Effects of Tithonia diversifolia Extract as a Feed Additive on Digestibility and Performance of Hair Lambs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
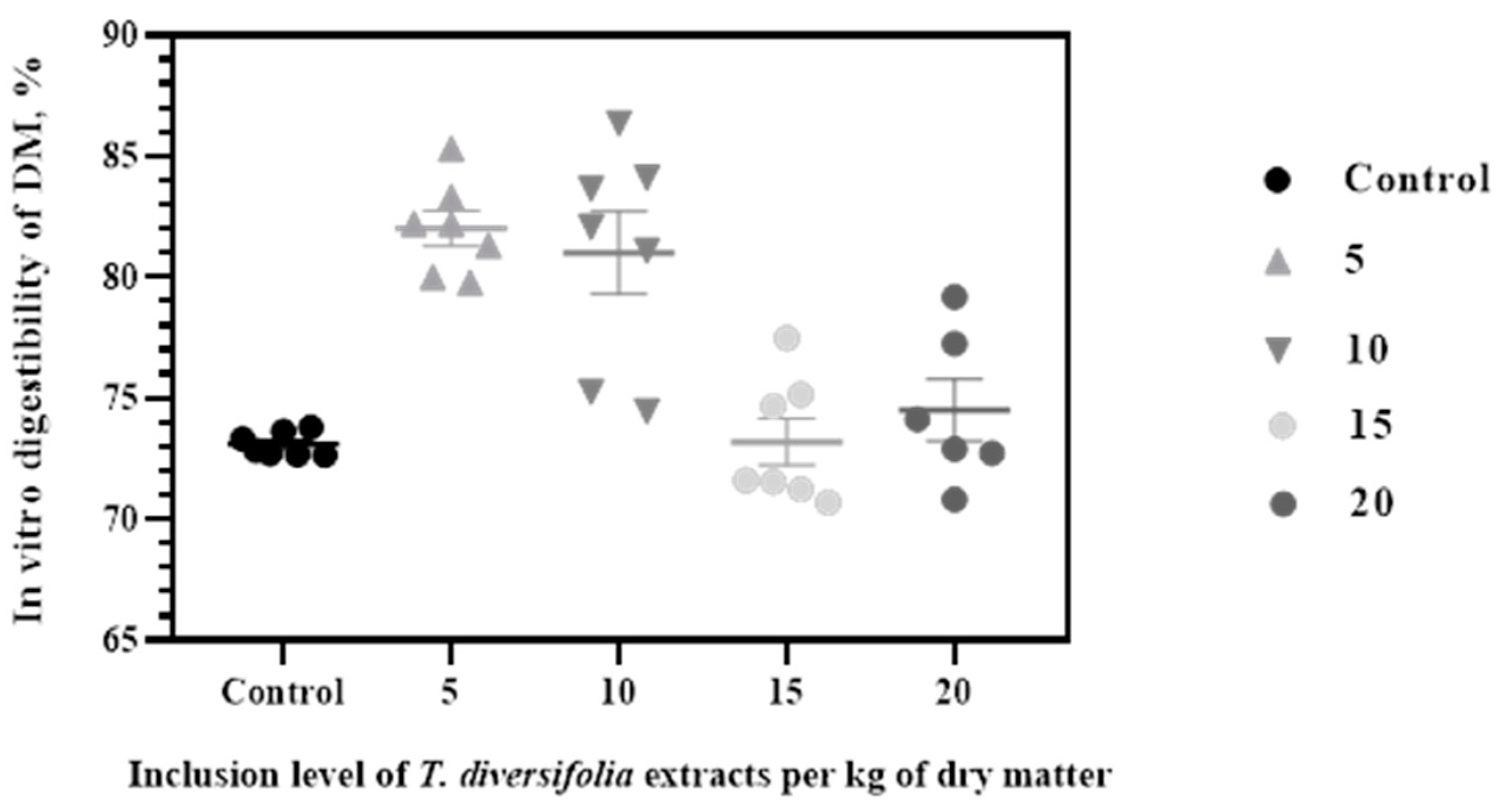
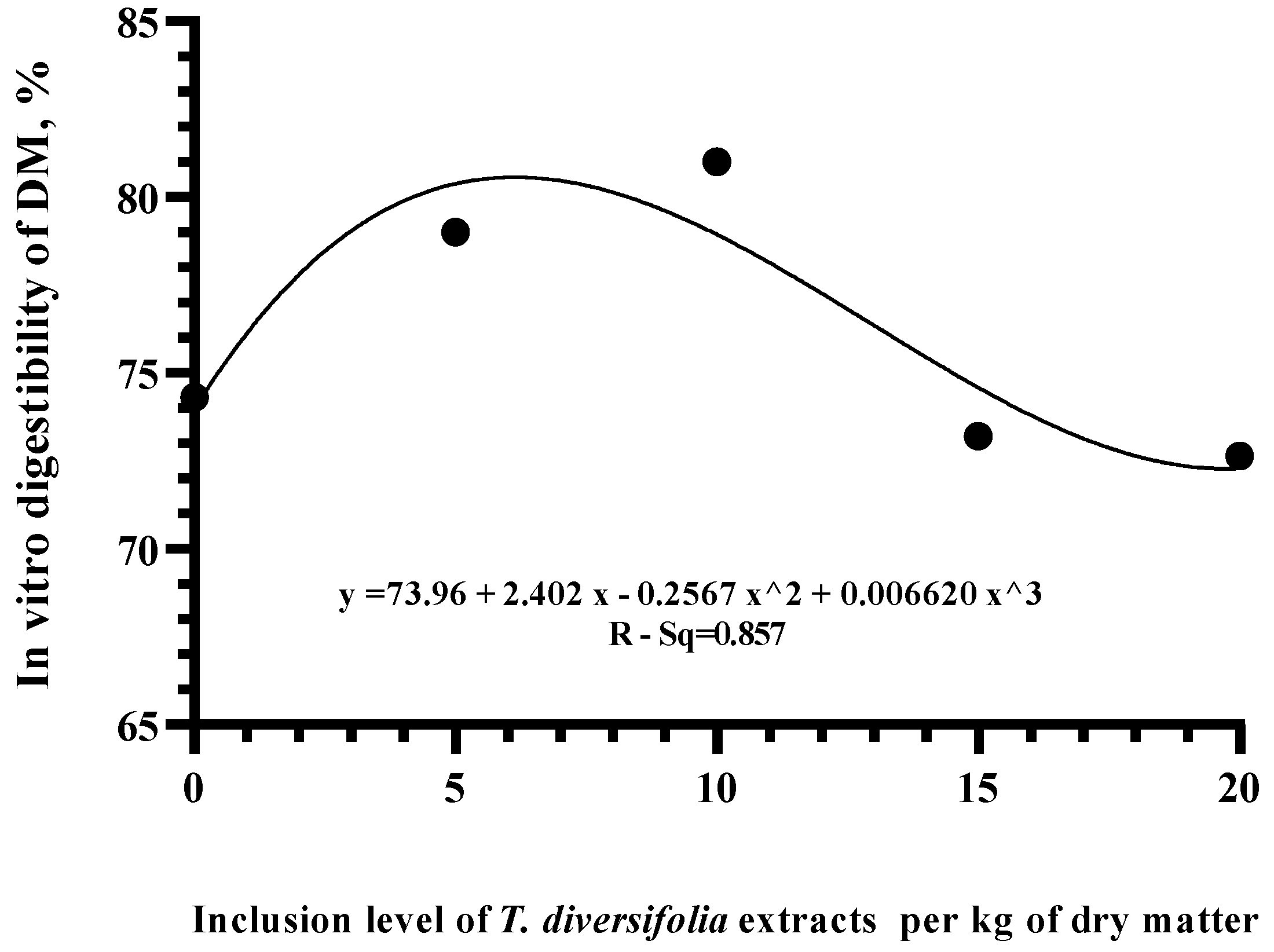
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Silva, C.S.; de Souza, E.J.O.; Pereira, G.F.C.; Cavalcante, E.O.; de Lima, E.I.M.; Torres, T.R.; da Silva, J.R.C.; da Silva, D.C. Plant Extracts as Phytogenic Additives Considering Intake, Digestibility, and Feeding Behavior of Sheep. Trop. Anim. Health Prod. 2017, 49, 353–359. [Google Scholar] [CrossRef]
- Gonzalez Ronquillo, M.; Angeles Hernandez, J.C. Antibiotic and Synthetic Growth Promoters in Animal Diets: Review of Impact and Analytical Methods. Food Control 2017, 72, 255–267. [Google Scholar] [CrossRef]
- François, B.; Jafri, H.S.; Bonten, M. Alternatives to Antibiotics. Intensive Care Med. 2016, 42, 2034–2036. [Google Scholar] [CrossRef] [PubMed]
- Jouany, J.-P.; Morgavi, D.P. Use of ‘Natural’ Products as Alternatives to Antibiotic Feed Additives in Ruminant Production. Animal 2007, 1, 1443–1466. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Kumar, P.; Saxena, M.J. Feed Additives in Animal Health. Nutraceuticals Vet. Med. 2019, 345–362. [Google Scholar] [CrossRef]
- Akram, F.; Imtiaz, M.; ul Haq, I. Emergent Crisis of Antibiotic Resistance: A Silent Pandemic Threat to 21st Century. Microb. Pathog. 2023, 174, 105923. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Strobel, H.J. Effect of Ionophores on Ruminal Fermentation. Appl. Environ. Microbiol. 1989, 55, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to Antibiotics for Maximizing Growth Performance and Feed Efficiency in Poultry: A Review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic Alternatives: The Substitution of Antibiotics in Animal Husbandry? Front. Microbiol. 2014, 5, 1–15. [Google Scholar] [CrossRef]
- Vasta, V.; Luciano, G. The Effects of Dietary Consumption of Plants Secondary Compounds on Small Ruminants’ Products Quality. Small Rumin. Res. 2011, 101, 150–159. [Google Scholar] [CrossRef]
