Effect of Substituting Soybean Meal in Oreochromis niloticus Diets with Pumpkin (Cucurbita maxima) Seed Cake on Water Quality, Growth, Antioxidant Capacity, Immunity, and Carcass Composition
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet Preparation
2.2. Fish and Rearing Conditions
2.3. Water Quality Parameters
2.4. Growth, Feed Utilization, and Body Indices
2.5. Chemical Composition of the Diets and the Whole Carcass of Tilapia
2.6. Serum Biochemical, Immunity, and Antioxidant Parameters
2.7. Statistical Analysis
3. Results
3.1. Water Quality
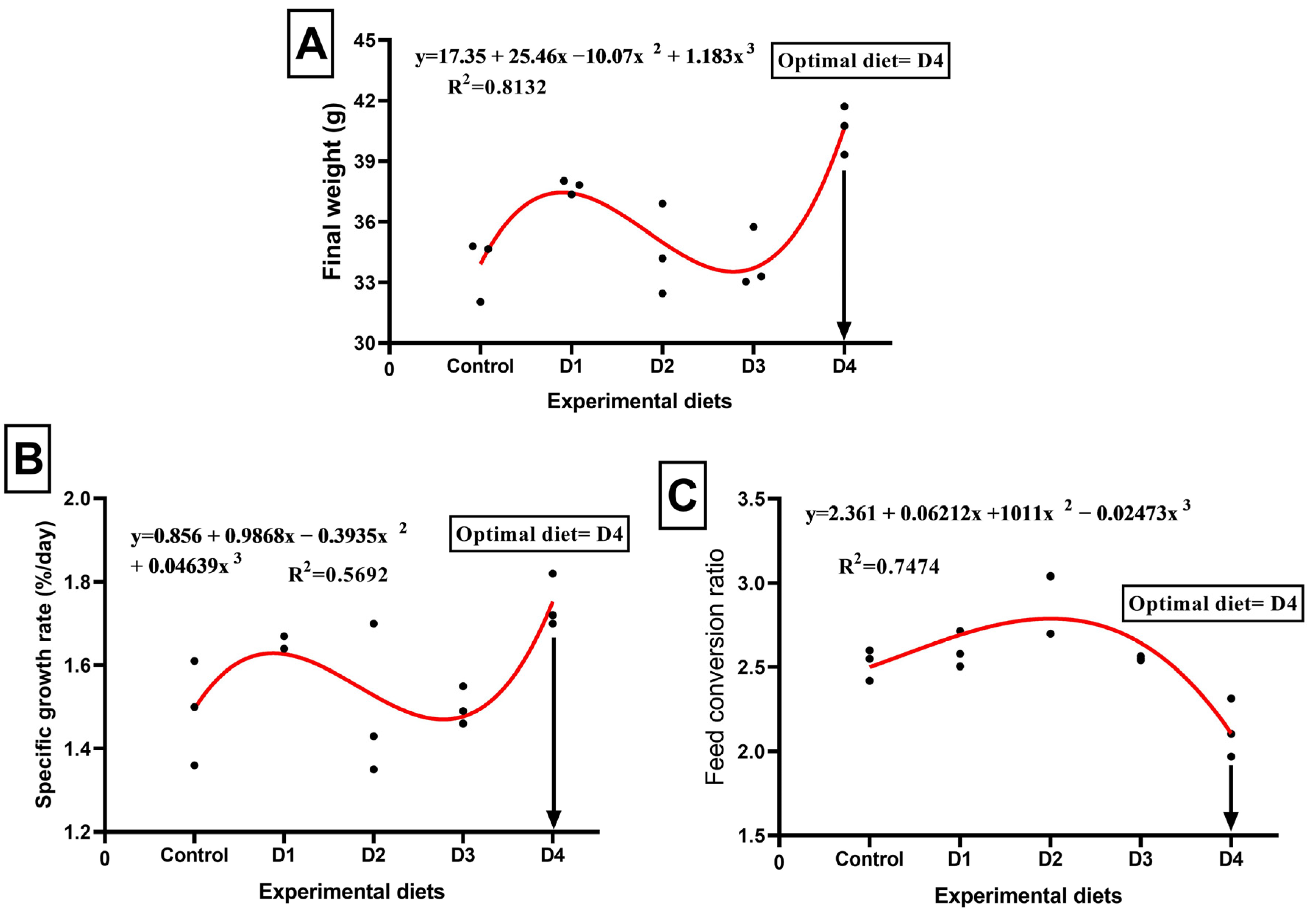
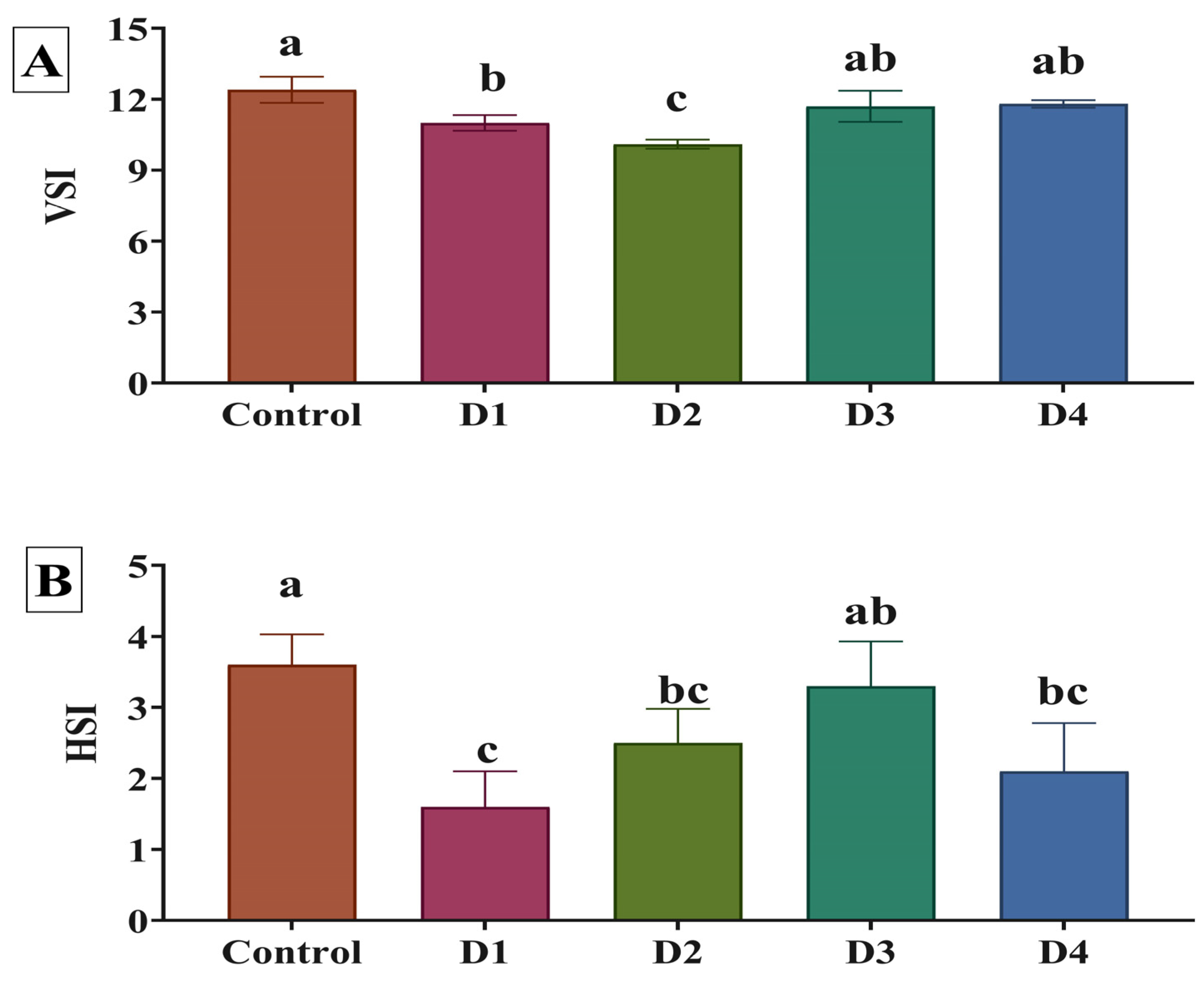
3.2. Growth Performance and Body Indices
3.3. Carcass Composition
3.4. Blood Biochemical Biomarkers
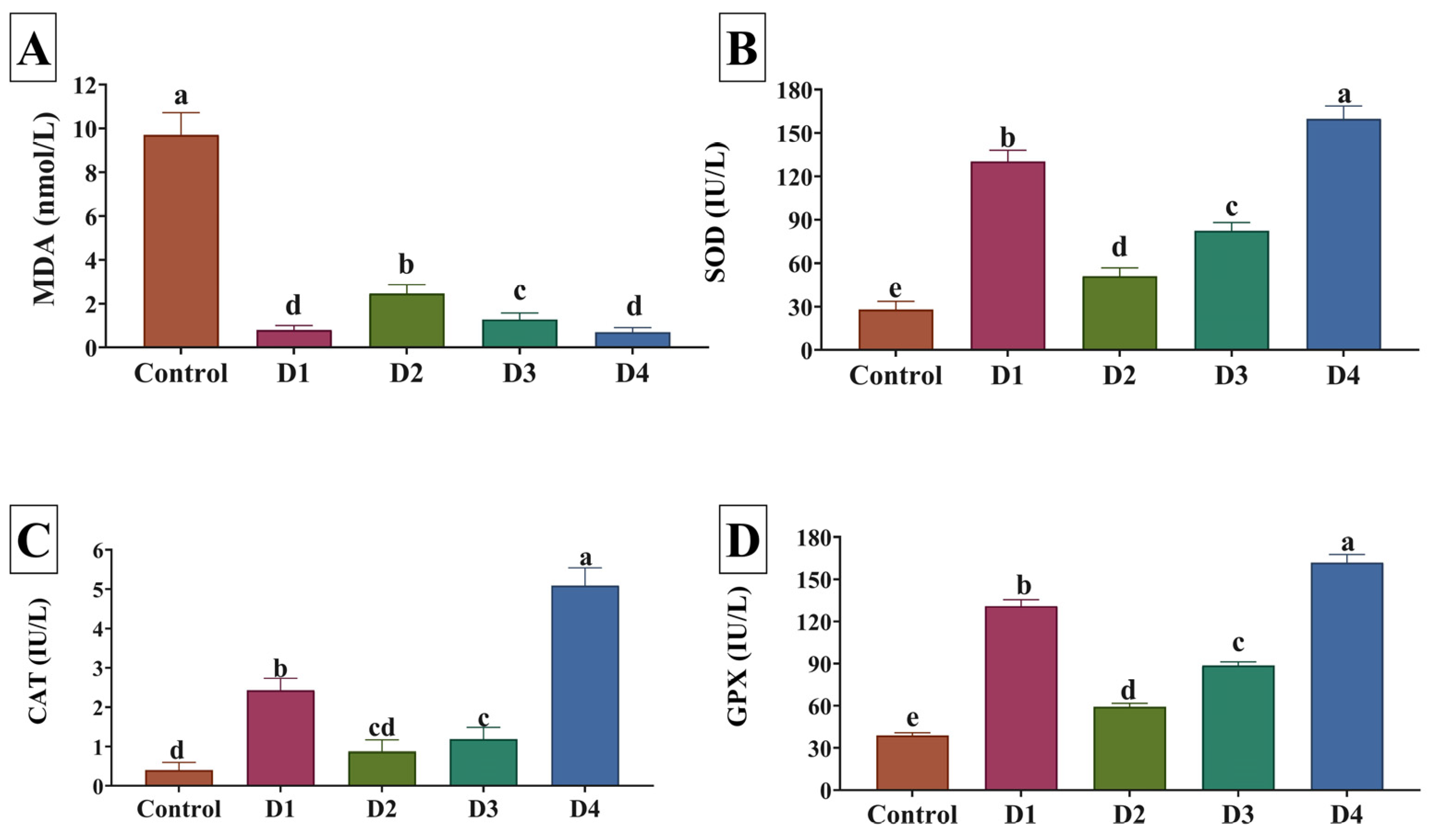
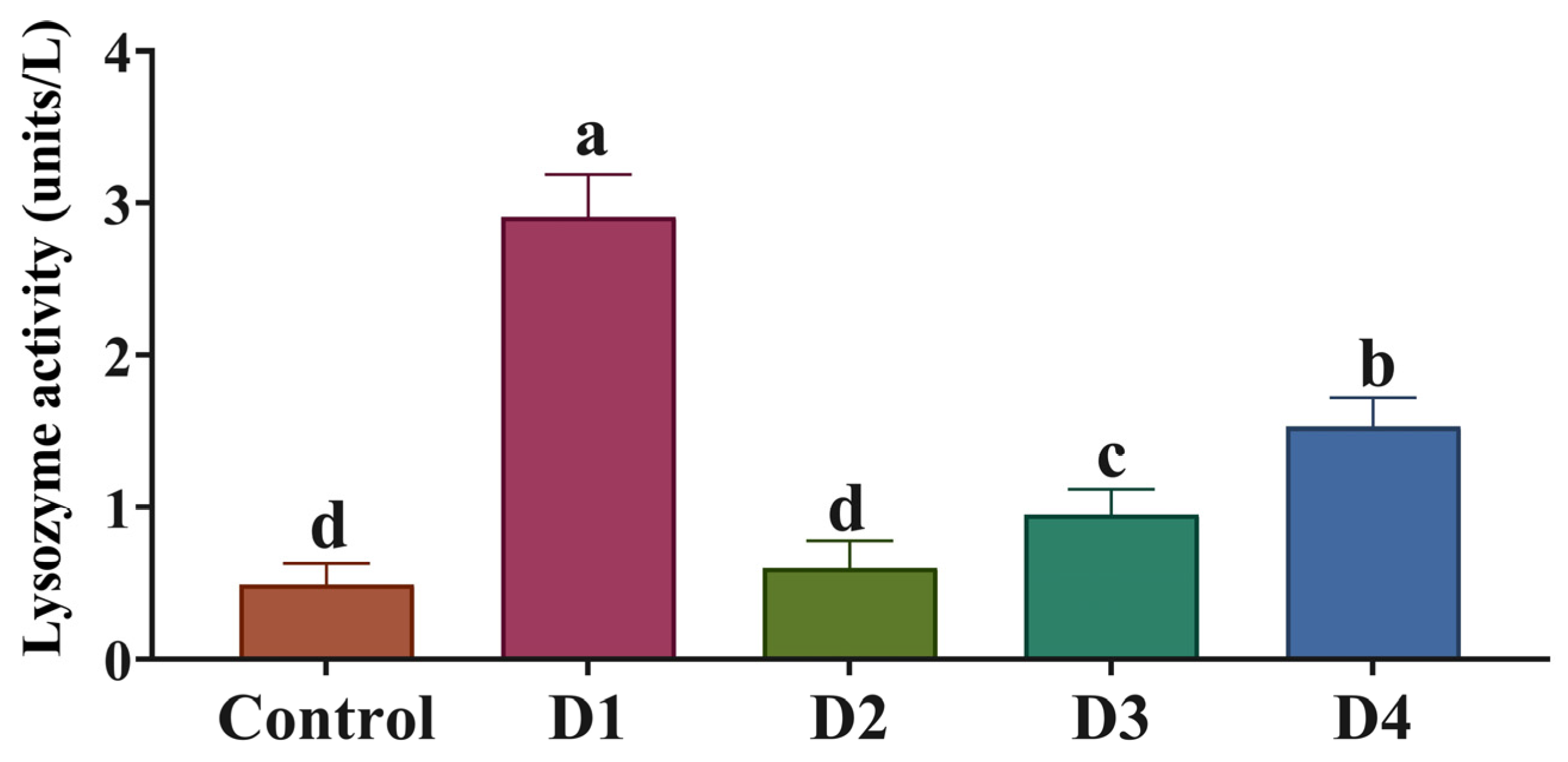
3.5. Antioxidant Activity and Immune Status
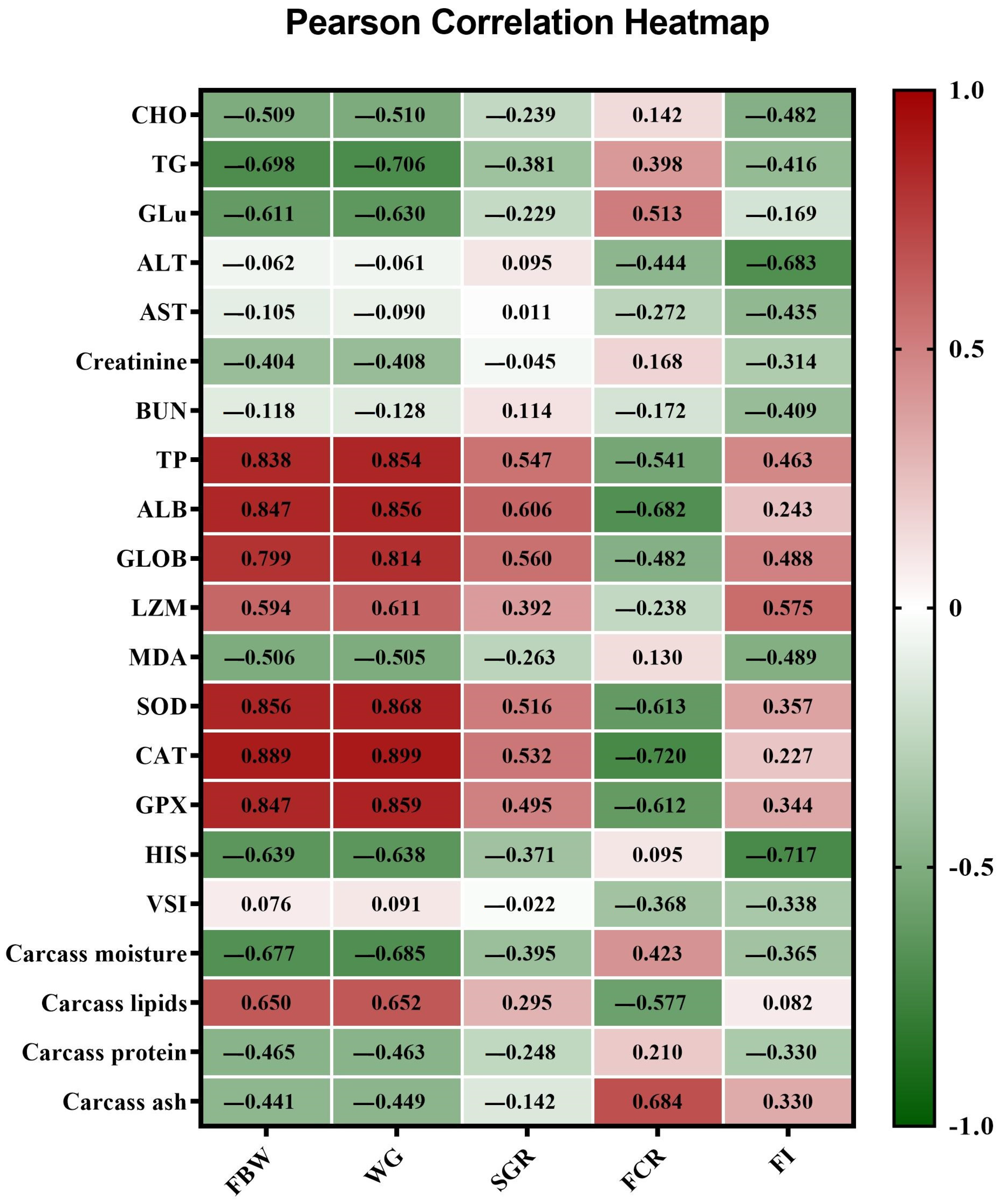
3.6. Pearson Correlation between the Estimated Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Houseiny, W.; Arisha, A.H.; Behairy, A.; Metwally, M.M.M.; Abdel-Warith, A.-W.A.; Younis, E.M.; Davies, S.J.; Hassan, B.A.; Abd-Elhakim, Y.M. The immunosuppressive, growth-hindering, hepatotoxic, and oxidative stress and immune related-gene expressions-altering effects of gibberellic acid in Oreochromis niloticus: A mitigation trial using alpha-lipoic acid. Pestic. Biochem. Physiol. 2024, 198, 105725. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Ayyat, M.S.; Al-Sagheer, A.A.; Ayyat, A.M.; Abdelrhman, A.M.; Ahmed, N.H.; Naiel, M.A. Effects of a blend of herbal feed supplements on growth, associated blood indices and body chemical analysis in nile tilapia reared under high stocking density. Aquac. Res. 2022, 53, 5475–5485. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Ayyat, A.M.N.; Abdel-Rahman, M.S.; Al-Sagheer, A.A. Appraisal of leaf protein concentrate derived from sugar beet and carrot as a novel fish meal substitute for juvenile Nile tilapia (Oreochromis niloticus). Anim. Feed Sci. Technol. 2024, 307, 115833. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M.; Fitzsimmons, K. From africa to the world—The journey of nile tilapia. Rev. Aquac. 2023, 15, 6–21. [Google Scholar] [CrossRef]
