Simple Summary
Many reptiles actively regulate their body temperature and during thermoregulation, they lose water from the body. The amount of body water lost in maintaining an adequate body temperature can vary from species to species. In this study, we compared the preferred body temperatures and the amount of water loss in males of two lacertid lizard species—the meadow lizard (Darevskia praticola) and the common wall lizard (Podarcis muralis)—that share the habitat at the western edge of the meadow lizard’s distribution area. We hypothesized that the meadow lizard would exhibit higher water loss than the common wall lizard, as it generally prefers humid and forested places. However, the results showed that, at this locality, water loss is similar for both species, although the meadow lizard preferred lower ambient temperatures than the common wall lizard. We concluded that the meadow lizard has developed mechanisms to control water loss. Its preference for lower temperatures could be due to both historical factors and local adaptations. This information could help us to better understand how lizards cope with environmental changes and, more importantly, what we should do to prevent the species’ decline in the wake of climate change.
Abstract
Many reptiles actively regulate their body temperature. During thermoregulation, they suffer evaporative water loss (EWL). Since evaporation increases with temperature, EWL could limit the activity of ectotherms when water is not available. In this study, we compared the preferred body temperatures (Tp) and EWL of two lacertid lizard species, Darevskia praticola and Podarcis muralis, at the western edge of D. praticola’s range, where they live in syntopy. We hypothesized that D. praticola, a species that inhabits forested and humid environments, would have a higher EWL than the more widespread P. muralis. Our results show that D. praticola prefers lower temperatures (mean Tp = 28.1 °C) than P. muralis (mean Tp = 30.6 °C). Despite the differences in their thermal preferences, both species showed similar total EWL (2.76% for D. praticola and 2.67% for P. muralis), although their daily patterns of water loss differed. Our results suggest that D. praticola has developed mechanisms to control water loss and that its lower thermal preference may be due to both historical factors and local adaptations. These results contribute to the understanding of how environmental factors influence the physiology of lizards, which in turn has implications for predicting the effects of climate change on species distribution.
1. Introduction
Reptiles rely on external sources of heat to achieve body temperatures necessary for their physiological processes, as well as other activities such as feeding, avoiding predators, and reproducing [1,2,3]. Additionally, numerous studies have shown that habitat humidity is another important environmental factor for terrestrial reptiles and that the interaction of ambient humidity and temperature has been shaping activity patterns and the distribution of many species [4,5,6,7]. Many reptiles have the ability to actively regulate their body temperature in response to varying environmental conditions. They achieve this by altering their behavior, adjusting their activity patterns, and selecting appropriate microhabitats [8,9,10]. However, during thermoregulation, they suffer evaporative water loss (EWL) mainly through the skin but also through respiratory passages and the eyes [11,12]. Since evaporation increases with temperature, a trade-off between thermoregulation and water balance may exist where EWL could constrain the activity of ectotherms when water is not available [6,13,14,15]. Mechanisms that limit the water loss from the body are essential for survival in terrestrial environments, and the intensity of water loss greatly depends on the temperature and the humidity of the habitat [11,12].
Preferred body temperature (Tp) and evaporative water loss (EWL) are two ecophysiological parameters that have often been used to describe lizards’ responses to the environment. The first one represents the range of temperatures selected by lizards in the absence of thermoregulatory constraints [16]. This temperature range is an important indicator of the thermal preference of the species because it is correlated with the optimal performance of many physiological functions [3,17,18]. Thermal preference of a species can remain similar under different environmental conditions and over longer time scales [3,19,20,21], but it has also been observed that a variety of factors, such as seasonality (especially in temperate species of lizards), ontogeny, or reproductive status, can influence the preferred body temperatures [8,18,20,22]. The evaporative water loss (EWL) provides evidence of the degree of resistance to water loss. According to Mautz [11], EWL (in the absence of defecation) and metabolic gas exchange (which is usually negligible) are reflected in the body weight loss; therefore, following the body weight loss is the most common method used for measuring the water loss rate (for a recent review of methods used in squamate reptiles, see Le Galliard et al. [23]). These ecophysiological measurements can be used to assess the thermal preference of individuals and their capacity to resist water loss, but they can also determine if these traits differ between populations or species [5,24,25,26,27].
Rapid global climate change poses significant extinction risks for reptiles [28,29,30]. If effective mitigation measures are not implemented, reptiles may experience large-scale declines in the near future due to climate change [30]. Understanding the relationship between species’ thermal requirements and geographic gradients is crucial for predicting their vulnerability to extinction from climate change [31]. Some niche-specialist lizard species may be able to adapt to changing environmental conditions successfully, provided they possess sufficient physiological plasticity [32]. A predicted rise in temperature and decrease in rainfall in southern and central parts of Europe will affect the availability of water for organisms [33,34]. In addition, climate warming will also increase the evaporation rates of organisms [6,29]. Hence, an integrated approach that takes into account these ecophysiology data can be highly useful in developing mechanistic distribution models. These models can be used to predict the distribution and the degree of vulnerability of species under climate change and provide directions for future conservation measures [6,35,36,37,38]. It is important to note that these changes can have different impacts at the edges of a species’ range, where populations tend to be smaller and more fragmented. As species approach their ecological limits, this fragmentation may lead to genetic isolation, reducing the potential for local adaptation [39,40].
In this study, we examined Tp and EWL in syntopic populations of two lacertid species, Darevskia praticola and Podarcis muralis, with an emphasis on D. praticola. There is a general scarcity of data on thermal physiology and water loss regarding the whole Darevskia genus. Until now, only three studies have been published on the thermal ecology of 4 Darevskia species—D. praticola in Serbia [41], D. valentini in Armenia [42], D. armeniaca, D. unisexualis, and D. valentini in Armenia [43], plus an extensive study on climate variation shaping diversification and genome variation in lacertid lizards [7] where a few basic thermal and water loss data of D. praticola, D. rudis, and D. valentini were included. This study was conducted on D. praticola because there is little data on its ecophysiology, and therefore it should be studied in more detail. Since P. muralis is the only small lacertid found in syntopy with D. praticola at the western edge of the distribution area, we have used it as a reference point for comparison. Podarcis muralis has previously been an object of studies concerning the thermal preferences of the species [17,44,45,46,47,48] and combined studies of thermal ecology and water loss [24,49].
Our goal was to investigate certain ecophysiological characteristics of a species at the edge of its distribution range living in syntopy with a potential competitor. Our main hypothesis was that D. praticola has higher water loss rates than the widespread P. muralis, considering its preference toward forested and humid habitats. Additionally, we supposed that D. praticola does not show significant interannual variation in preferred temperatures. Therefore, we experimentally analyzed Tp and EWL in both species to (1) evaluate the differences between the species in Tp and variation of Tp; (2) examine whether there is a difference in Tp of D. praticola, compared to our previous research at the same study site; (3) determine EWL rates and their daily variation in both species.
2. Materials and Methods
2.1. Analyzed Species
Both analyzed species are small lacertid lizards with partially overlapping ranges in the Balkans (Figure 1): P. muralis is distributed from the Iberian Peninsula to Asia Minor and is widespread across Central and Southern Europe [50,51], while D. praticola has a disjunct distribution, with its range split into two subranges—Eastern (Caucasus) and Western (Eastern and South-Eastern Europe) [51,52]. The western portion of its range is in Southeastern Europe (covering areas in Romania, Serbia, Bulgaria, Northern Greece, and the European part of Turkey), where the species reaches its western distribution limit in Central Serbia (Ćorović et al. [53] and references therein). Podarcis muralis is widespread through most of its range and can be found in a wide variety of habitats, from sunny slopes in broad-leaved and coniferous forests to fields, rocky surfaces, and stone walls, and also in urban environments [54,55]. It can sometimes be found syntopic with D. praticola. Unlike other species in its genus, which are typically saxicolous, D. praticola is a ground-dwelling lizard. It is most commonly found in open oak woodlands with a well-developed herbaceous understory and tends to avoid dry forest habitats [56,57,58,59]. In cases of deforestation and habitat degradation, it can be found in the humid, grassy vegetation surrounding streams or drainage canals [60]. Overall, D. praticola prefers moister and more shaded environments compared to other small lacertids [56,60,61,62]. More detailed information on the distribution and habitat preference of D. praticola can be found in the ecological niche study of Ćorović et al. [53].
