GnRH Immunocastration in Male Xizang Sheep: Impacts on Rumen Microbiome and Metabolite Profiles for Enhanced Health and Productivity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. GnRH Vaccine Preparation
2.3. Experimental Design and Animal Sample Collection
2.4. Live Weight and Serum Testosterone Determination in Sheep
2.5. Rumen Fluid Sample Processing and Metabolite Analysis
2.6. Microbiome Sample Processing and Sequencing
2.7. Sequencing Data Processing and Analysis
2.8. Data Analysis
2.9. Relevance Analysis
3. Results
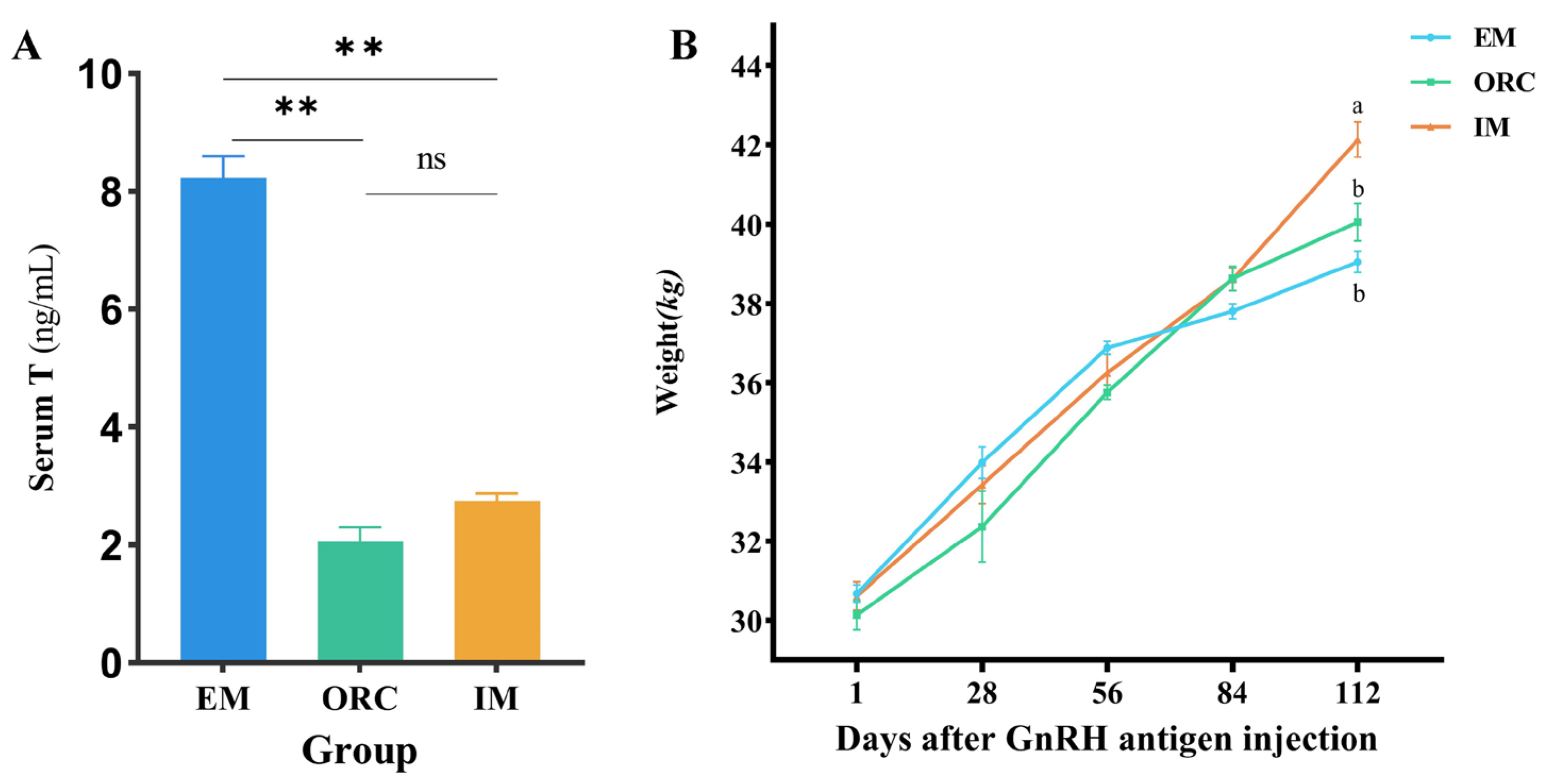
3.1. Effect of Different Castration Methods on Serum Testosterone and Body Weight in Male Xizang Sheep
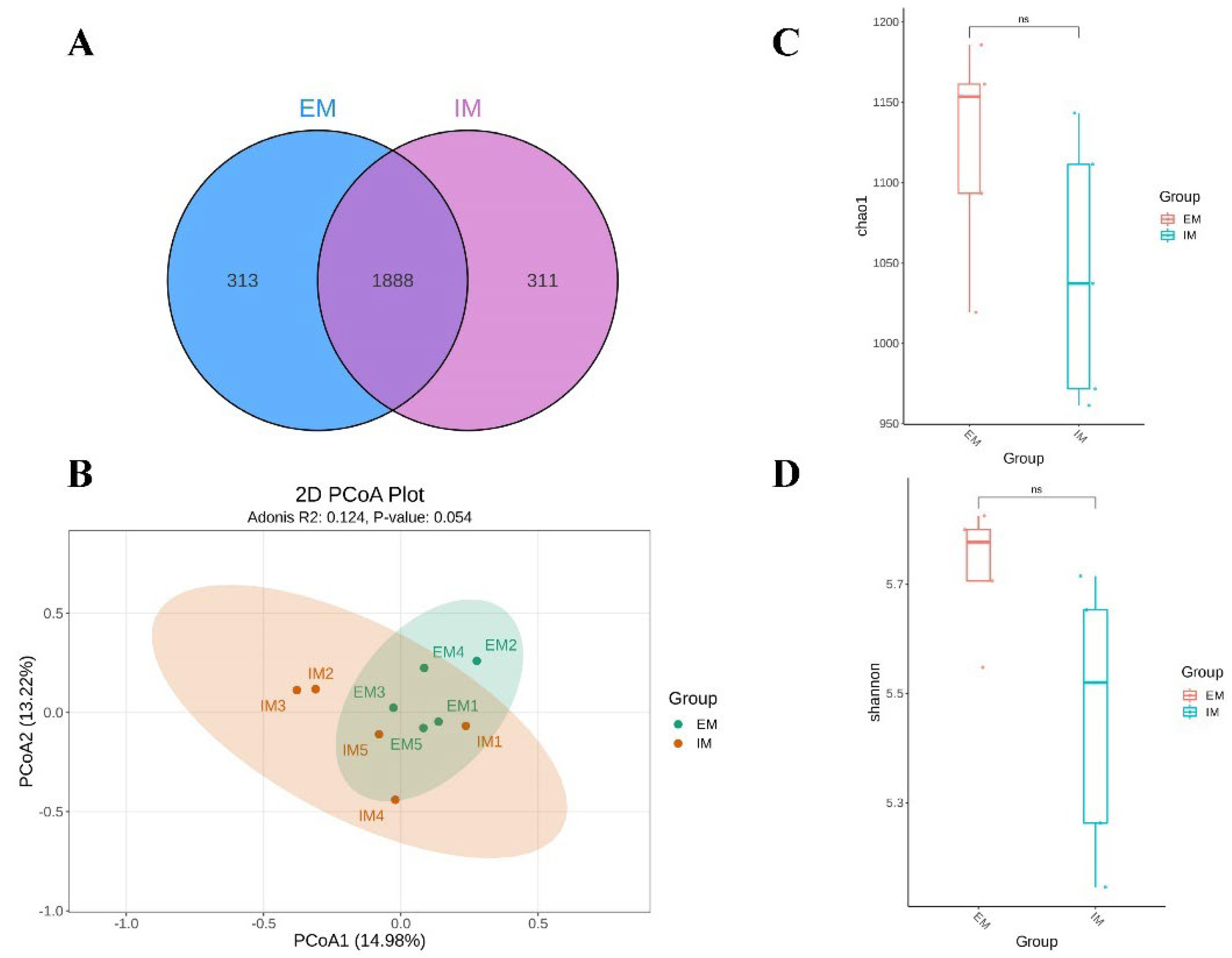
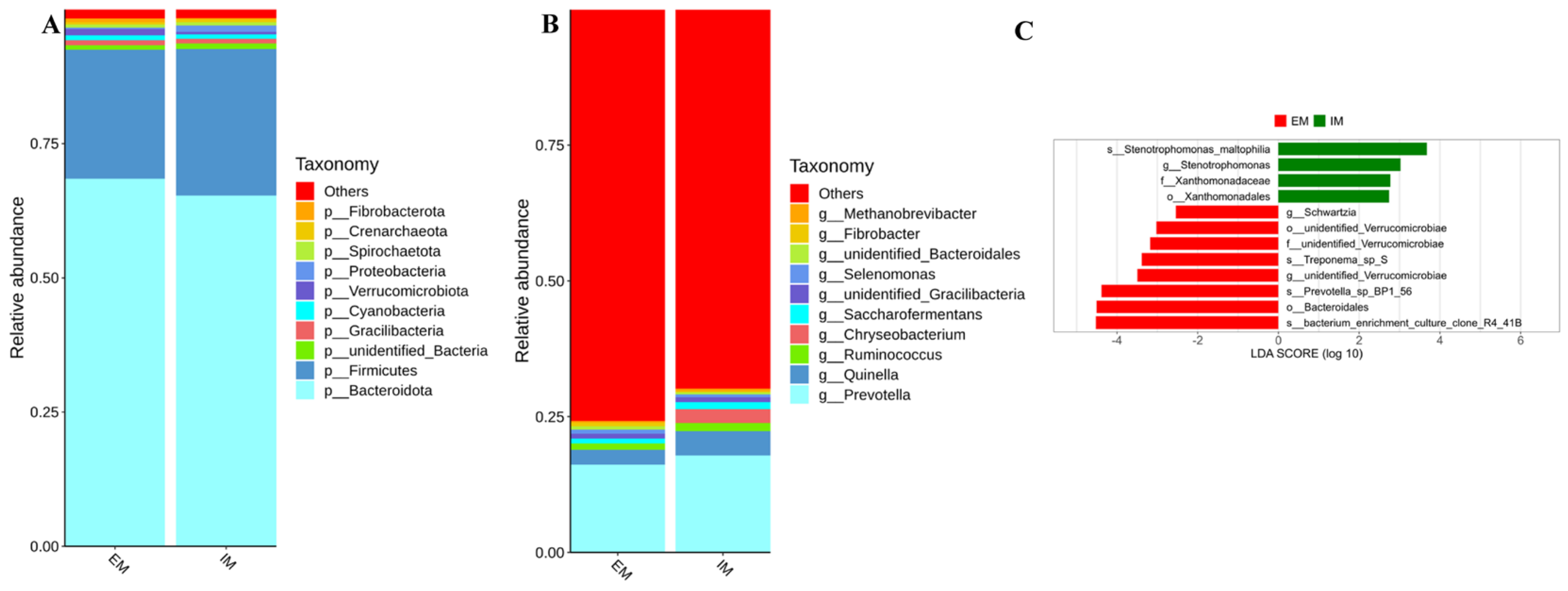
3.2. Effects of GnRH Immunization on Rumen Microorganisms in Male Xizang Sheep
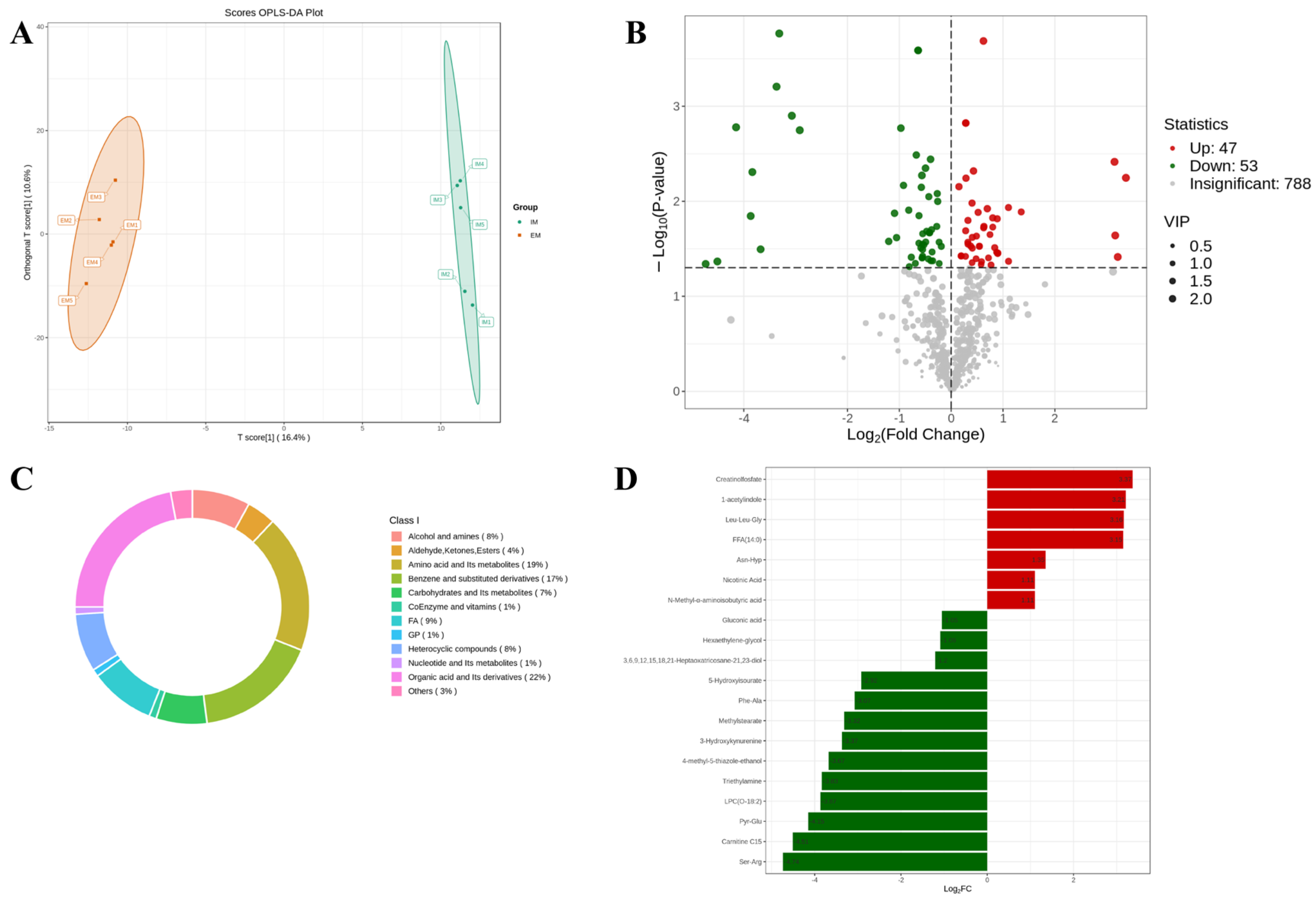
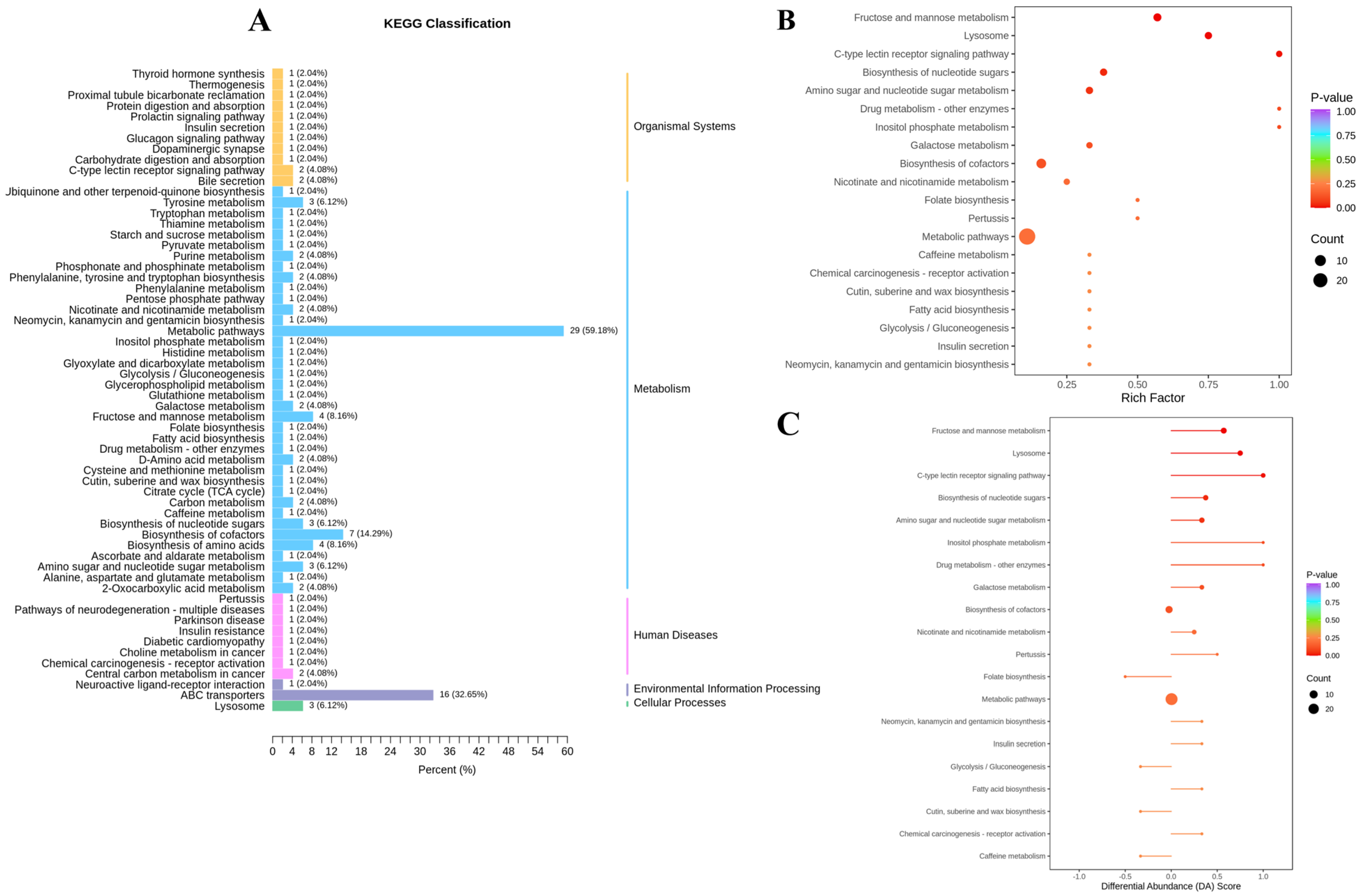
3.3. Effect of GnRH Immunocastration on Rumen Metabolomics in Male Xizang Sheep
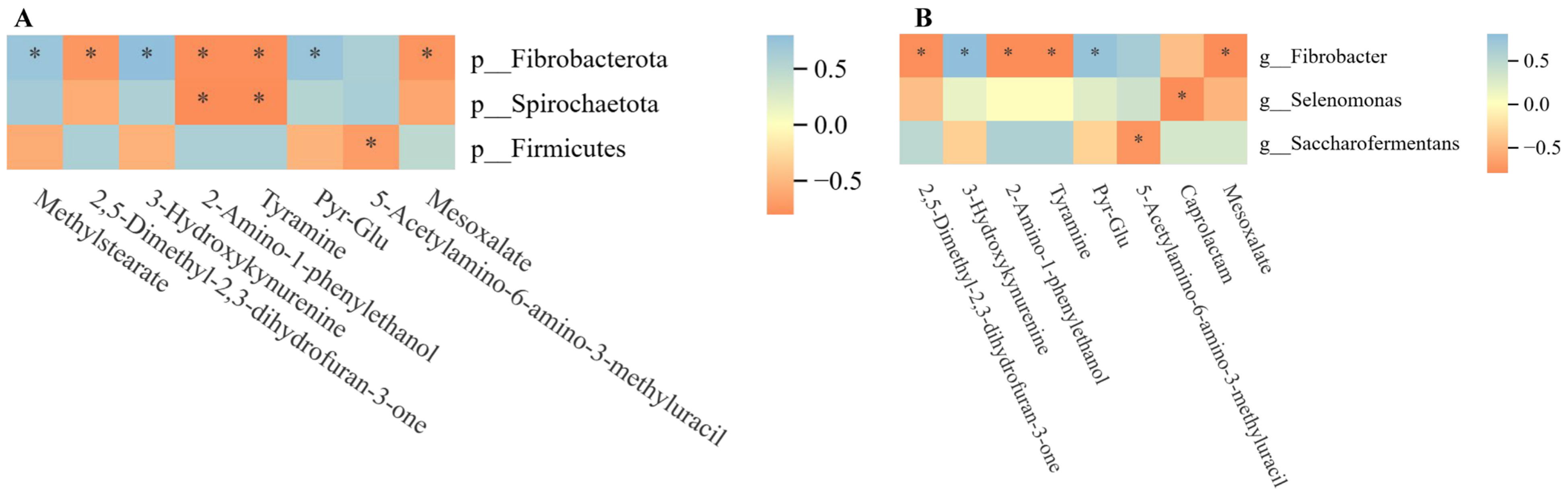
3.4. Metabolome–Microbiome Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, Y.; Li, S.; Zhao, F.; Wang, J.; Liu, X.; Hu, J.; Bao, G.; Luo, Y. Changes in the Mitochondrial Dynamics and Functions Together with the mRNA/miRNA Network in the Heart Tissue Contribute to Hypoxia Adaptation in Tibetan Sheep. Animals 2022, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Munday, J.S.; Perrott, M.; Wang, G.; Liu, X. Association of Age with the Expression of Hypoxia-Inducible Factors HIF-1α, HIF-2α, HIF-3α and VEGF in Lung and Heart of Tibetan Sheep. Animals 2019, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Prestel, L.; Joerling, J.; Failing, K.; Wagner, H.; Wehrend, A. Suppression of reproductive function in juvenile rams by a slow-release gonadotropin-releasing hormone implant. Open Vet. J. 2022, 12, 171–181. [Google Scholar] [CrossRef]
- Ahmed, S.; Jiang, X.; Liu, G.; Sadiq, A.; Farooq, U.; Wassie, T.; Saleem, A.H.; Zubair, M. New trends in immunocastration and its potential to improve animal welfare: A mini review. Tropocal Anim. Health Prod. 2022, 54, 369. [Google Scholar] [CrossRef]
