; Redmer, D.A.; Grazul-Bilska, A.T.; Vonnahme, K.A.; Borowicz, P.P.; Luther, J.S.; Wallace, J.M.; Wu, G.; Spencer, T.E. Evidence for Altered Placental Blood Flow and Vascularity in Compromised Pregnancies. J. Physiol. 2006, 572, 51–58. [Google Scholar] [CrossRef]
- Doherty, C.B.; Lewis, R.M.; Sharkey, A.; Burton, G.J. Placental Composition and Surface Area but Not Vascularization Are Altered by Maternal Protein Restriction in the Rat. Placenta 2003, 24, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Mugnier, A.; Gaillard, V.; Chastant, S. Relative Impact of Birth Weight and Early Growth on Neonatal Mortality in Puppies. Animals 2023, 13, 1928. [Google Scholar] [CrossRef] [PubMed]
- Tønnessen, R.; Borge, K.S.; Nødtvedt, A.; Indrebø, A. Canine Perinatal Mortality: A Cohort Study of 224 Breeds. Theriogenology 2012, 77, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Axnér, E.; Axelsson, R.; Hermansson, U. Evaluation of Canine Neonatal Health by Breeders: A Prospective Questionnaire Study on the Association between Neonatal Scores (Modified APGAR), Parturition, Birth Weight, Growth, and Puppy Mortality. Animals 2023, 13, 3605. [Google Scholar] [CrossRef]

| APGAR Score | ||
|---|---|---|
| Natural Delivery | Caesarean Section | |
| 5 min | 7.8 ± 1.6 a | 6.4 ± 1.2 b |
| 60 min | 8.9 ± 1.1 a | 8.6 ± 1.4 a |
| 120 min | 9.1 ± 0.9 a | 9 ± 1.2 a |
| Mean Population | Male Puppies | Female Puppies | |
|---|---|---|---|
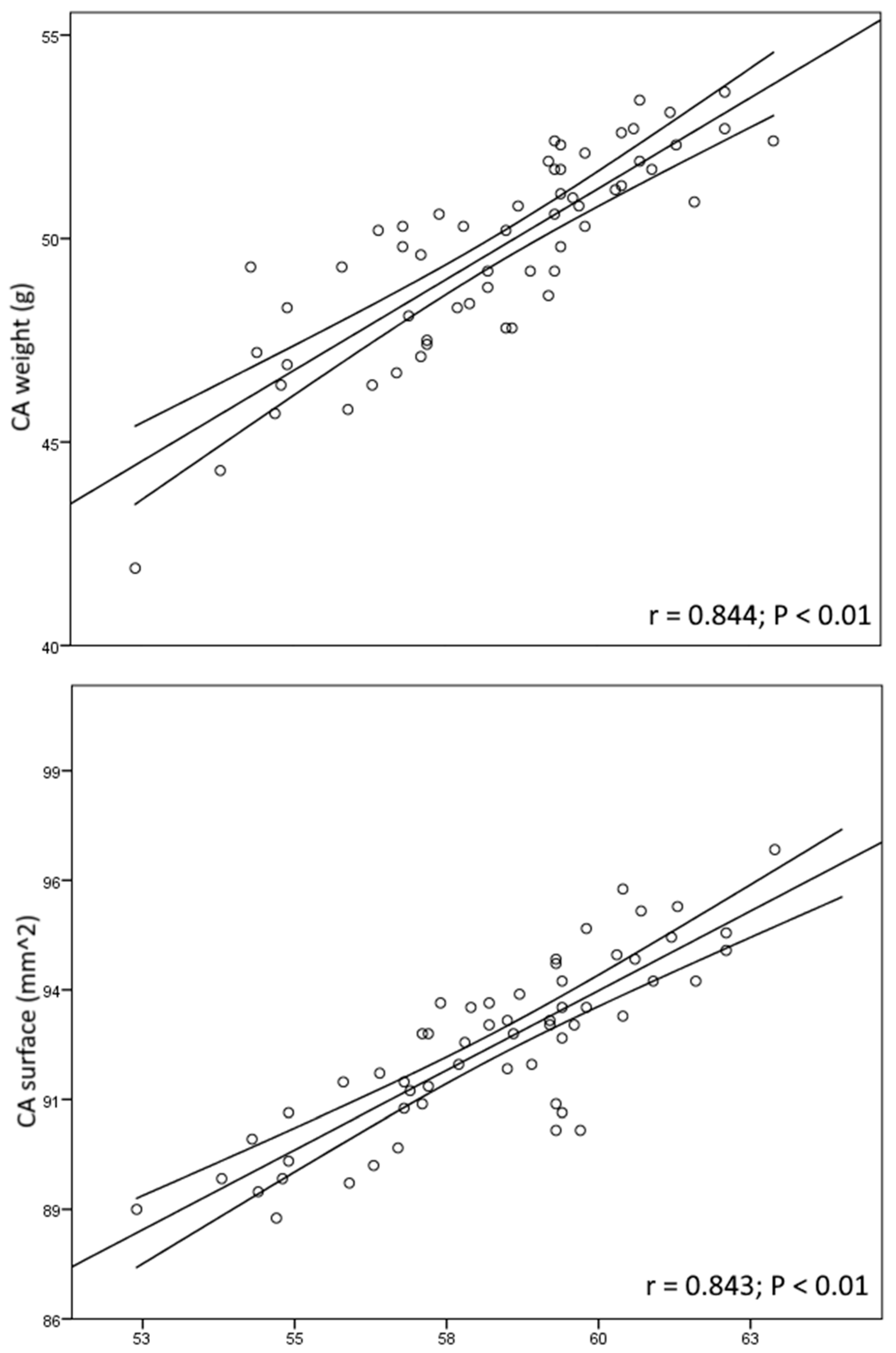
| CAW (g) | 49.7 ± 7.5 | 51.2 ± 5.2 a | 46.4 ± 5.6 a |
| CAS (cm2) | 92.5 ± 17.7 | 95.6 ± 13.9 a | 90.3 ± 9.4 a |
| WPE | 9.6 ± 1.9 | 9.8 ± 1.7 a | 9.4 ± 1.4 a |
| SPE | 5.2 ± 1.2 | 5.2 ± 0.9 a | 4.9 ± 1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gloria, A.; Veronesi, M.C.; Contri, A. Does Placental Efficiency and Vascularization Affect Puppy Health? A Study in Boxer and Dobermann Dogs. Animals 2024, 14, 423. https://doi.org/10.3390/ani14030423
Gloria A, Veronesi MC, Contri A. Does Placental Efficiency and Vascularization Affect Puppy Health? A Study in Boxer and Dobermann Dogs. Animals. 2024; 14(3):423. https://doi.org/10.3390/ani14030423
Chicago/Turabian StyleGloria, Alessia, Maria Cristina Veronesi, and Alberto Contri. 2024. "Does Placental Efficiency and Vascularization Affect Puppy Health? A Study in Boxer and Dobermann Dogs" Animals 14, no. 3: 423. https://doi.org/10.3390/ani14030423
APA StyleGloria, A., Veronesi, M. C., & Contri, A. (2024). Does Placental Efficiency and Vascularization Affect Puppy Health? A Study in Boxer and Dobermann Dogs. Animals, 14(3), 423. https://doi.org/10.3390/ani14030423