- Piao, M.; Tu, Y.; Zhang, N.; Diao, Q.; Bi, Y. Advances in the Application of Phytogenic Extracts as Antioxidants and Their Potential Mechanisms in Ruminants. Antioxidants 2023, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Olagaray, K.E.; Bradford, B.J. Plant Flavonoids to Improve Productivity of Ruminants—A Review. Anim. Feed Sci. Technol. 2019, 251, 21–36. [Google Scholar] [CrossRef]
- Cui, K.; Guo, X.D.; Tu, Y.; Zhang, N.F.; Ma, T.; Diao, Q.Y. Effect of Dietary Supplementation of Rutin on Lactation Performance, Ruminal Fermentation and Metabolism in Dairy Cows. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; You, W.; Tan, X.; Liu, G.; Zhang, X.; Liu, X.; Wan, F.; Wei, C. Caffeic Acid Modulates Methane Production and Rumen Fermentation in an Opposite Way with High-Forage or High-Concentrate Substrate in Vitro. J. Sci. Food Agric. 2021, 101, 3013–3020. [Google Scholar] [CrossRef] [PubMed]
- Sinz, S.; Kunz, C.; Liesegang, A.; Braun, U.; Marquardt, S.; Soliva, C.R.; Kreuzer, M. In Vitro Bioactivity of Various Pure Flavonoids in Ruminal Fermentation, with Special Reference to Methane Formation. Czech J. Anim. Sci. 2018, 63, 293–304. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Oskoueian, A. Effects of Flavonoids on Rumen Fermentation Activity, Methane Production, and Microbial Population. Biomed Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Barreto-cruz, O.T.; Carlos, J.; Zambrano, H.; Castañeda-serrano, R.D.; Maria, L.; Sierra, P. Veterinary Sciences Assessing the In Vitro and In Vivo Effect of Supplementation with a Garlic (Allium Sativum) and Oregano (Origanum Vulgare) Essential Oil Mixture on Digestibility in West African Sheep. Vet. Sci. 2023, 10, 695. [Google Scholar] [CrossRef]
- Farha, A.K.; Yang, Q.Q.; Kim, G.; Li, H.B.; Zhu, F.; Liu, H.Y.; Gan, R.Y.; Corke, H. Tannins as an Alternative to Antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Rasmann, S.; Agrawal, A.A. Plant Defense against Herbivory: Progress in Identifying Synergism, Redundancy, and Antagonism between Resistance