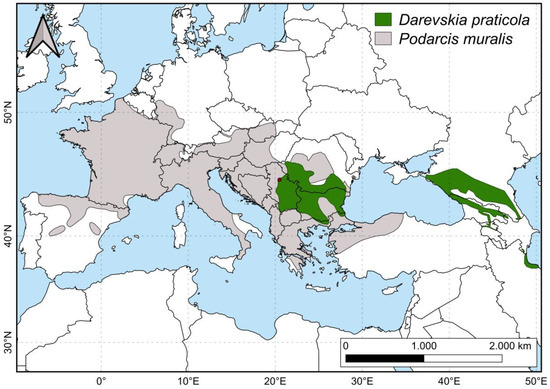
Figure 1.
Distribution ranges of Darevskia praticola and Podarcis muralis. The red dot on the map represents the geographic position of this study locality. The shapefiles used for the map were downloaded from the IUCN Red List of Threatened Species [50,52].
2.2. Field Procedures
Ten adult males of both species (D. praticola and P. muralis) were captured by noose during the reproductive season in May of 2019 at the same study site—the slopes of Avala mountain, near Belgrade, Central Serbia (44°40′52.5″ N, 20°33′00.3″ E; 230 m altitude), overgrown with a forest of a thermophilic oak community Quercetum frainetto-cerris. This study locality is set at the westernmost edge of D. praticola’s range, characterized by higher mean annual temperatures and lower amounts of annual precipitation compared to central habitats of D. praticola in this area (eastern Serbia and southwestern Romania), and it is under intense habitat fragmentation caused by human activity [41].
2.3. Preferred Body Temperatures
In this experiment, we followed the procedure described in Carretero, Roig, and Llorente [20] and Veríssimo and Carretero [63], following the same approach as in our previous study on the thermal ecology of D. praticola [41]. To eliminate the potential effects of reproduction, body condition, and ontogeny on Tp and EWL, we only analyzed adult males (D. praticola: N = 10 and P. muralis: N = 10) [20,27]. The lizards were housed in individual cages under natural light conditions for no more than three days prior to the experiment, with ad libitum food and water. Snout-vent length (SVL) of every lizard was measured to the nearest 0.01 mm using callipers, and weight was measured to the nearest 0.001 g using an analytical scale. Only lizards with unbroken or fully regenerated tails were included. Each lizard was individually exposed to a thermal gradient (~20–45 °C) created by a 150 W infrared reflector bulb positioned at one end of the terrarium (100 × 40 × 30 cm) [60]. The terraria were placed in a room with a natural light photoperiod and a stable temperature of around 20 °C. The bulbs were turned on at 07:00 h, and the lizards were placed in the experimental terraria at 08:00 h, with the first measurements taken at 09:00 h [20]. Preferred body temperature (Tp) was measured hourly from 09:00 to 17:00 h by inserting the tip of an electronic digital thermometer into the cloaca (Dostmann digital Einstich–Thermometer TFA with an accuracy of ±0.1 °C) within 10 s of catching each individual to minimize heat transfer from the researcher’s hand [63]. The thermal gradient was monitored hourly throughout the experiment. Set-point temperature ranges (Tset) were estimated for each lizard as the central 50% of all body temperatures (Tb) selected in the thermogradient [16].
2.4. Evaporative Water Loss Rates
The evaporative water loss experiments were conducted the day after the thermal experiments (following Osojnik et al. [24]; Carneiro et al. [49]; and Carneiro et al. [5]). During the thermal experiments, the lizards were not fed, and after the experiment, until the next day, they only had access to water. This way the lizards could rehydrate, but the possibility of defecation that could affect the results of the water loss experiment was diminished. After the experiments, the lizards had a recuperation period with food and water, after which they were released at their capture sites.
During the water loss experiment, individual lizards were placed in opaque plastic boxes (12 cm × 7 cm × 8 cm) with a perforated lid and bottom to enable air circulation. Under each individual box was an additional box, containing silica gel (5 g) that maintained a lower relative humidity in the box with the lizard. Groups of four individual boxes were placed in a bigger (40 cm × 30 cm × 19 cm), closed box. The bigger box also contained a solid calcium chloride moisture absorber that was used to keep the relative humidity in the box between 20 and 30%. The temperature of the experimental room was set to 24 °C because it falls around the low threshold of activity of most lacertids [18], so the activity of animals and the amount of stress were reduced, but the evaporative water loss was still enabled. Relative humidity and temperature were monitored in the box to the nearest 0.1% and 0.1 °C, respectively, using a Testo 410-2 hygrothermometer (Testo, Neustadt, Germany). The experiment was conducted from 09:00 to 17:00 h for eight consecutive hours (covering the daily activity period of the species, based on previous field experience at the chosen locality). To avoid stress from handling, the lizards were weighed in their individual boxes, at hourly intervals, with an analytical scale with ±0.001 g precision. Each weighing operation took around 10 s to minimize the disturbance of animals [5]. The lizard’s body mass during the experiment was calculated by subtracting the weight of the corresponding box that was individually marked and measured before the experiment, to the precision of ±0.001 g, from the weight of the box with the lizard. Before the analyses, the data were checked for errors and outliers.
Using these data, two measures of the relative water loss were calculated. Accumulated evaporative water loss—EWLa, which enables the assessment of the total water loss (EWLt) during the experiment and is calculated for every hour of the experiment (from 10:00 to 17:00 h) using the formula: EWLa = [(W0 − Wn)/W0] × 100. The second measure is the instantaneous evaporative water loss—EWLi which shows the water loss between two consecutive measurements, and it can be used to see if the pattern of water loss changes during the experiment. Instantaneous evaporative water loss is calculated using the formula: EWLi = [(Wn − Wn+1)/W0] × 100. In both formulas, the W0 is the initial mass before the experiment (at 09:00 h), Wn is the mass at a certain time of measurement, and the acquired values are represented in percentages.
2.5. Statistical Analyses
We first used GLM (co)variance analyses with repeated measurements to determine the variation in Tp according to species and time interval (within-subject factor). In the second step, SVL and body mass of the lizards were incorporated as covariates to account for the effect of size and shape. For water loss experiments, we also used these analyses to determine the differences in instantaneous water loss (EWLi) between species and time intervals, adding as covariates the SVL and body masses of lizards. We also calculated the accumulated water loss for the eight intervals (EWLa) and compared it between species using GLM (co)variance analyses, with SVL and body mass as covariates. The possible trade-off between Tp and EWL was investigated through Spearman’s correlation between mean Tp and total EWL ([(W0 − W8)/W0] × 100) separately for both species. We also assessed the influence of SVL and body mass on mean Tp and the total EWL. All the analyses were performed in Statistica 12 software [64].
3. Results
3.1. Species Morphology
The SVL of adult males used in this experiment was statistically different between the two species (D. praticola: 48.65 ± 1.70 mm, P. muralis: 61.30 ± 2.85 mm; univariate tests of significance, F1,18 = 145.45; p < 0.001) and body mass (W0) (D. praticola: 2.35 ± 0.24 g, P. muralis: 4.95 ± 0.57 g; univariate test of significance, F1,18 = 175.23, p < 0.001) (Table 1). Males of P. muralis were larger and heavier than males of D. praticola.

Table 1.
Between-species comparison of main variables examined in this study. Sample size (N); mean, maximum, minimum, and standard deviation (SD) of the body mass (W0), snout-vent length (SVL), preferred body temperature (Tp), and total accumulated evaporative water loss (EWLt) calculated for Darevskia praticola and Podarcis muralis. Mean ± standard deviation and range (in parenthesis). Note: The ranges of Tp are the min and max values from the whole data set, not of the Tp mean.