- Morgan, L.; Itin-Shwartz, B.; Koren, L.; Meyer, J.S.; Matas, D.; Younis, A.; Novak, S.; Weizmann, N.; Rapaic, O.; Abu Ahmad, W.; et al. Physiological and economic benefits of abandoning invasive surgical procedures and enhancing animal welfare in swine production. Sci. Rep. 2019, 9, 16093. [Google Scholar] [CrossRef] [PubMed]
- Batorek, N.; Čandek-Potokar, M.; Bonneau, M.; Van Milgen, J. Meta-analysis of the effect of immunocastration on production performance, reproductive organs and boar taint compounds in pigs. Animal 2012, 6, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yao, H.; Han, X.; Cao, X.; Du, X.; Meng, F.; Bu, G.; Kong, F.; Song, T.; Zeng, X. Active immunization against gonadotropin-releasing hormone affects thymic T cell production, migration, and colonization in male rat lymphoid tissue. J. Reprod. Immunol. 2023, 159, 104132. [Google Scholar] [CrossRef]
- Goto, A.; Yoshida, N.; Nakada, K.; Inoue, Y.; Hisaeda, K.; Inaba, T.; Domoto, N.; Ishiguro, Y.; Itoh, M.; Takahashi, E.; et al. Efficiency of immunocastration with an anti-gonadotropin-releasing hormone vaccine on cryptorchid bulls. J. Vet. Med. Sci. 2023, 85, 551–556. [Google Scholar] [CrossRef]
- Broeke, A.V.D.; Aluwé, M.; Kress, K.; Stefanski, V.; Škrlep, M.; Batorek, N.; Ampe, B.; Millet, S. Effect of dietary energy level in finishing phase on performance, carcass and meat quality in immunocastrates and barrows in comparison with gilts and entire male pigs. Animal 2022, 16, 100437. [Google Scholar] [CrossRef]
- Sha, Y.; Hu, J.; Shi, B.; Dingkao, R.; Wang, J.; Li, S.; Zhang, W.; Luo, Y.; Liu, X. Characteristics and Functions of the Rumen Microbial Community of Cattle-Yak at Different Ages. Biomed Res. Int. 2020, 2020, 3482692. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, Y.; Qiao, H. Bacterial community and diversity in the rumen of 11 Mongolian cattle as revealed by 16S rRNA amplicon sequencing. Sci. Rep. 2024, 14, 1546. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Wu, S.; Zeng, X.; Zhao, H.; Wang, G.; Chen, M.; Qian, N. Different gut microbiome composition in obese Guizhou minipigs between female and castrated male. Folia Microbiol. 2019, 64, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shi, J.; Lei, Y.; Wu, J.; Zhang, R.; Zhang, X.; Jia, L.; Wang, Y.; Ma, Y.; He, P.; et al. Castration alters the cecal microbiota and inhibits growth in Holstein cattle. J. Anim. Sci. 2022, 100, skac367. [Google Scholar] [CrossRef]