Traits. Curr. Opin. Plant Biol. 2009, 12, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Tagne, A.M.; Marino, F.; Cosentino, M. Tithonia diversifolia (Hemsl.) A. Gray as a Medicinal Plant: A Comprehensive Review of Its Ethnopharmacology, Phytochemistry, Pharmacotoxicology and Clinical Relevance. J. Ethnopharmacol. 2018, 220, 94–116. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved Extraction of PCR-Quality Community DNA from Digesta and Fecal Samples. Biotechniques 2004, 3, 808–812. [Google Scholar] [CrossRef]
- Delgado, D.C.; Galindo, J.; González, R.; González, N.; Scull, I.; Dihigo, L.; Cairo, J.; Aldama, A.I.; Moreira, O. Feeding of Tropical Trees and Shrub Foliages as a Strategy to Reduce Ruminal Methanogenesis: Studies Conducted in Cuba. Trop. Anim. Health Prod. 2012, 44, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Galindo, J.; González, N.; Marrero, Y.; Sosa, A.; Ruiz, T.; Febles, G.; Torres, V.; Aldana, A.I.; Achang, G.; Moreira, O.; et al. Effect of Tropical Plant Foliage on the Control of Methane Production and in Vitro Ruminal Protozoa Population. Cuba J. Agric. Sci. 2014, 48, 359–364. [Google Scholar]
- Kohn, R.A.; Dinneen, M.M.; Russek-Cohen, E. Using Blood Urea Nitrogen to Predict Nitrogen Excretion and Efficiency of Nitrogen Utilization in Cattle, Sheep, Goats, Horses, Pigs, and Rats. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef]
- Hrkovic-Porobija, A.; Hodzic, A.; Hadzimusic, N. Functional Liver Stress in Dairy Sheep. Indian J. Small Rumin. 2017, 23, 194. [Google Scholar] [CrossRef]
- Trotta, R.J.; Harmon, D.L.; Matthews, J.C.; Swanson, K.C. Nutritional and Physiological Constraints Contributing to Limitations in Small Intestinal Starch Digestion and Glucose Absorption in Ruminants. Ruminants 2022, 2, 1. [Google Scholar] [CrossRef]
- Ajao, A.A.; Moteetee, A.N. Tithonia diversifolia (Hemsl) A. Gray. (Asteraceae: Heliantheae), an Invasive Plant of Significant Ethnopharmacological Importance: A Review. S. Afr. J. Bot. 2017, 113, 396–403. [Google Scholar] [CrossRef]