3.2. Preferred Body Temperature
Results of preferred body temperatures (Table 1 and Table A1, Figure 2) showed a significant difference between the two species (F1,18 = 18.98; p < 0.001) and a significant variation among time intervals with a trend of decrease of Tp in D. praticola (F8,144 = 2.19; p < 0.05). The interaction of time*species showed no difference (F8,144 = 1.19; p = 0.311). Incorporating SVL and W0 as covariates had an influence on these results (species F1,16 = 1.13; p = 0.305; time F8,128 = 0.51; p = 0.846; time*species F8,128 = 1.28; p = 0.261). There were no significant correlations between SVL and W0 with the mean Tp in either of the species.
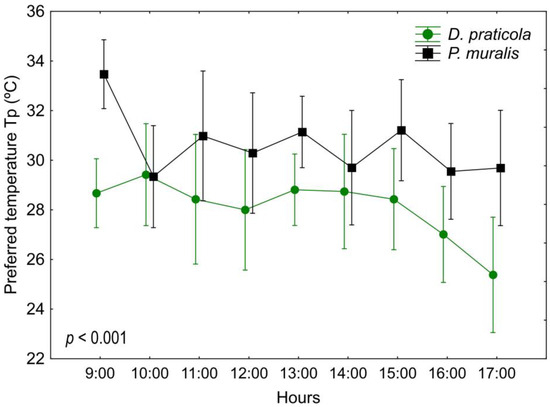
Figure 2.
Daily variation of the preferred body temperatures (Tp) of Darevskia praticola and Podarcis muralis, showing a different pattern between the two species. Displayed are the mean values and 0.95 confidence intervals. The presented p-value illustrates the magnitude of the difference in Tp between the species.
Set-point range (Tset) calculated as the interquartile 50% from all preferred temperatures varied between 26.9 °C and 29.4 °C, with a mean of 28.1 ± 2.5 °C for D. praticola, and between 29.0 °C and 32.7 °C, with a mean of 30.6 ± 3.8 °C for P. muralis. To gain a better insight into the thermal preferences of D. praticola, we have also taken into account data from our earlier experiments—from 2014 [38] and unpublished data from 2018 [62] (Table 2). Ambient temperatures, corresponding to the month of the experiments, were—June 2014 (min–max: 16.4–26.4 °C, mean: 21.4 °C), May 2018 (min–max: 16.2–26.8 °C, mean: 21.5 °C), and May 2019 (min–max: 11.6–20.2 °C, mean: 15.6 °C) (data obtained from a local weather station).

Table 2.
Preferred body temperatures (Tp) and set-point temperatures (Tset) for Darevskia praticola adult males, recorded in different years. Sample size (N).
3.3. Evaporative Water Loss
Our analysis of EWLi rate (Table A2, Figure 3) showed a difference between time intervals and in the interaction of time*species (time F7,126 = 7.26; p < 0.001; time*species F7,126 = 5.05; p < 0.001) but not between the species (F1,18 = 0.10; p = 0.750), indicating that the species have different EWL patterns throughout the day.
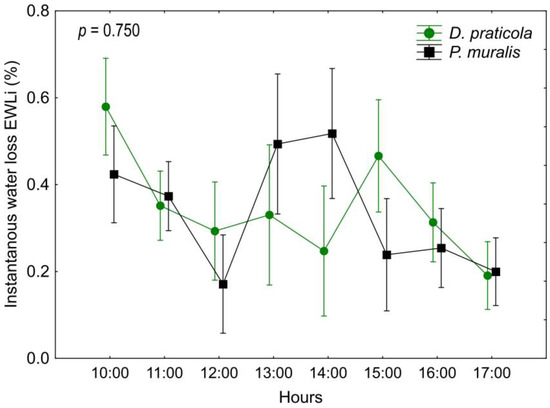
Figure 3.
Daily variation of the instantaneous water loss (EWLi) of Darevskia praticola and Podarcis muralis, showing a different pattern between the two species. Displayed are the mean values and 0.95 confidence intervals. The presented p-value illustrates the magnitude of the difference in EWLi between the species.
Rate of EWLa (Table A3, Figure 4) as a function of species and interaction of time*species did not show a significant difference (species F1,18 = 0.27; p = 0.611; time*species F7,126 = 0.98; p = 0.452), but as EWLa increases over time, the influence of time was significant (F7,126 = 161.98; p < 0.001). Adding SVL and W0 as covariates had no influence on the results of the EWLa analyses (species F1,16 = 1.78; p = 0.201; time F7,112 = 4.762; p < 0.001; time*species F7,112 = 0.71; p = 0.663).
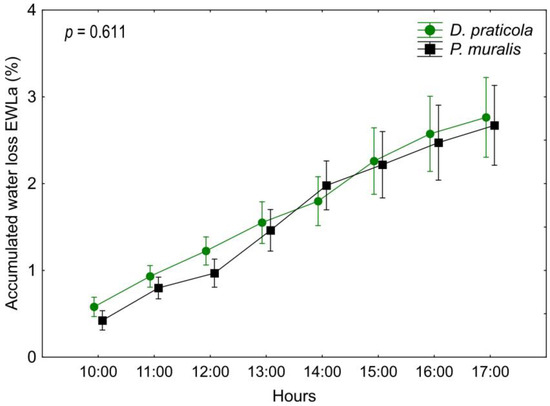
Figure 4.
Daily variation of the accumulated water loss (EWLa) of Darevskia praticola and Podarcis muralis shows a similar pattern for the two species. Displayed are the mean values and 0.95 confidence intervals. The presented p-value illustrates the magnitude of the difference in EWLa between the species.
Analysis of EWLt rate did not show a significant difference between the species (F1,18 = 0.089; p = 0.770). Moreover, there were no significant correlations between SVL and the EWLt in either of the species. Total EWL was correlated with W0 only in D. praticola (R = −0.7; p < 0.05). Total EWL for D. praticola was 2.76 ± 0.62, and for P. muralis, 2.67 ± 0.75 (mean ± SD %) (Table 1).
No significant correlations between mean Tp and EWLt were detected in either of the species, indicating a lack of support for a trade-off between these variables (D. praticola: R = −0.2; p = 0.580; P. muralis: R = 0.2; p = 0.580).
4. Discussion
Due to the limited data available for the Darevskia genus, we compared the thermal biology of the meadow lizard with that of other European lacertid species, focusing mainly on P. muralis. This species is the only small lacertid that shares a habitat with D. praticola at the western edge of its distribution range.
The results of this study suggest that D. praticola and P. muralis differ in some aspects of their ecophysiology. Regarding the thermal preference, we detected a significant difference, with D. praticola selecting lower temperatures than P. muralis. Its mean Tset in the laboratory (28.1 °C) is 2 °C lower than that of P. muralis (30.6 °C), from the present study and also lower than the mean set-point temperatures reported for 15 species of Podarcis lizards (ranging from 31.7 °C to 35.5 °C) [65]. Moreover, its set-point range (26.9–29.4 °C) falls mostly below that of P. muralis from the current study (29.0–32.7 °C), and completely below the values detected for the populations of the common wall lizard from the South of Europe (31.9–36.5 °C from Central Spain; 31.3–34.0 °C from Peloponnese, Greece; reported by Bauwens et al. [17] and Sagonas et al. [47], respectively). The mean Tp of D. praticola (28.1 °C) is more than 3 °C lower than that reported for D. valentini (31.5 °C) from Armenia [42]. On the other hand, in our previous research on the thermal ecology of D. praticola, we observed that its set-point temperature range closely resembles that of Iberolacerta bonnali, a cold-adapted alpine lizard. This similarity in Tset between a species living in temperate forests and an alpine species may be attributed to the relatively cool, shaded forest habitat of the meadow lizard, as well as the moderate temperatures during spring at this specific locality [41].