- Oonk, H.B.; Turkstra, J.A.; Schaaper, W.M.; Erkens, J.H.; Schuitemaker-de Weerd, M.H.; van Nes, A.; Verheijden, J.H.; Meloen, R.H. New GnRH-like peptide construct to optimize efficient immunocastration of male pigs by immunoneutralization of GnRH. Vaccine 1998, 16, 1074–1082. [Google Scholar] [CrossRef]
- Han, X.-F.; Cao, X.-H.; Tang, J.; Du, X.-G.; Zeng, X.-Y. Active immunization against GnRH reduces the synthesis of GnRH in male rats. Theriogenology 2013, 80, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xu, J.; Liao, W.; Wen, Y.; Jiang, S.; Wen, J.; Zhao, C. Suppression of hepatocellular carcinoma by Ulva lactuca ulvan via gut microbiota and metabolite interactions. J. Adv. Res. 2023, 52, 103–117. [Google Scholar] [CrossRef]
- Pang, Z.; Mao, X.; Zhou, S.; Yu, S.; Liu, G.; Lu, C.; Wan, J.; Hu, L.; Xu, P. Microbiota-mediated nitrogen fixation and microhabitat homeostasis in aerial root-mucilage. Microbiome 2023, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Hines, M.; Brook, C.; Conway, G.S. Androgen and psychosexual development: Core gender identity, sexual orientation and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia (CAH). J. Sex Res. 2004, 41, 75–81. [Google Scholar] [CrossRef]
- Lamb, D.J.; Weigel, N.L.; Marcelli, M. Androgen receptors and their biology. Vitam. Horm. 2001, 62, 199–230. [Google Scholar]
- Navarro, G.; Allard, C.; Xu, W.; Mauvais-Jarvis, F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity 2015, 23, 713–719. [Google Scholar] [CrossRef]
- Harada, N.; Hanaoka, R.; Horiuchi, H.; Kitakaze, T.; Mitani, T.; Inui, H.; Yamaji, R. Castration influences intestinal microflora and induces abdominal obesity in high-fat diet-fed mice. Sci. Rep. 2016, 6, 23001. [Google Scholar] [CrossRef] [PubMed]
- Whon, T.W.; Kim, H.S.; Shin, N.R.; Jung, E.S.; Tak, E.J.; Sung, H.; Jung, M.J.; Jeong, Y.S.; Hyun, D.W.; Kim, P.S.; et al. Male castration increases adiposity via small intestinal microbial alterations. EMBO Rep. 2021, 22, e50663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Q.; Li, S.-S.; Hu, B.; Xu, L.-W.; Liu, J.-J.; Sun, Y.-J.; Bai, X. Effect of GnRH Active Immunisation on Reproductive Performance of Male Sprague Dawley Rats. Int. J. Mol. Sci. 2024, 25, 3193. [Google Scholar] [CrossRef] [PubMed]