- George, T.S.; Gregory, P.J.; Robinson, J.S.; Buresh, R.J.; Jama, B.A. Tithonia diversifolia: Variations in Leaf Nutrient Concentration and Implications for Biomass Transfer. Agrofor. Syst. 2001, 52, 199–205. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press, Ed.; National Academies Press: Washington, DC, USA, 2007; ISBN 978-0-309-47323-1. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC INTERNATIONAL; George Latimer, J., Ed.; AOAC International: Rockville, MD, USA, 2016; ISBN 0-935584-87-0. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Mertens, D.R. Gravimetric Determination of Amylase-Treated Neutral Detergent Fiber in Feeds with Refluxing in Beakers or Crucibles: Collaborative Study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar] [PubMed]
- Sniffen, C.J.; O’connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A Net Carbohydrate and Protein System for Evaluating Cattle Diets: I. Ruminal Fermentation. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef] [PubMed]
- Tietz, N.W.; Rinker, A.D.; Shaw, L.M. IFCC Methods for the Measurement of Catalytic Concentration of Enzymes Part 5. IFCC Method for Alkaline Phosphatase. Clin. Chem. Lab. Med. 1983, 21, 731–760. [Google Scholar] [CrossRef]
- Tietz, N.W. Tietz Clinical Guide to Laboratory Tests, 4th ed.; Wu, A.H., Ed.; Saunders Co.: Philadelphia, PA, USA, 2006. [Google Scholar]
- Minitab, I. Minitab 17 Statistical Software; MlNlTAB: State College, PA, USA, 2017; p. 73. [Google Scholar]
- Cobellis, G.; Petrozzi, A.; Forte, C.; Acuti, G.; Orrù, M.; Marcotullio, M.C.; Aquino, A.; Nicolini, A.; Mazza, V.; Trabalza-Marinucci, M. Evaluation of the Effects of Mitigation on Methane and Ammonia Production by Using Origanum Vulgare L. and Rosmarinus Officinalis L. Essential Oils on in Vitro Rumen Fermentation Systems. Sustainability 2015, 7, 12856–12869. [Google Scholar] [CrossRef]
- Serrano, R.D.C.; Cruz, O.T.B.; Coneglian, S.M.; Branco, A.F. Use of Cashew and Castor Essential Oils to Improve Fibre Digestibility in High Forage Diets: Digestibility, Ruminal Fermentation and Microbial Protein Synthesis. Semin. Agrar. 2020, 41, 3429–3440. [Google Scholar] [CrossRef]