Darevskia praticola and P. muralis showed a different temporal pattern of daily fluctuations in Tp. Probably due to their preference for higher temperatures, P. muralis showed a peak in Tp at the beginning of the experiment when they started thermoregulating. Later, they showed a stable pattern of body temperatures. In D. praticola we observed something peculiar. Until early afternoon, the lizards showed a stable pattern of body temperatures. However, after 15:00 their Tp started to decrease. This could be related to their daily activity pattern, as they are less active in the field in the afternoon. This pattern could also indicate a sensitivity to water loss. It could therefore be a compensatory mechanism for dehydration as the experiment progressed. This is consistent with the research of Sannolo et al. [15], where it was experimentally found that dehydrated lizards select lower temperatures in the gradient.
Our findings showed that the thermal preference of D. praticola was conserved within the same sex and season, as Tp and Tset were almost identical for males in the spring of 2014 [41] and 2018 (unpublished data) [66]. However, these temperatures also showed a potential for variation with seasonal weather, with lower values in the spring of 2019 (which was atypically cold and rainy); see Table 2. Additionally, the difference in Tset between the population of P. muralis from this study and those in Spain and Greece indicates some degree of plasticity of this trait. This is in accordance with previous research where it was shown that the thermal preference of a species can remain similar under different environmental conditions [3,19,20,21] but also shows plasticity for seasonal acclimatization [8,18,20,22].
Our main hypothesis was that D. praticola is sensitive to water loss, considering its preference towards forested and humid habitats, and that it has higher water loss rates than the widespread P. muralis. The patterns of water loss differed between the two investigated species regarding the time from the beginning of the experiment. In fact, D. praticola and P. muralis exhibited different trends in daily EWL patterns, even though the total EWL at the end of the experiment was almost the same. Various regulatory mechanisms can influence water loss. These may be structural, such as dynamic skin resistance to water loss [67], or behavioral, such as spending less time with eyes open to reduce water loss [68]. Water loss could also be increased by stress or increased physical activity, causing the animals to hyperventilate and lose more water through respiration. These mechanisms and induced physiological states, as well as possible intrinsic diurnal variations in activity and water loss, may lead to different temporal patterns of EWL during the experiment. Darevskia praticola showed a water loss pattern exhibiting an initially high EWLi, referred to as “initial acclimation”. This is a common EWLi pattern observed in other lacertids, where the water loss is presumably higher due to the initial stress of handling the individuals [69]. As the experiment progressed, the EWLi rate no longer fluctuated significantly and decreased towards the end of the experiment, probably due to reduced activity. Podarcis muralis, on the other hand, showed a pattern with a peak in the middle of the experiment, referred to as the “mid-peak” [69]. This pattern is still not well explained or understood, but a certain observation was made. In the work of Žagar et al. [69], this pattern was observed in diurnal lizards that live in mesic habitats and are active at ground surface. We hypothesize that the increased water loss in the early afternoon is a consequence of the daily activity cycle of this species. In spring, the activity of P. muralis is highest at this time because of the increased amount of sun exposure. So, they may experience an “internal clock” increase in activity and with it the increase in water loss during this time.
For the comparison of EWL rates, we used recent studies that applied the same experimental methodology, with a consideration of the different time frames of the measurements, which influence the total EWL. We compared the EWL values up to the eighth hour of the experiments to match the time frame of our experiment. EWLt for P. muralis was 2.67%, which is lower than the EWLt observed for P. muralis in Slovenia (3.12%—Osojnik et al. [24]) and higher than that reported for P. muralis in Spain (2.3%—Carneiro et al. [49]). These differences indicate the existence of local adaptations for this trait. Compared to other species, the EWLt of both D. praticola and P. muralis was higher than those reported for some Podarcis and Iberolacerta species [24,25,49] but lower than those observed in some species of Algyroides, which are highly sensitive to water loss [5].
The low EWLt value of D. praticola was peculiar, given the species’ preference for humid habitats and presumed sensibility to water loss. Comparing D. praticola temperature preference and evaporative water loss to other lacertid species showed that its preferred temperatures are lower than in most lacertids, while its EWLt is moderate (but lower than expected). These results seem to indicate that it has developed mechanisms for water loss management and that its observed preference for humid habitats might be a result of its thermal constraints and the choice of more thermally favorable habitats. However, these findings may also be a result of the geographical origin of the species and its phylogeny, as it is the only representative of the Darevskia genus living in Europe. A large study conducted on the Lacertidae family showed that multiple lacertid clades have independently conquered cold environments [7]. These clades include the genus Zootoca, whose range extends into the Subarctic, as well as the montane genera Iberolacerta, Darevskia, and Dinarolacerta.
Our primary focus has been on the significance of ambient humidity and temperature for the activity patterns and the distribution of reptiles [4,5,6,7]. However, it is important to acknowledge that other environmental factors, such as vegetation cover, sun exposure, wind, and substrate humidity, also influence the thermal and hydric conditions of reptile microhabitats [70] and can directly impact their physiology [71,72]. Therefore, when assessing species’ thermoregulation and hydroregulation costs in the field, it is crucial to consider the variation of these environmental factors as well.
Given that D. praticola is a forest-dwelling lacertid lizard, we emphasize the importance of vegetation cover for its conservation. Forests mitigate the effects of the surrounding climate by creating distinct microclimatic conditions—characterized by lower maximum temperatures, higher minimum temperatures, and increased relative humidity compared to adjacent open habitats. This enables forest-dwelling species to be less reliant on broader climate patterns [73,74]. According to Maiorano et al. [75], most of the meadow lizard’s distribution range in Europe is situated in regions predicted to face a 70% to 90% risk of exposure to extreme climate conditions in the coming decades. Given this, ecophysiological studies such as this one could help in developing effective conservation strategies for the threatened species. For the meadow lizard, conservation efforts should focus on preserving the remaining natural habitats, particularly deciduous oak forests, which provide the necessary temperature and humidity conditions [53]. Additionally, in areas under intense anthropogenic alteration, conservation efforts should include the restoration of sufficiently large and interconnected suitable forest fragments.
5. Conclusions
Studies on the preferred temperatures showed that they tend to be more related to biogeographic origins and that the phylogenetic signal across Lacertidae indicates that Tp adaptations are characteristic of major clades that diverged early in lacertid evolution. It was also found that EWL was phylogenetically less conserved than Tp, which may indicate a stronger selection pressure and faster adaptation of this physiological trait [7]. Thus, the “unusual” physiological traits of D. praticola may be a combination of historical influences, as well as recent local adaptations. This emphasizes the need for further studies on this species throughout its range as well as on other species of the genus.
Author Contributions
Conceptualization, J.Ć. and J.C.-I.; methodology, J.Ć. and J.C.-I.; validation, J.Ć., J.C.-I. and N.Ć.; formal analysis, J.Ć.; investigation, J.Ć. and N.Ć.; data curation, J.Ć.; writing—original draft preparation, J.Ć.; writing—review and editing, J.Ć., J.C.-I. and N.Ć.; visualization, J.Ć.; supervision, J.C.-I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, grant number 451-03-66/2024-03/200007. J.C.-I. was also funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, grant number 451-03-65/2024-03/200124.
Institutional Review Board Statement
Sampling and measuring procedures on the lizards were approved by the Ministry of Environmental Protection of the Republic of Serbia, with permits for collecting protected wild animal and plant species for scientific purposes No. 353-01-1359/2017-04 and No. 353-01-2892/2018-04. Additionally, the experiment was approved by the Ethical Committee of the Institute for Biological Research “Siniša Stanković” with permits No. 04-05/18 and No. 03-5/19.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
We would like to thank Miguel A. Carretero for his advice on the statistical analysis of the data.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
This appendix contains three tables (Table A1, Table A2 and Table A3) with all values of Tp, EWLi, and EWLa of D. praticola and P. muralis measured in these experiments.

Table A1.
Daily variation of the preferred body temperatures (Tp) of Darevskia praticola and Podarcis muralis.
Table A1.