- Amatayakul-Chantler, S.; Hoe, F.; Jackson, J.; Roca, R.; Stegner, J.; King, V.; Howard, R.; Lopez, E.; Walker, J. EEffects on performance and carcass and meat quality attributes following immunocastration with the gonadotropin releasing factor vaccine Bopriva or surgical castration of Bos indicus bulls raised on pasture in Brazil. Meat Sci. 2013, 95, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sales, J. Quantification of the effects of castration on carcass and meat quality of sheep by meta-analysis. Meat Sci. 2014, 98, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Chen, Y.F.; Yue, H.N.; He, Y.Q.; McNeilly, A.S. Sexual development and the effects of active immunization against GnRH in Chinese Tanyang ram lambs. Anim. Reprod. Sci. 2003, 77, 129–139. [Google Scholar] [CrossRef]
- Keum, G.B.; Pandey, S.; Kim, E.S.; Doo, H.; Kwak, J.; Ryu, S.; Choi, Y.; Kang, J.; Kim, S.; Kim, H.B. Understanding the Diversity and Roles of the Ruminal Microbiome. J. Microbiol. 2024, 62, 217–230. [Google Scholar] [CrossRef]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. GUT Microbes 2016, 7, 313–322. [Google Scholar] [CrossRef]
- Yang, W.; Sha, Y.; Chen, X.; Liu, X.; Wang, F.; Wang, J.; Shao, P.; Chen, Q.; Gao, M.; Huang, W. Effects of the Interaction between Rumen Microbiota Density-VFAs-Hepatic Gluconeogenesis on the Adaptability of Tibetan Sheep to Plateau. Int. J. Mol. Sci. 2024, 25, 6726. [Google Scholar] [CrossRef]
- van der Meijs, R.M.; van Leeuwen, W.; Prins, C.; Wittink, F.; Pirovano, W.; Duijsings, D.; Bogert, B.v.D.; Sonsbeek, L.G.B.-V. Gut microbiome diversity of three rhinoceros species in european zoos. J. Zoo Wildl. Med. 2024, 55, 301–312. [Google Scholar] [CrossRef]
- Han, X.; Zhou, Y.; Zeng, Y.; Sui, F.; Liu, Y.; Tan, Y.; Cao, X.; Du, X.; Meng, F.; Zeng, X. Effects of active immunization against GnRH versus surgical castration on hypothalamic-pituitary function in boars. Theriogenology 2017, 97, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Morgavi, D.P.; Rathahao-Paris, E.; Popova, M.; Boccard, J.; Nielsen, K.F.; Boudra, H. Rumen microbial communities influence metabolic phenotypes in lambs. Front. Microbiol. 2015, 6, 1060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Li, F.; Li, C.; Li, G.; Zhang, D.; Song, Q.; Li, X.; Zhao, Y.; Wang, W. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal 2021, 15, 100161. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Z.; Duan, X.; Wang, C.; Chen, Z.; Zhang, M.; Li, X.; Ma, Y. Rumen Development of Tianhua Mutton Sheep Was Better than That of Gansu Alpine Fine Wool Sheep under Grazing Conditions. Animals 2024, 14, 1259. [Google Scholar] [CrossRef] [PubMed]
- Cholewińska, P.; Wołoszyńska, M.; Michalak, M.; Czyż, K.; Rant, W.; Janczak, M. Evaluation of Changes in the Levels of Firmicutes and Bacteroidetes Phyla of Sheep Feces Depending on the Breed. Animal 2020, 10, 1901. [Google Scholar] [CrossRef]
- Wang, S.; Yao, B.; Gao, H.; Zang, J.; Tao, S.; Zhang, S.; Huang, S.; He, B.; Wang, J. Combined supplementation of Lactobacillus fermentum and Pediococcus acidilactici promoted growth performance, alleviated inflammation, and modulated intestinal microbiota in weaned pigs. BMC Vet. Res. 2019, 15, 239. [Google Scholar] [CrossRef]
- Chen, C.; Fang, S.; Wei, H.; He, M.; Fu, H.; Xiong, X.; Zhou, Y.; Wu, J.; Gao, J.; Yang, H.; et al. Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome 2021, 9, 175. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Cao, W.; Zhang, Z. Alterations of Gastric Microbiota in Gastric Cancer and Precancerous Stages. Front. Cell. Infect. Microbiol. 2021, 11, 559148. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Liu, Z.; Lv, H.; Cai, X.; Zhong, R.; Chen, L.; Zhang, H. Baicalin restore intestinal damage after early-life antibiotic therapy: The role of the MAPK signaling pathway. Pharmacol. Res. 2024, 204, 107194. [Google Scholar] [CrossRef]
- Wang, X.; Deng, T.; Zhou, X.; Chu, L.; Zeng, X.; Zhang, S.; Guan, W.; Chen, F. A Mixture of Formic Acid, Benzoic Acid, and Essential Oils Enhanced Growth Performance via Modulating Nutrient Uptake, Mitochondrion Metabolism, and Immunomodulation in Weaned Piglets. Antioxidants 2024, 13, 246. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Hou, S.; Raza, S.H.A.; Gui, L.; Sun, S.; Wang, Z.; Yang, B.; Yuan, Z.; Simal-Gandara, J.; et al. Metabolomics approach reveals high energy diet improves the quality and enhances the flavor of black Tibetan sheep meat by altering the composition of rumen microbiota. Front. Nutr. 2022, 9, 915558. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Altermann, E.; Leahy, S.C.; Jauregui, R.; Jonker, A.; Henderson, G.; Kittelmann, S.; Attwood, G.T.; Kamke, J.; Waters, S.M.; et al. Genomic insights into the physiology of Quinella, an iconic uncultured rumen bacterium. Nat. Commun. 2022, 13, 6240. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, Z.; Jia, L.; Ma, Y.; Huang, Y.; He, P.; Ran, T.; Liu, W.; Zhang, W.; Cheng, Q.; et al. Castration alters the ileum microbiota of Holstein bulls and promotes beef flavor compounds. BMC Genom. 2024, 25, 426. [Google Scholar] [CrossRef]
- Xue, F.; Pan, X.; Jiang, L.; Guo, Y.; Xiong, B. GC-MS analysis of the ruminal metabolome response to thiamine supplementation during high grain feeding in dairy cows. Metabolomics 2018, 14, 67. [Google Scholar] [CrossRef]
- Shen, Z.; Kuhla, S.; Zitnan, R.; Seyfert, H.-M.; Schneider, F.; Hagemeister, H.; Chudy, A.; Löhrke, B.; Blum, J.W.; Hammon, H.M.; et al. Intraruminal infusion of n-butyric acid induces an increase of ruminal papillae size independent of IGF-1 system in castrated bulls. Arch. Anim. Nutr. 2005, 59, 213–225. [Google Scholar] [CrossRef]
- Sutton, J.D. Digestion and absorption of energy substrates in the lactating cow. J. Dairy Sci. 1985, 68, 3376–3393. [Google Scholar] [CrossRef]
- Ma, Y.; Han, L.; Zhang, S.; Zhang, X.; Hou, S.; Gui, L.; Sun, S.; Yuan, Z.; Wang, Z.; Yang, B. Insight into the differences of meat quality between Qinghai white Tibetan sheep and black Tibetan sheep from the perspective of metabolomics and rumen microbiota. Food Chem. X 2023, 19, 100843. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Gui, L.; Raza, S.H.A.; Hou, S.; Yang, B.; Wang, Z.; Ma, Y.; Makhlof, R.T.M.; Alhuwaymil, Z.; et al. Metabolome and microbiome analysis revealed the effect mechanism of different feeding modes on the meat quality of Black Tibetan sheep. Front. Microbiol. 2022, 13, 1076675. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, C.; Elmhadi, M.; Zhang, H.; Han, Y.; Shen, B.; He, B.; Liu, X.; Wang, H. Thiamine ameliorates metabolic disorders induced by a long-term high-concentrate diet and promotes rumen epithelial development in goats. J. Dairy Sci. 2021, 104, 11522–11536. [Google Scholar] [CrossRef]