- Coneglian, S.M.; Castañeda Serrano, R.D.; Cruz, O.T.B.; Branco, A.F. Effects of Essential Oils of Cashew and Castor on Intake, Digestibility, Ruminal Fermentation and Purine Derivatives in Beef Cattle Fed High Grain Diets. Semin. Ciências Agrárias 2019, 40, 2057. [Google Scholar] [CrossRef]
- Tian, X.; Qin, J.; Luo, Q.; Xu, Y.; Xie, S.; Chen, R.; Wang, X.; Lu, Q. Differences in Chemical Composition, Polyphenol Compounds, Antioxidant Activity, and In Vitro Rumen Fermentation among Sorghum Stalks. Animals 2024, 14, 415. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Ren, H.; Liu, S.M.; Cai, C.J.; Han, J.T.; Li, F.; Yao, J.H. Dynamics of Methanogenesis, Ruminal Fermentation, and Alfalfa Degradation during Adaptation to Monensin Supplementation in Goats. J. Dairy Sci. 2018, 101, 1048–1059. [Google Scholar] [CrossRef]
- de Paula, E.M.; Samensari, R.B.; Machado, E.; Pereira, L.M.; Maia, F.J.; Yoshimura, E.H.; Franzolin, R.; Faciola, A.P.; Zeoula, L.M. Effects of Phenolic Compounds on Ruminal Protozoa Population, Ruminal Fermentation, and Digestion in Water Buffaloes. Livest. Sci. 2016, 185, 136–141. [Google Scholar] [CrossRef]
- Andrés, S.; Bodas, R.; Tejido, M.L.; Giráldez, F.J.; Valdés, C.; López, S. Effects of the Inclusion of Flaxseed and Quercetin in the Diet of Fattening Lambs on Ruminal Microbiota, in Vitro Fermentation and Biohydrogenation of Fatty Acids. J. Agric. Sci. 2016, 154, 542–552. [Google Scholar] [CrossRef]
- Berger, L.M.; Blank, R.; Zorn, F.; Wein, S.; Metges, C.C.; Wolffram, S. Ruminal Degradation of Quercetin and Its Influence on Fermentation in Ruminants. J. Dairy Sci. 2015, 98, 5688–5698. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kuppusamy, P.; Jung, J.S.; Kim, K.H.; Choi, K.C. Microbial Dynamics and in Vitro Degradation of Plant Secondary Metabolites in Hanwoo Steer Rumen Fluids. Animals 2021, 11, 2350. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, N.W.; Denman, S.E.; Pilajun, R.; Wanapat, M.; McSweeney, C.S.; Elliott, R. Manipulating Rumen Fermentation and Methanogenesis Using an Essential Oil and Monensin in Beef Cattle Fed a Tropical Grass Hay. Anim. Feed Sci. Technol. 2015, 200, 25–34. [Google Scholar] [CrossRef]