Daily variation of the preferred body temperatures (Tp) of Darevskia praticola and Podarcis muralis.
| SP | CODE | SVL | W0 | TP9 | TP10 | TP11 | TP12 | TP13 | TP14 | TP15 | TP16 | TP17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DP | DP1 | 47.7 | 2.0 | 28.6 | 26.8 | 28.1 | 26.2 | 24.6 | 27.8 | 28.5 | 25.6 | 25.7 |
| DP | DP2 | 49.7 | 2.6 | 29.2 | 28.7 | 28.5 | 26.0 | 29.8 | 23.5 | 27.8 | 26.9 | 22.6 |
| DP | DP3 | 49.8 | 2.4 | 29.6 | 32.8 | 29.4 | 29.3 | 30.5 | 32.1 | 29.2 | 28.2 | 30.5 |
| DP | DP4 | 47.2 | 2.3 | 28.2 | 31.2 | 26.6 | 32.6 | 29.2 | 29.2 | 28.9 | 28.8 | 30.9 |
| DP | DP5 | 47.0 | 2.0 | 31.3 | 29.1 | 31.2 | 27.5 | 28.2 | 27.7 | 28.6 | 27.0 | 21.0 |
| DP | DP6 | 48.2 | 2.6 | 29.2 | 31.0 | 29.9 | 28.5 | 26.2 | 29.1 | 28.7 | 26.8 | 23.3 |
| DP | DP7 | 47.2 | 2.2 | 30.7 | 27.1 | 31.5 | 29.8 | 29.8 | 30.4 | 28.5 | 29.7 | 26.3 |
| DP | DP8 | 47.5 | 2.4 | 23.7 | 29.8 | 28.6 | 23.2 | 31.1 | 29.3 | 30.1 | 23.1 | 27.3 |
| DP | DP9 | 52.0 | 2.6 | 30.3 | 30.1 | 27.0 | 29.2 | 31.6 | 30.1 | 28.4 | 29.5 | 25.1 |
| DP | DP10 | 50.2 | 2.4 | 25.9 | 27.6 | 23.5 | 27.7 | 27.1 | 28.2 | 25.6 | 24.5 | 21.1 |
| PM | PM1 | 60.3 | 5.0 | 35.2 | 28.7 | 34.2 | 30.1 | 29.4 | 27.9 | 26.1 | 26.0 | 23.8 |
| PM | PM2 | 57.8 | 4.0 | 30.8 | 29.7 | 34.1 | 24.5 | 31.8 | 34.3 | 31.7 | 34.6 | 29.1 |
| PM | PM3 | 61.6 | 5.3 | 34.6 | 26.7 | 25.5 | 34.6 | 31.5 | 32.5 | 31.1 | 32.2 | 33.1 |
| PM | PM4 | 59.8 | 5.2 | 35.5 | 32.3 | 22.1 | 35.7 | 32.5 | 32.1 | 33.7 | 30.2 | 31.2 |
| PM | PM5 | 64.1 | 5.2 | 34.1 | 21.9 | 25.4 | 28.6 | 30.7 | 27.6 | 36.7 | 28.7 | 28.3 |
| PM | PM6 | 60.5 | 5.1 | 33.6 | 24.7 | 35.3 | 30.2 | 27.2 | 33.1 | 33.3 | 29.5 | 32.0 |
| PM | PM7 | 62.2 | 5.1 | 32.8 | 29.9 | 30.4 | 29.8 | 30.8 | 22.6 | 34.6 | 22.6 | 33.8 |
| PM | PM8 | 61.3 | 5.1 | 32.7 | 33.6 | 30.9 | 22.4 | 35.1 | 22.4 | 26.1 | 30.4 | 32.7 |
| PM | PM9 | 58.0 | 3.9 | 35.2 | 32.3 | 35.8 | 30.8 | 32.1 | 32.0 | 24.6 | 28.1 | 28.2 |
| PM | PM10 | 67.4 | 5.8 | 30.2 | 33.6 | 36.1 | 36.2 | 30.3 | 32.5 | 34.2 | 33.2 | 24.7 |
SP—species: DP—Darevskia praticola, PM—Podarcis muralis; SVL—snout to vent length (mm); W0—initial weight (g); TP9–TP17—preferred body temperatures (°C) from 09:00 to 17:00 h.

Table A2.
Daily variation of the instantaneous water loss (EWLi) of Darevskia praticola and Podarcis muralis.
Table A2.
Daily variation of the instantaneous water loss (EWLi) of Darevskia praticola and Podarcis muralis.
| SP | CODE | SVL | W0 | EWLi 9–10 | EWLi 10–11 | EWLi 11–12 | EWLi 12–13 | EWLi 13–14 | EWLi 14–15 | EWLi 15–16 | EWLi 16–17 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DP | DP1 | 47.7 | 2.0 | 0.41 | 0.51 | 0.20 | 0.20 | 0.10 | 0.51 | 0.46 | 0.05 |
| DP | DP2 | 49.7 | 2.6 | 0.55 | 0.19 | 0.16 | 0.35 | 0.51 | 0.35 | 0.19 | 0.12 |
| DP | DP3 | 49.8 | 2.4 | 0.58 | 0.41 | 0.33 | 0.37 | 0.08 | 0.41 | 0.41 | 0.25 |
| DP | DP4 | 47.2 | 2.3 | 0.47 | 0.34 | 0.34 | 0.21 | 0.17 | 0.26 | 0.26 | 0.30 |
| DP | DP5 | 47.0 | 2.0 | 0.46 | 0.61 | 0.71 | 0.25 | 0.10 | 0.76 | 0.51 | 0.46 |
| DP | DP6 | 48.2 | 2.6 | 0.62 | 0.27 | 0.27 | 0.12 | 0.16 | 0.39 | 0.23 | 0.12 |
| DP | DP7 | 47.2 | 2.2 | 0.73 | 0.14 | 0.60 | 0.60 | 0.32 | 0.69 | 0.23 | 0.09 |
| DP | DP8 | 47.5 | 2.4 | 0.86 | 0.33 | 0.16 | 0.41 | 0.29 | 0.70 | 0.20 | 0.20 |
| DP | DP9 | 52.0 | 2.6 | 0.27 | 0.30 | 0.08 | 0.38 | 0.42 | 0.15 | 0.23 | 0.08 |
| DP | DP10 | 50.2 | 2.4 | 0.86 | 0.41 | 0.08 | 0.41 | 0.33 | 0.45 | 0.41 | 0.25 |
| PM | PM1 | 60.3 | 5.0 | 0.60 | 0.26 | 0.04 | 0.20 | 0.40 | 0.10 | 0.36 | 0.06 |
| PM | PM2 | 57.8 | 4.0 | 0.43 | 0.55 | 0.20 | 0.45 | 0.48 | 0.27 | 0.40 | 0.30 |
| PM | PM3 | 61.6 | 5.3 | 0.32 | 0.40 | 0.38 | 0.38 | 0.09 | 0.30 | 0.27 | 0.30 |
| PM | PM4 | 59.8 | 5.2 | 0.27 | 0.41 | 0.21 | 0.99 | 0.58 | 0.50 | 0.47 | 0.31 |
| PM | PM5 | 64.1 | 5.2 | 0.23 | 0.33 | 0.25 | 0.33 | 0.25 | 0.23 | 0.06 | 0.15 |
| PM | PM6 | 60.5 | 5.1 | 0.39 | 0.43 | 0.28 | 1.10 | 0.51 | 0.57 | 0.43 | 0.10 |
| PM | PM7 | 62.2 | 5.1 | 0.51 | 0.35 | 0.06 | 0.55 | 1.14 | 0.27 | 0.22 | 0.18 |
| PM | PM8 | 61.3 | 5.1 | 0.59 | 0.41 | 0.06 | 0.33 | 0.63 | 0.04 | 0.12 | 0.33 |
| PM | PM9 | 58.0 | 3.9 | 0.56 | 0.38 | 0.08 | 0.15 | 0.72 | 0.05 | 0.10 | 0.05 |
| PM | PM10 | 67.4 | 5.8 | 0.33 | 0.21 | 0.16 | 0.45 | 0.38 | 0.03 | 0.12 | 0.21 |
SP—species: DP—Darevskia praticola, PM—Podarcis muralis; SVL—snout to vent length (mm); W0—initial weight (g); EWLi—instantaneous water loss between time intervals from 09:00 to 17:00 h.