- Lee, S.; Zhang, C.; Kilicarslan, M.; Piening, B.D.; Bjornson, E.; Hallström, B.M.; Groen, A.K.; Ferrannini, E.; Laakso, M.; Snyder, M.; et al. Integrated Network Analysis Reveals an Association between Plasma Mannose Levels and Insulin Resistance. Cell Metab. 2016, 24, 172–184. [Google Scholar] [CrossRef]
- Baaske, L.; Gäbel, G.; Dengler, F. Ruminal epithelium: A checkpoint for cattle health. J. Dairy Res. 2020, 87, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Phuntsok, T.; Froetschel, M.A.; Amos, H.E.; Zheng, M.; Huang, Y.W. Biogenic amines in silage, apparent postruminal passage, and the relationship between biogenic amines and digestive function and intake by steers. J. Bairy Sci. 1998, 81, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, J.R.; Penner, G.B.; Stumpff, F.; Gäbel, G. Ruminant Nutrition Symposium: Role of fermentation acid absorption in the regulation of ruminal pH. J. Anim. Sci. 2011, 89, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.; Beauchemin, K.A.; Koenig, K.M. Using organic acids to control subacute ruminal acidosis and fermentation in feedlot cattle fed a high-grain diet. J. Anim. Sci. 2015, 93, 3950–3958. [Google Scholar] [CrossRef]
- Kratzer, J.T.; Lanaspa, M.A.; Murphy, M.N.; Cicerchi, C.; Graves, C.L.; Tipton, P.A.; Ortlund, E.A.; Johnson, R.J.; Gaucher, E.A. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc. Natl. Acad. Sci. USA 2014, 111, 3763–3768. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, Y.; Xu, C.; Luo, D.; Qiu, Q.; Pan, K.; Xiong, X.; Qu, M.; Ouyang, K. Niacin mitigates rumen epithelial damage in vivo by inhibiting rumen epithelial cell apoptosis on a high concentrate diet. Vet. Res. Commun. 2022, 46, 699–709. [Google Scholar] [CrossRef]
- Luo, D.; Peng, Z.; Yang, L.; Qu, M.; Xiong, X.; Xu, L.; Zhao, X.; Pan, K.; Ouyang, K. Niacin Protects against Butyrate-Induced Apoptosis in Rumen Epithelial Cells. Oxidative Med. Cell. Longev. 2019, 2019, 2179738. [Google Scholar] [CrossRef]
- Yuan, K.; Shaver, R.; Bertics, S.; Espineira, M.; Grummer, R. Effect of rumen-protected niacin on lipid metabolism, oxidative stress, and performance of transition dairy cows. J. Dairy Sci. 2012, 95, 2673–2679. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Li, W.; Ma, H.; Gong, Q.; Kan, X.; Cao, Y.; Wang, J.; Fu, S. Niacin Alleviates Dairy Cow Mastitis by Regulating the GPR109A/AMPK/NRF2 Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 3321. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, G.; Wang, Z.; Liu, J.; Chen, J.; Zhang, Z. Treatment of corn with lactic acid or hydrochloric acid modulates the rumen and plasma metabolic profiles as well as inflammatory responses in beef steers. BMC Vet. Res. 2018, 14, 408. [Google Scholar] [CrossRef]
- Mao, S.Y.; Huo, W.J.; Zhu, W.Y. Microbiome-metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model. Environ. Microbiol. 2016, 18, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Dai, D.; Wu, H.; Chai, S.; Liu, S.; Meng, Q.; Zhou, Z. Dietary Concentrate-to-Forage Ratio Affects Rumen Bacterial Community Composition and Metabolome of Yaks. Front. Nutr. 2022, 9, 927206. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Lin, G.; Langdon, W.Y.; Tao, L.; Zhang, J. Regulation of C-Type Lectin Receptor-Mediated Antifungal Immunity. Front. Immunol. 2018, 9, 123. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Song, T.; Liu, G.; Wu, J.; Zhaxi, Y.; Mustafa, S.B.; Shahzad, K.; Chen, X.; Zhao, W.; Jiang, X. GnRH Immunocastration in Male Xizang Sheep: Impacts on Rumen Microbiome and Metabolite Profiles for Enhanced Health and Productivity. Animals 2024, 14, 2942. https://doi.org/10.3390/ani14202942
Zhang X, Song T, Liu G, Wu J, Zhaxi Y, Mustafa SB, Shahzad K, Chen X, Zhao W, Jiang X. GnRH Immunocastration in Male Xizang Sheep: Impacts on Rumen Microbiome and Metabolite Profiles for Enhanced Health and Productivity. Animals. 2024; 14(20):2942. https://doi.org/10.3390/ani14202942
Chicago/Turabian StyleZhang, Xiaoming, Tianzeng Song, Guiqiong Liu, Jing Wu, Yangzong Zhaxi, Shehr Bano Mustafa, Khuram Shahzad, Xiaoying Chen, Wangsheng Zhao, and Xunping Jiang. 2024. "GnRH Immunocastration in Male Xizang Sheep: Impacts on Rumen Microbiome and Metabolite Profiles for Enhanced Health and Productivity" Animals 14, no. 20: 2942. https://doi.org/10.3390/ani14202942
APA StyleZhang, X., Song, T., Liu, G., Wu, J., Zhaxi, Y., Mustafa, S. B., Shahzad, K., Chen, X., Zhao, W., & Jiang, X. (2024). GnRH Immunocastration in Male Xizang Sheep: Impacts on Rumen Microbiome and Metabolite Profiles for Enhanced Health and Productivity. Animals, 14(20), 2942. https://doi.org/10.3390/ani14202942