- Ayyat, M.S.; A. Abdel-Rahman, G.; Ayyat, A.M.N.; Abdel-Rahman, M.S.; Al-Sagheer, A.A. Evaluation of leaf protein concentrate from Beta vulgaris and Daucus carota as a substitute for soybean meal in Oreochromis niloticus fingerlings diets. Aquac. Res. 2021, 52, 3256–3269. [Google Scholar] [CrossRef]
- Gyan, W.R.; Ayiku, S.; Yang, Q. Effects of replacing fishmeal with soybean products in fish and crustaceans performance. J. Aquat. Res. Dev. 2019, 10, 573. [Google Scholar]
- Li, H.; Hu, Z.; Liu, S.; Sun, J.; Ji, H. Influence of dietary soybean meal replacement with yellow mealworm (Tenebrio molitor) on growth performance, antioxidant capacity, skin color, and flesh quality of mirror carp (Cyprinus carpio var. Specularis). Aquaculture 2022, 561, 738686. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Ayyat, A.M.N.; Abd El-Latif, K.M.; Hessein, A.A.A.; Al-Sagheer, A.A. Inorganic mercury and dietary safe feed additives enriched diet impacts on growth, immunity, tissue bioaccumulation, and disease resistance in Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2020, 224, 105494. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Arisha, A.H.; Metwally, M.M.M.; Abdel-Warith, A.-W.A.; Younis, E.M.; Davies, S.J.; Hassan, B.A.; Abd-Elhakim, Y.M. Alpha-lipoic acid suppresses gibberellic acid nephrotoxicity in Nile tilapia (Oreochromis niloticus) via modulating oxidative stress, inflammation, cytokine production, and apoptosis. Pestic. Biochem. Physiol. 2023, 196, 105598. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Bio-processing of agro-byproducts to animal feed. Crit. Rev. Biotechnol. 2012, 32, 382–400. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Adel, R.; Abdel-Sattar, E. Pumpkin bio-wastes as source of functional ingredients. In Mediterranean Fruits Bio-Wastes: Chemistry, Functionality and Technological Applications; Ramadan, M.F., Farag, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 667–696. [Google Scholar]
- Rabrenović, B.B.; Dimić, E.B.; Novaković, M.M.; Tešević, V.V.; Basić, Z.N. The most important bioactive components of cold pressed oil from different pumpkin (Cucurbita pepo L.) seeds. LWT-Food Sci. Technol. 2014, 55, 521–527. [Google Scholar] [CrossRef]
- Salehi, B.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; Calina, D.; et al. Cucurbits plants: A key emphasis to its pharmacological potential. Molecules 2019, 24, 1854. [Google Scholar] [CrossRef] [PubMed]
- Zdunczyk, Z.; Minakowski, D.; Frejnagel, S.; Flis, M. Comparative study of the chemical composition and nutritional value of pumpkin seed cake, soybean meal and casein. Food/Nahr. 1999, 43, 392–395. [Google Scholar] [CrossRef]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo l.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, A.; Aydın, B. Effect of replacing dietary soybean meal with pumpkin (Cucurbita pepo) seed cake on growth, feed utilization, haematological parameters and fatty acid composition of mirror carp (Cyprinus carpio). Aquac. Res. 2021, 52, 5870–5881. [Google Scholar] [CrossRef]
- Akin, G.; Arslan, F.N.; Elmasa, S.K.; Yilmaz, I. Cold-pressed pumpkin seed (Cucurbita pepo L.) oils from the central anatolia region of Turkey: Characterization of phytosterols, squalene, tocols, phenolic acids, carotenoids and fatty acid bioactive compounds. Grasas Aceites 2018, 69, e232. [Google Scholar] [CrossRef]
- Lovatto, N.D.M.; Goulart, F.R.; Loureiro, B.B.; Adorian, T.J.; de Freitas, S.T.; Pianesso, D.; Dalcin, M.O.; Athayde, M.L.; da Silva, L.P. Effects of phosphorylated protein concentrate of pumpkin seed meal on growth and digestive enzymes activity of silver catfish (Rhamdia quelen). Aquac. Nutr. 2017, 23, 201–209. [Google Scholar] [CrossRef]