- McGuffey, R.K.; Richardson, L.F.; Wilkinson, J.I.D. Ionophores for Dairy Cattle: Current Status and Future Outlook. J. Dairy Sci. 2001, 84, E194–E203. [Google Scholar] [CrossRef]
- Hook, S.E.; Northwood, K.S.; Wright, A.D.G.; McBride, B.W. Long-Term Monensin Supplementation Does Not Significantly Affect the Quantity or Diversity of Methanogens in the Rumen of the Lactating Dairy Cow. Appl. Environ. Microbiol. 2009, 75, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, Y.; Jiang, Y.; Yao, J.; Li, Z. Ruminal Bacterial Community Successions in Response to Monensin Supplementation in Goats. Animals 2022, 12, 2291. [Google Scholar] [CrossRef] [PubMed]
- Brockman, R.P.; Laarveld, B. Hormonal Regulation of Metabolism in Ruminants: A Review. Livest. Prod. Sci. 1986, 14, 313–334. [Google Scholar] [CrossRef]
- Oke, O.E.; Uyanga, V.A.; Iyasere, O.S.; Oke, F.O.; Majekodunmi, B.C.; Logunleko, M.O.; Abiona, J.A.; Nwosu, E.U.; Abioja, M.O.; Daramola, J.O.; et al. Environmental Stress and Livestock Productivity in Hot-Humid Tropics: Alleviation and Future Perspectives. J. Therm. Biol. 2021, 100, 103077. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, J.J. Carbohydrate Metabolism and Its Diseases. In Clinical Biochemistry of Domestic Animals; Academic Press: Cambridge, MA, USA, 1997; pp. 45–81. [Google Scholar]
- Maza, L.A.; Cardona, J.A.; Vergara, O.G. Analysis of Metabolic Profile in Pregnant Creole Sheep in Extensive Grazing Conditions. Rev. Científica 2011, XXI, 335–339. [Google Scholar]
- Hammond, A.C. Update on BUN and MUN as a Guide for Protein Supplementation in Cattle. In U.S. Department of Agriculture, Agricultural Research Service; Subtropical Agricultural Research Station: Brooksville, FL, USA, 1997; pp. 45–54. [Google Scholar]