Table A3.
Daily variation of the accumulated water loss (EWLa) of Darevskia praticola and Podarcis muralis.
Table A3.
Daily variation of the accumulated water loss (EWLa) of Darevskia praticola and Podarcis muralis.
| SP | CODE | SVL | W0 | EWLa 10 | EWLa 11 | EWLa 12 | EWLa 13 | EWLa 14 | EWLa 15 | EWLa 16 | EWLa 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DP | DP1 | 47.7 | 2.0 | 0.4 | 0.9 | 1.1 | 1.3 | 1.4 | 1.9 | 2.4 | 2.4 |
| DP | DP2 | 49.7 | 2.6 | 0.5 | 0.7 | 0.9 | 1.2 | 1.8 | 2.1 | 2.3 | 2.4 |
| DP | DP3 | 49.8 | 2.4 | 0.6 | 1.0 | 1.3 | 1.7 | 1.8 | 2.1 | 2.6 | 2.8 |
| DP | DP4 | 47.2 | 2.3 | 0.5 | 0.8 | 1.2 | 1.4 | 1.5 | 1.8 | 2.1 | 2.4 |
| DP | DP5 | 47.0 | 2.0 | 0.5 | 1.1 | 1.8 | 2.0 | 2.1 | 2.9 | 3.4 | 3.9 |
| DP | DP6 | 48.2 | 2.6 | 0.6 | 0.9 | 1.2 | 1.3 | 1.4 | 1.8 | 2.1 | 2.2 |
| DP | DP7 | 47.2 | 2.2 | 0.7 | 0.9 | 1.5 | 2.1 | 2.4 | 3.1 | 3.3 | 3.4 |
| DP | DP8 | 47.5 | 2.4 | 0.9 | 1.2 | 1.4 | 1.8 | 2.0 | 2.7 | 2.9 | 3.2 |
| DP | DP9 | 52.0 | 2.6 | 0.3 | 0.6 | 0.6 | 1.0 | 1.4 | 1.6 | 1.8 | 1.9 |
| DP | DP10 | 50.2 | 2.4 | 0.9 | 1.3 | 1.4 | 1.8 | 2.1 | 2.5 | 2.9 | 3.2 |
| PM | PM1 | 60.3 | 5.0 | 0.6 | 0.9 | 0.9 | 1.1 | 1.5 | 1.6 | 2.0 | 2.0 |
| PM | PM2 | 57.8 | 4.0 | 0.4 | 1.0 | 1.2 | 1.6 | 2.1 | 2.4 | 2.8 | 3.1 |
| PM | PM3 | 61.6 | 5.3 | 0.3 | 0.7 | 1.1 | 1.5 | 1.6 | 1.9 | 2.1 | 2.4 |
| PM | PM4 | 59.8 | 5.2 | 0.3 | 0.7 | 0.9 | 1.9 | 2.5 | 3.0 | 3.4 | 3.7 |
| PM | PM5 | 64.1 | 5.2 | 0.2 | 0.6 | 0.8 | 1.1 | 1.4 | 1.6 | 1.7 | 1.8 |
| PM | PM6 | 60.5 | 5.1 | 0.4 | 0.8 | 1.1 | 2.2 | 2.7 | 3.3 | 3.7 | 3.8 |
| PM | PM7 | 62.2 | 5.1 | 0.5 | 0.9 | 0.9 | 1.5 | 2.6 | 2.9 | 3.1 | 3.3 |
| PM | PM8 | 61.3 | 5.1 | 0.6 | 1.0 | 1.1 | 1.4 | 2.0 | 2.1 | 2.2 | 2.5 |
| PM | PM9 | 58.0 | 3.9 | 0.6 | 0.9 | 1.0 | 1.2 | 1.9 | 1.9 | 2.0 | 2.1 |
| PM | PM10 | 67.4 | 5.8 | 0.3 | 0.5 | 0.7 | 1.1 | 1.5 | 1.6 | 1.7 | 1.9 |
SP—species: DP—Darevskia praticola, PM—Podarcis muralis; SVL—snout to vent length (mm); W0—initial weight (g); EWLa—accumulated water loss from 10:00 to 17:00 h.
References
- Huey, R.B. Temperature, physiology, and the ecology of reptiles. In Biology of the Reptilia, Physiology C, Physiological Ecology; Gans, C., Pough, H.F., Eds.; Academic Press: London, UK, 1982; pp. 25–91. [Google Scholar]
- Adolph, S.C.; Porter, W.P. Temperature, activity and lizard life histories. Am. Nat. 1993, 142, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M.J., Jr. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Daltry, J.C.; Ross, T.; Thorpe, R.S.; Wüster, W. Evidence that humidity influences snake activity patterns: A field study of the Malayan pit viper Calloselasma rhodostoma. Ecography 1998, 21, 25–34. [Google Scholar] [CrossRef]
- Carneiro, D.; García-Muñoz, E.; Žagar, A.; Pafilis, P.; Carretero, M.A. Is ecophysiology congruent with the present-day relictual distribution of a lizard group? Evidence from preferred temperatures and water loss rates. Herpetol. J. 2017, 27, 47–56. [Google Scholar]
- Kearney, M.R.; Munns, S.L.; Moore, D.; Malishev, M.; Bull, C.M. Field tests of a general ectotherm niche model show how water can limit lizard activity and distribution. Ecol. Monogr. 2018, 88, 672–693. [Google Scholar] [CrossRef]
- Garcia-Porta, J.; Irisarri, I.; Kirchner, M.; Rodríguez, A.; Kirchhof, S.; Brown, J.L.; MacLeod, A.; Turner, A.P.; Ahmadzadeh, F.; Albaladejo, G.; et al. Environmental temperatures shape thermal physiology as well as diversification and genome-wide substitution rates in lizards. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Díaz, J.A.; Cabezas-Díaz, S. Seasonal variation in the contribution of different behavioural mechanisms to lizard thermoregulation. Funct. Ecol. 2004, 18, 867–875. [Google Scholar] [CrossRef]
- Ortega, Z.; Pérez-Mellado, V. Seasonal patterns of body temperature and microhabitat selection in a lacertid lizard. Acta Oecol. 2016, 77, 201–206. [Google Scholar] [CrossRef]
- Black, I.R.G.; Berman, J.M.; Cadena, V.; Tattersall, G.J. Behavioral thermoregulation in lizards: Strategies for achieving preferred temperature. In Behavior of Lizards: Evolutionary and Mechanistic Perspectives; Bels, V.L., Russell, A.P., Eds.; CRC Press: London, UK, 2019; pp. 13–46. [Google Scholar]
- Mautz, W.J. Patterns of evaporative water loss. In Biology of the Reptilia, Physiology C, Physiological Ecology; Gans, C., Pough, H.F., Eds.; Academic Press: London, UK, 1982; Volume 12, pp. 443–481. [Google Scholar]
- Pirtle, E.I.; Tracy, C.R.; Kearney, M.R. Hydroregulation: A neglected behavioral response of lizards to climate change. In Behavior of Lizards: Evolutionary and Mechanistic Perspectives; Bels, V.L., Russell, A.P., Eds.; CRC Press: London, UK, 2019; pp. 343–374. [Google Scholar]
- Dupoué, A.; Stahlschmidt, Z.R.; Michaud, B.; Lourdais, O. Physiological state influences evaporative water loss and microclimate preference in the snake Vipera aspis. Physiol. Behav. 2015, 144, 82–89. [Google Scholar] [CrossRef]
- Rozen-Rechels, D.; Dupoué, A.; Lourdais, O.; Chamaillé-Jammes, S.; Meylan, S.; Clobert, J.; Le Galliard, J.F. When water interacts with temperature: Ecological and evolutionary implications of thermo-hydroregulation in terrestrial ectotherms. Ecol. Evol. 2019, 9, 10029–10043. [Google Scholar] [CrossRef]
- Sannolo, M.; Carretero, M.A. Dehydration constrains thermoregulation and space use in lizards. PLoS ONE 2019, 14, 1–17. [Google Scholar] [CrossRef]
- Hertz, P.E.; Huey, R.B.; Stevenson, R.D. Evaluating temperature regulation by field-active ectotherms: The fallacy of the inappropriate question. Am. Nat. 1993, 142, 796–818. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, D.; Garland, T.J.; Castilla, A.M.; Van Damme, R. Evolution of sprint speed in lacertid lizards: Morphological, physiological and behavioral covariation. Evolution 1995, 49, 848–863. [Google Scholar] [PubMed]
- Castilla, A.M.; Van Damme, R.; Bauwens, D. Field body temperatures, mechanisms of thermoregulation and evolution of thermal characteristics in lacertid lizards. Nat. Croat. 1999, 8, 253–274. [Google Scholar]
- Gvoždík, L.; Castilla, A.M. A comparative study of preferred body temperatures and critical thermal tolerance limits among populations of Zootoca vivipara (Squamata: Lacertidae) along an altitudinal gradient. J. Herpetol. 2001, 35, 486–492. [Google Scholar] [CrossRef]
- Carretero, M.A.; Roig, J.M.; Llorente, G.A. Variation in preferred body temperature in an oviparous population of Lacerta (Zootoca) vivipara. Herpetol. J. 2005, 15, 51–55. [Google Scholar]