- Greiling, A.M.; Schwarz, C.; Gierus, M.; Rodehutscord, M. Pumpkin seed cake as a fishmeal substitute in fish nutrition: Effects on growth performance, morphological traits and fillet colour of two freshwater salmonids and two catfish species. Arch. Anim. Nutr. 2018, 72, 239–259. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Fish and Shrimp; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington DC, USA, 2017. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists International, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Ghareghanipoora, M.; Akbary, P.; Akhlaghi, M.; Fereidouni, M. Non-specific immune responses and immune related genes expression of rainbow trout (Oncorhynchus mykiss, walbaum) fed Zataria multiflora boiss extract. Bull Environ. Pharmacol. Life Sci. 2014, 3, 140–146. [Google Scholar]
- SAS. Note: SAS Proprietary Software Version 9.00 (TS m0); Sas Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Yossa, R.; Verdegem, M. Misuse of multiple comparison tests and underuse of contrast procedures in aquaculture publications. Aquaculture 2015, 437, 344–350. [Google Scholar] [CrossRef]
- Prism, G. Prism 8 for Windows; GraphPad Software Inc.: San Diego, CA, USA, 2019. [Google Scholar]
- Boyd, C. Water Quality in Ponds for Aquaculture; Birmingham Publ. Co.: Birmingham, AL, USA, 1990; p. 482. [Google Scholar]
- Boyd, C.E.; Tucker, C.S. Ecology of aquaculture ponds. In Pond Aquaculture Water Quality Management; Springer: Berlin/Heidelberg, Germany, 1998; pp. 8–86. [Google Scholar]
- El-Houseiny, W.; Anter, R.G.A.; Arisha, A.H.; Mansour, A.T.; Safhi, F.A.; Alwutayd, K.M.; Elshopakey, G.E.; Abd El-Hakim, Y.M.; Mohamed, E.M.M. Growth retardation, oxidative stress, immunosuppression, and inflammatory disturbances induced by herbicide exposure of catfish, Clarias gariepinus, and the alleviation effect of dietary wormwood Artemisia cina. Fishes 2023, 8, 297. [Google Scholar] [CrossRef]