- Putri, E.M.; Zain, M.; Warly, L.; Hermon, H. Effects of Rumen-Degradable-to-Undegradable Protein Ratio in Ruminant Diet on in Vitro Digestibility, Rumen Fermentation, and Microbial Protein Synthesis. Vet. World 2021, 14, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen Metabolism in the Rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, S.K. A Toxicologist Guide to the Diagnostic Interpretation of Hepatic Biochemical Parameters. Food Chem. Toxicol. 2007, 45, 1551–1557. [Google Scholar] [CrossRef]
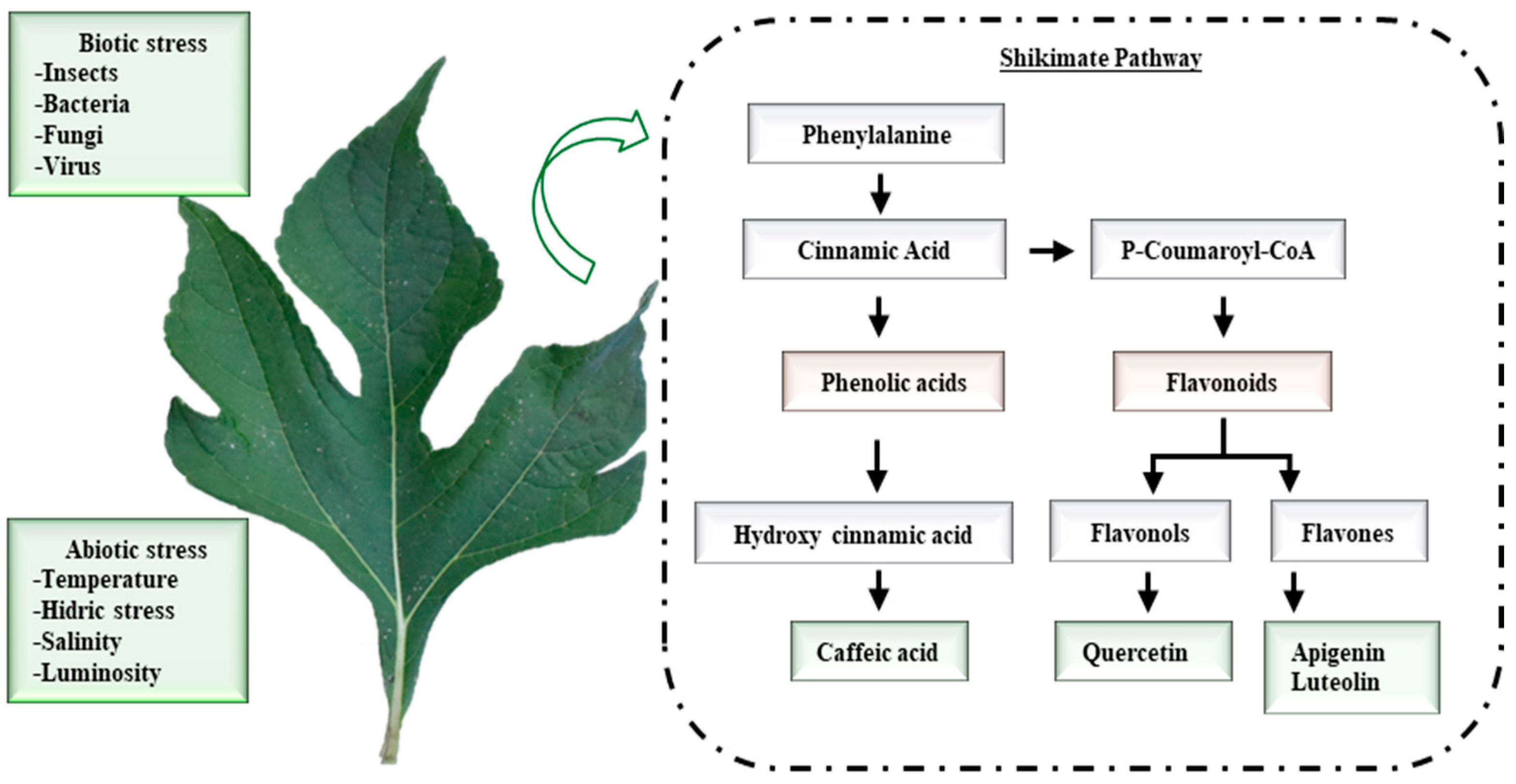
| Retention Time, min (tR) | Compound | mg/kg |
|---|---|---|
| 4.00 | Caffeic acid | 49.80 |
| 6.00 | Quercetin | 1.00 |
| 6.20 | Luteolin | 0.07 |
| 6.60 | Apigenin | 0.30 |
| Item | % of DM |
|---|---|
| Ingredient | |
| Corn silage | 66.18 |
| Corn grain, ground | 19.00 |
| Soybean meal | 12.50 |
| Urea | 1.77 |
| Bicalcium phosphate | 0.35 |
| Mineral mixture 1 | 0.20 |
| Bromatological composition | |
| Crude protein (CP) | 14.29 |
| Neutral detergent fibre (NDF) | 40.08 |
| Non-structural carbohydrates | 35.08 |
| Ether extract (EE) | 1.83 |
| Ash | 8.02 |
| Total digestible nutrients (TDN) | 71.21 |
| Parameter | Treatments | SEM 1 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | Monensin | 5 + | 10 + | |||
| Animal Performance, kg | ||||||
| Initial BW 2 | 18.95 | 18.26 | 18.92 | 17.98 | 1.70 | 0.299 |
| Final BW | 27.47 | 26.33 | 27.34 | 25.20 | 1.96 | 0.075 |
| ADG 3 | 0.20 | 0.19 | 0.20 | 0.17 | 0.03 | 0.223 |
| DM conversion | 4.41 | 4.43 | 4.46 | 4.65 | 0.54 | 0.952 |
| Intake kg day−1 | ||||||
| Dry matter | 0.89 a | 0.85 b | 0.90 a | 0.79 b | 0.04 | 0.000 |
| Crude Protein | 0.16 a | 0.10 b | 0.15 a | 0.13 b | 0.01 | 0.000 |
| Neutral detergent fibre | 0.38 a | 0.30 c | 0.37 ab | 0.32 b | 0.02 | 0.000 |
| Non-structural carbohydrates | 0.38 a | 0.28 c | 0.36 a | 0.32 b | 0.01 | 0.000 |
| Ether extract | 0.02 | 0.01 | 0.02 | 0.01 | 0.00 | 0.052 |
| Parameter | Treatments | SEM 1 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | Monensin | 5 + | 10 + | |||
| Digestibility, g/kg | ||||||
| Dry matter | 72.97 | 72.85 | 73.48 | 73.90 | 0.94 | 0.849 |
| Crude Protein | 93.40 | 92.91 | 93.05 | 93.12 | 0.29 | 0.676 |