- Díaz, J.A.; Iraeta, P.; Monasterio, C. Seasonality provokes a shift of thermal preferences in a temperate lizard, but altitude does not. J. Therm. Biol. 2006, 31, 237–242. [Google Scholar] [CrossRef]
- Clusella-Trullas, S.; Chown, S.L. Lizard thermal trait variation at multiple scales: A review. J. Comp. Physiol. B 2014, 184, 5–21. [Google Scholar] [CrossRef]
- Le Galliard, J.F.; Rozen-Rechels, D.; Lecomte, A.; Demay, C.; Dupoue, A.; Meylan, S. Short-term changes in air humidity and water availability weakly constrain thermoregulation in a dry-skinned ectotherm. PLoS ONE 2021, 16, 1–17. [Google Scholar] [CrossRef]
- Osojnik, N.; Žagar, A.; Carretero, M.A.; García-Muñoz, E.; Vrezec, A. Ecophysiological dissimilarities of two sympatric lizards. Herpetologica 2013, 69, 445–454. [Google Scholar] [CrossRef]
- Ferreira, C.C.; Santos, X.; Carretero, M.A. Does ecophysiology mediate reptile responses to fire regimes? Evidence from Iberian lizards. PeerJ 2016, 4, e2107. [Google Scholar] [CrossRef]
- Sannolo, M.; Civantos, E.; Martín, J.; Carretero, M.A. Variation in field body temperature and total evaporative water loss along an environmental gradient in a diurnal ectotherm. J. Zool. 2020, 310, 221–231. [Google Scholar] [CrossRef]
- S’khifa, A.; Koziel, G.; Vences, M.; Carretero, M.A.; Slimani, T. Ecophysiology of a lacertid community in the high Moroccan mountains suggests conservation guidelines. J. Therm. Biol. 2020, 94, 102743. [Google Scholar] [CrossRef] [PubMed]
- Sinervo, B.; Méndez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Cruz, M.V.S.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Huey, R.B.; Kearney, M.R.; Krockenberger, A.; Holtum, J.A.M.; Jess, M.; Williams, S.E. Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philos. Trans. R. Soc. B 2012, 367, 1665–1679. [Google Scholar] [CrossRef]
- Biber, M.F.; Voskamp, A.; Hof, C. Potential effects of future climate change on global reptile distributions and diversity. Glob. Ecol. Biogeogr. 2023, 32, 519–534. [Google Scholar] [CrossRef]
- Plasman, M.; Gonzalez-Voyer, A.; Bautista, A.; Díaz D La Vega-Pérez, A.H. Flexibility in thermal requirements: A comparative analysis of the wide-spread lizard genus Sceloporus. Integr. Zool. 2024, 1–17. [Google Scholar] [CrossRef]
- Obregón, R.L.; Scolaro, J.A.; Ibargüengoytía, N.R.; Medina, M. Thermal biology and locomotor performance in Phymaturus calcogaster: Are Patagonian lizards vulnerable to climate change? Integr. Zool. 2021, 16, 53–66. [Google Scholar] [CrossRef]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Le Galliard, J.-F.; Massot, M.; Baron, J.-P.; Clobert, J. Ecological effects of climate change on European reptiles. In Wildlife Conservtion in a Changing Climate; Brodie, J.F., Post, E.S., Doak, D.F., Eds.; University of Chicago Press: Chicago, IL, USA, 2012; pp. 179–203. [Google Scholar]
- Kearney, M.R.; Simpson, S.J.; Raubenheimer, D.; Kooijman, S.A.L.M. Balancing heat, water and nutrients under environmental change: A thermodynamic niche framework. Funct. Ecol. 2013, 27, 950–966. [Google Scholar] [CrossRef]
- Urban, M.C.; Bocedi, G.; Hendry, A.P.; Mihoub, J.-B.; Pe’er, G.; Singer, A.; Bridle, J.R.; Crozier, L.G.; De Meester, L.; Godsoe, W.; et al. Improving the forecast for biodiversity under climate change. Science 2016, 353, aad8466. [Google Scholar] [CrossRef]
- Huang, S.P.; Kearley, R.E.; Hung, K.W.; Porter, W.P. Evaporative water loss simulation improves models’ prediction of habitat suitability for a high-elevation forest skink. Oecologia 2020, 192, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.O.; White, C.R.; Chapple, D.G.; Kearney, M.R. A Hierarchical approach to understanding physiological associations with climate. Glob. Ecol. Biogeogr. 2022, 31, 332–346. [Google Scholar] [CrossRef]
- Bridle, J.R.; Vines, T.H. Limits to evolution at range margins: When and why does adaptation fail? Trends. Ecol. Evol. 2007, 22, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Sexton, J.P.; McIntyre, P.J.; Angert, A.L.; Rice, K.J. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 415–436. [Google Scholar] [CrossRef]
- Ćorović, J.; Crnobrnja-Isailović, J. Aspects of thermal ecology of the meadow lizard (Darevskia praticola). Amphibia-Reptilia 2018, 39, 229–238. [Google Scholar] [CrossRef]
- Galoyan, E.; Bolshakova, A.; Abrahamyan, M.; Petrosyan, R.; Komarova, V.; Spangenberg, V.; Arakelyan, M. Natural history of Valentin’s rock lizard (Darevskia valentini) in Armenia. Zool. Res. 2019, 40, 277–292. [Google Scholar] [CrossRef]
- Nikolaev, O.D.; Belova, D.A.; Novikov, B.A.; Simis, I.B.; Petrosyan, R.K.; Arakelyan, M.S.; Komarova, V.A.; Galoyan, E.A. Peculiarities of thermal biology in two parthenogenetic rock lizard species, Darevskia armeniaca and Darevskia unisexualis, and one bisexual species, Darevskia valentini (Lacertidae, Squamata). Biol. Bull. 2022, 49, 1037–1045. [Google Scholar] [CrossRef]
- Grbac, I.; Bauwens, D. Constraints on temperature regulation in two sympatric Podarcis lizards during autumn. Copeia 2001, 2001, 178–186. [Google Scholar] [CrossRef]
- Carretero, M.A. Measuring body temperatures in small lacertids: Infrared vs. contact thermometers. Basic Appl. Herpetol. 2012, 26, 99–105. [Google Scholar] [CrossRef]
- Žagar, A.; Carretero, M.A.; Osojnik, N.; Sillero, N.; Vrezec, A. A place in the sun: Interspecific interference affects thermoregulation in coexisting lizards. Behav. Ecol. Sociobiol. 2015, 69, 1127–1137. [Google Scholar] [CrossRef]
- Sagonas, K.; Kapsalas, G.; Valakos, E.; Pafilis, P. Living in sympatry: The effect of habitat partitioning on the thermoregulation of three Mediterranean lizards. J. Therm. Biol. 2017, 65, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Trochet, A.; Dupoué, A.; Souchet, J.; Bertrand, R.; Deluen, M.; Murarasu, S.; Calvez, O.; Martinez-Silvestre, A.; Verdaguer-Foz, I.; Darnet, E.; et al. Variation of preferred body temperatures along an altitudinal gradient: A multi-species study. J. Therm. Biol. 2018, 77, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, D.; García-Muñoz, E.; Kaliontzopoulou, A.; Llorente, G.A.; Carretero, M.A. Comparing ecophysiological traits in two Podarcis wall lizards with overlapping ranges. Salamandra 2015, 51, 335–344. [Google Scholar]
- Böhme, W.; Pérez-Mellado, V.; Cheylan, M.; Nettmann, H.K.; Krecsák, L.; Sterijovski, B.; Schmidt, B.; Lymberakis, P.; Podloucky, R.; Sindaco, R.; et al. Podarcis muralis. The IUCN Red List of Threatened Species 2009: E.T61550A12514105. Available online: https://www.iucnredlist.org/species/61550/12514105 (accessed on 11 November 2024).