- Dernekbaşı, S.; Karayücel, İ.; Akyüz, A.P. Evaluation of sesame (Sesamum indicum) seed meal as a replacer for soybean meal in the diets of rainbow trout (Oncorhynchus mykiss walbaum, 1792). Ege J. Fish. Aquat. Sci. 2017, 34, 31–39. [Google Scholar]
- Ustaoglu Tiril, S.; Kerim, M. Evaluation of safflower meal as a protein source in diets of rainbow trout [Oncorhynchus mykiss, walbaum, 1792)]. J. Appl. Ichthyol. 2015, 31, 895–899. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Sun, J.; Huang, X.; Tang, H.; He, Y.; Pan, Q.; Gan, L. Partial substitution of soybean meal with faba bean meal in grass carp (Ctenopharyngodon idella) diets, and the effects on muscle fatty acid composition, flesh quality, and expression of myogenic regulatory factors. J. World Aquac. Soc. 2020, 51, 1145–1160. [Google Scholar] [CrossRef]
- Lu, R.; Chen, Y.; Yu, W.; Lin, M.; Yang, G.; Qin, C.; Meng, X.; Zhang, Y.; Ji, H.; Nie, G. Defatted black soldier fly (Hermetia illucens) larvae meal can replace soybean meal in juvenile grass carp (Ctenopharyngodon idellus) diets. Aquac. Rep. 2020, 18, 100520. [Google Scholar] [CrossRef]
- Wang, T.; Xu, M.; Wang, J.; Wan, W.; Guan, D.; Han, H.; Wang, Z.; Sun, H. A combination of rapeseed, cottonseed and peanut meal as a substitute of soybean meal in diets of yellow river carp Cyprinus carpio var. Aquac. Nutr. 2020, 26, 1520–1532. [Google Scholar] [CrossRef]
- Musthafa, M.S.; Asgari, S.M.; Kurian, A.; Elumalai, P.; Ali, A.R.J.; Paray, B.A.; Al-Sadoon, M.K. Protective efficacy of Mucuna pruriens (L.) seed meal enriched diet on growth performance, innate immunity, and disease resistance in Oreochromis mossambicus against Aeromonas hydrophila. Fish Shellfish Immunol. 2018, 75, 374–380. [Google Scholar] [CrossRef]