| Neutral detergent fibre | 55.92 c | 58.40 ab | 56.93 ab | 61.32 a | 1.69 | 0.025 |
| Non-structural carbohydrates | 85.74 | 83.65 | 85.74 | 83.90 | 0.75 | 0.082 |
| Ether extract | 86.68 | 87.29 | 87.29 | 83.63 | 0.65 | 0.061 |
| TDN 2 | 61.52 | 63.88 ab | 61.99 c | 66.41 a | 1.37 | 0.048 |
| Volatile Fatty Acid. VFA (%) | ||||||
| Acetate | 76.29 a | 77.31 a | 76.42 a | 67.82 b | 1.65 | 0.042 |
| Propionate | 15.72 | 14.52 | 16.59 | 21.07 | 1.22 | 0.056 |
| Butyrate | 7.99 | 8.18 | 7.00 | 11.12 | 1.00 | 0.147 |
| Acetate: Propionate | 4.86 | 5.37 | 4.70 | 3.22 | 0.46 | 0.106 |
| Parameter | Treatments | SEM 1 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | Monensin | 5 + | 10 + | |||
| Glucose, mg/dL | 81.43 | 76.80 | 81.54 | 81.63 | 3.79 | 0.763 |
| BUN, mg/dL | 14.45 | 12.69 | 12.35 | 11.49 | 2.39 | 0.231 |
| AST, mg/dL | 16.18 | 12.94 | 12.13 | 10.93 | 1.79 | 0.221 |
| ALT, mg/dL | 21.33 | 33.36 | 15.84 | 23.80 | 8.78 | 0.569 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto-Cruz, O.T.; Henao Zambrano, J.C.; Ospina Barrero, M.A.; Castañeda-Serrano, R.D. Effects of Tithonia diversifolia Extract as a Feed Additive on Digestibility and Performance of Hair Lambs. Animals 2024, 14, 3648. https://doi.org/10.3390/ani14243648
Barreto-Cruz OT, Henao Zambrano JC, Ospina Barrero MA, Castañeda-Serrano RD. Effects of Tithonia diversifolia Extract as a Feed Additive on Digestibility and Performance of Hair Lambs. Animals. 2024; 14(24):3648. https://doi.org/10.3390/ani14243648
Chicago/Turabian StyleBarreto-Cruz, Olga Teresa, Juan Carlos Henao Zambrano, Maria Alejandra Ospina Barrero, and Román David Castañeda-Serrano. 2024. "Effects of Tithonia diversifolia Extract as a Feed Additive on Digestibility and Performance of Hair Lambs" Animals 14, no. 24: 3648. https://doi.org/10.3390/ani14243648
APA StyleBarreto-Cruz, O. T., Henao Zambrano, J. C., Ospina Barrero, M. A., & Castañeda-Serrano, R. D. (2024). Effects of Tithonia diversifolia Extract as a Feed Additive on Digestibility and Performance of Hair Lambs. Animals, 14(24), 3648. https://doi.org/10.3390/ani14243648