- Sillero, N.; Campos, J.; Bonardi, A.; Corti, C.; Creemers, R.; Crochet, P.-A.; Crnobrnja Isailović, J.; Denoël, M.; Ficetola, G.F.; Gonçalves, J.; et al. Updated distribution and biogeography of amphibians and reptiles of Europe. Amphibia-Reptilia 2014, 35, 1–31. [Google Scholar] [CrossRef]
- Agasyan, A.; Avci, A.; Tuniyev, B.; Crnobrnja-Isailović, J.; Lymberakis, P.; Andrén, C.; Cogalniceanu, D.; Wilkinson, J.; Ananjeva, N.; Üzüm, N.; et al. Darevskia praticola. The IUCN Red List of Threatened Species 2009: E.T157245A5058913. Available online: https://www.iucnredlist.org/species/157245/5058913 (accessed on 11 November 2024).
- Ćorović, J.; Popović, M.; Cogălniceanu, D.; Carretero, M.A.; Crnobrnja-Isailović, J. Distribution of the meadow lizard in Europe and its realized ecological niche model. J. Nat. Hist. 2018, 52, 1909–1925. [Google Scholar] [CrossRef]
- Arnold, E.N. Field Guide of Reptiles and Amphibians of Britain and Europe, 2nd ed.; Collins: London, UK, 2004; pp. 145–147. [Google Scholar]
- Speybroeck, J.; Beukema, W.; Bok, B.; Van Der Voort, J. Field Guide to the Amphibians and Reptiles of Britain and Europe; Bloomsbury Publishing: London, UK, 2016; pp. 265–268. [Google Scholar]
- Strijbosch, H.; Helmer, W.; Scholte, P.T. Distribution and ecology of lizards in the Greek province of Evros. Amphibia-Reptilia 1989, 10, 151–174. [Google Scholar] [CrossRef]
- Gherghel, I.; Strugariu, A.; Sahlean, T.; Stefanescu, A. New Romanian distribution record for Darevskia praticola pontica (LANTZ & CYRÉN, 1919) at its north-western range limit. Herpetozoa 2011, 23, 91–93. [Google Scholar]
- Stojanov, A.J.; Tzankov, N.; Naumov, B.; Nöllert, A. Die Amphibien Und Reptilien Bulgariens; Edition Chimaira: Frankfurt, 2011. [Google Scholar]
- Gaceu, O.; Josan, I. Note on the occurrence of Darevskia pontica (Reptilia) north of the Mureş river, in Metaliferi mountains, Western Romania. North. West. J. Zool. 2013, 9, 450–452. [Google Scholar]
- Covaciu-Marcov, S.-D.; Cicort-Lucaciu, A.-Ş.; Gaceu, O.; Sas, I.; Ferenţi, S.; Bogdan, H.V. The herpetofauna of the south-western part of Mehedinţi county, Romania. North West J. Zool. 2009, 5, 142–164. [Google Scholar]
- Arnold, E.N. Resource partition among lacertid lizards in southern Europe. J. Zool. 1987, 1, 739–782. [Google Scholar] [CrossRef]
- Darevsky, I.S. Lacerta praticola Eversmann, 1834. In Atlas of Amphibians and Reptiles in Europe; Gasc, J.P., Ed.; Museum National D’Histoire Naturelle: Paris, France, 1997; pp. 254–255. [Google Scholar]
- Veríssimo, C.V.; Carretero, M.A. Preferred temperatures of Podarcis vaucheri from Morocco: Intraspecific variation and interspecific comparisons. Amphibia-Reptilia 2009, 30, 17–23. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 12. Available online: www.statsoft.com (accessed on 25 September 2023).
- Kapsalas, G.; Gavriilidi, I.; Adamopoulou, C.; Foufopoulos, J.; Pafilis, P. Effective thermoregulation in a newly established population of Podarcis siculus in Greece: A possible advantage for a successful invader. Acta. Herpetol. 2016, 11, 111–118. [Google Scholar] [CrossRef]
- Ćorović, J. (Institute for Biological Research “Siniša Stanković” National Institute of the Republic of Serbia, University of Belgrade, Belgrade, Serbia); Crnobrnja-Isailović, J. (Institute for Biological Research “Siniša Stanković” National Institute of the Republic of Serbia, University of Belgrade, Belgrade, Serbia). Personal communication, 2018.
- Dmiel, R. Skin resistance to evaporative water loss in reptiles: A physiological adaptive mechanism to environmental stress or a phyletically dictated trait? Isr. J. Zool. 2001, 47, 56–67. [Google Scholar] [CrossRef]
- Mathews, C.G.; Amlaner, C.J. Eye states and postures of the western fence lizard (Sceloporus occidentalis), with special reference to asynchronous eye closure and behavioral sleep. J. Herpetol. 2000, 34, 472–475. [Google Scholar] [CrossRef]
- Žagar, A.; Carretero, M.A.; de Groot, M. Time changes everything: A multispecies analyses of temporal patterns in evaporative water loss. Oecologia 2022, 198, 905–915. [Google Scholar] [CrossRef]
- Gates, D.M. Introduction. In Biophysical Ecology, 1st ed.; Reichle, D.E., Ed.; Springer: New York, NY, USA, 1980; pp. 1–12. [Google Scholar]
- Ortega, Z.; Mencía, A.; Pérez-Mellado, V. Wind constraints on the thermoregulation of high mountain lizards. Int. J. Biometeorol. 2017, 61, 565–573. [Google Scholar] [CrossRef]
- Bodineau, T.; Chabaud, C.; Decencière, B.; Agostini, S.; Lourdais, O.; Meylan, S.; Le Galliard, J.F. Microhabitat humidity rather than food availability drives thermo-hydroregulation responses to drought in a lizard. Oikos 2024, 2024, e10535. [Google Scholar] [CrossRef]
- Renaud, V.; Innes, J.L.; Dobbertin, M.; Rebetez, M. Comparison between open-site and below canopy climatic conditions in Switzerland for different types of forests over 10 years (1998–2007). Theor. Appl. Climatol. 2011, 105, 119–127. [Google Scholar] [CrossRef]
- Gaudio, N.; Gendre, X.; Saudreau, M.; Seigner, V.; Balandier, P. Impact of tree canopy on thermal and radiative microclimates in a mixed temperate forest: A new statistical method to analyse hourly temporal dynamics. Agric. For. Meteoro. 2017, 237, 71–79. [Google Scholar] [CrossRef]
- Maiorano, L.; Amori, G.; Capula, M.; Falcucci, A.; Masi, M.; Montemaggiori, A.; Pottier, J.; Psomas, A.; Rondinini, C.; Russo, D.; et al. Threats from climate change to terrestrial vertebrate hotspots in Europe. PLoS ONE 2013, 8, e74989. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).