- Bai, T.L.; Ch, J.; Reddy, D.K. Comparison of effects of cotton seed meal with fish meal on growth, feed conversion ratio and survival of redbellied pacu (Piaractus brachypomus) int. J. Sci. Environ. Technol. 2016, 5, 1092–1099. [Google Scholar]
- Dotto, J.M.; Chacha, J.S. The potential of pumpkin seeds as a functional food ingredient: A review. Sci. Afr. 2020, 10, e00575. [Google Scholar] [CrossRef]
- Caili, F.; Huan, S.; Quanhong, L. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006, 61, 70–77. [Google Scholar] [CrossRef]
- Nkosi, C.; Opoku, A.; Terblanche, S. In vitro antioxidative activity of pumpkin seed (Cucurbita pepo) protein isolate and its in vivo effect on alanine transaminase and aspartate transaminase in acetaminophen-induced liver injury in low protein fed rats. Phytother. Res. 2006, 20, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Musthafa, M.S.; Jawahar Ali, A.R.; Arun Kumar, M.S.; Paray, B.A.; Al-Sadoon, M.K.; Balasundaram, C.; Harikrishnan, R. Effect of Cucurbita mixta (L.) seed meal enrichment diet on growth, immune response and disease resistance in Oreochromis mossambicus. Fish Shellfish Immunol. 2017, 68, 509–515. [Google Scholar] [CrossRef] [PubMed]
- El-Houseiny, W.; Algharib, S.A.; Mohamed, E.A.A.; Metwally, M.M.M.; Mahmoud, Y.K.; Alghamdi, Y.S.; Soliman, M.M.; Abd-Elhakim, Y.M.; El-Murr, A.E. Dietary parsley seed mitigates methomyl-induced impaired growth performance, hemato-immune suppression, oxidative stress, hepato-renal damage, and pseudomonas aeruginosa susceptibility in Oreochromis niloticus. Antioxidants 2022, 11, 1185. [Google Scholar] [CrossRef] [PubMed]
- Niroomand, M.; Akbarzadeh, A.; Ebrahimi, E.; Sobhani, S.A.; Sheikhahmadi, A. Effects of dietary black cumin seed meal on growth performance, blood biochemistry and fatty acid composition of pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2020, 26, 1072–1082. [Google Scholar] [CrossRef]
- Latif, M.; Faheem, M.; Asmatullah; Hoseinifar, S.H.; Van Doan, H. Dietary black seed effects on growth performance, proximate composition, antioxidant and histo-biochemical parameters of a culturable fish, rohu (Labeo rohita). Animals 2020, 11, 48. [Google Scholar] [CrossRef]
- Esmaeili, M. Blood performance: A new formula for fish growth and health. Biology 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Al-Sagheer, A.A.; Mahmoud, H.K.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Supplementation of diets for Oreochromis niloticus with essential oil extracts from lemongrass (Cymbopogon citratus) and geranium (Pelargonium graveolens) and effects on growth, intestinal microbiota, antioxidant and immune activities. Aquac. Nutr. 2018, 24, 1006–1014. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Fang, X.; Zhao, C.; Wu, H.; Lan, Y.; Che, L.; Sun, Y.; Lv, J.; Zhang, Y. Effects of replacing soybean meal with pumpkin seed cake and dried distillers grains with solubles on milk performance and antioxidant functions in dairy cows. Animal 2021, 15, 100004. [Google Scholar] [CrossRef]
- Krimer-Malešević, V. Pumpkin seeds: Phenolic acids in pumpkin seed (Cucurbita pepo L.). In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 533–542. [Google Scholar]
- Zhang, G.; Gong, S.; Yu, D.; Yuan, H. Propolis and herba epimedii extracts enhance the non-specific immune response and disease resistance of Chinese sucker, Myxocyprinus asiaticus. Fish Shellfish Immunol. 2009, 26, 467–472. [Google Scholar] [CrossRef]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Ciênc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef]
- Reynaud, S.; Raveton, M.; Ravanel, P. Interactions between immune and biotransformation systems in fish: A review. Aquat. Toxicol. 2008, 87, 139–145. [Google Scholar] [CrossRef] [PubMed]

| Experimental Diets 1 | |||||
|---|---|---|---|---|---|
| Control | D1 | D2 | D3 | D4 | |
| Ingredients | |||||
| Soybean meal | 420 | 378 | 336 | 294 | 252 |
| Pumpkin seed cake 2 | 0 | 33.5 | 67 | 100.4 | 133.9 |
| Yellow corn | 375 | 375 | 375 | 375 | 375 |
| Fish meal | 100 | 100 | 100 | 100 | 100 |
| Wheat bran | 70 | 70 | 70 | 70 | 70 |
| Cellulose | 0 | 11.2 | 22.4 | 33.7 | 44.9 |
| Fish oil | 10 | 10 | 10 | 10 | 10 |
| Sunflower oil | 20 | 17.3 | 14.6 | 11.9 | 9.2 |
| Premix 3 | 5 | 5 | 5 | 5 | 5 |
| Proximate composition | |||||
| Dry matter | 914.0 | 915.0 | 912.0 | 921.0 | 925.0 |
| Crude protein (N × 6.25) | 315.0 | 316.5 | 314.5 | 315.5 | 314.5 |
| Crude lipid | 65.5 | 65.7 | 65.4 | 65.1 | 65.9 |
| Ash | 75.5 | 79.5 | 76.5 | 74.5 | 76.0 |
| Items | Experimental Diets 1 | SDM 2 | ||||
|---|---|---|---|---|---|---|
| Control | D1 | D2 | D3 | D4 | ||
| Initial weight (g fish−1) | 11.86 NS | 11.87 | 12.08 | 11.92 | 11.94 | 0.17 |
| Final weight (g fish−1) | 33.83 c | 37.74 b | 34.51 c | 34.02 c | 40.60 a | 3.01 |
| Body weight gain (g fish−1) | 21.97 c | 25.87 b | 22.43 c | 22.10 c | 28.66 a | 2.98 |
| Specific growth rate (%) | 1.51 b | 1.65 ab | 1.51 b | 1.51 b | 1.75 a | 0.20 |
| Feed intake (g fish−1) | 55.36 b | 67.26 a | 65.40 a | 56.41 b | 61.90 ab | 5.38 |
| Feed conversion ratio (g feed/g gain) | 2.52 ab | 2.60 ab | 2.93 a | 2.55 ab | 2.16 b | 0.29 |
| Survival rate (%) | 100 NS | 100 | 100 | 100 | 100 | 0.00 |
| Items | Experimental Diets 1 | SDM 2 | ||||
|---|---|---|---|---|---|---|
| Control | D1 | D2 | D3 | D4 | ||
| Moisture | 76.9 a | 72.4 cd | 74.9 b | 73.0 c | 71.8 d | 1.9 |
| Crude lipids | 14.4 d | 18.0 c | 16.8 c | 20.3 b | 23.7 a | 3.4 |
| Crude protein | 64.8 a | 58.7 c | 60.0 b | 57.4 d | 57.5 d | 2.8 |
| Ash | 20.0 b | 22.5 a | 22.5 a | 21.8 a | 18.3 c | 1.9 |
| Items | Experimental Diets 1 | SDM 2 | ||||
|---|---|---|---|---|---|---|
| Control | D1 | D2 | D3 | D4 | ||
| Total cholesterol (mg/dL) | 178.0 a | 126.25 c | 139.25 b | 130.0 bc | 123.05 c | 6.39 |
| Triglycerides (mg/dL) | 280.81 a | 188.25 d | 235.0 b | 208.92 c | 173.25 d | 6.37 |
| Glucose (mg/dL) | 122.83 a | 112.05 ab | 122.25 a | 116.05 ab | 105.36 b | 7.48 |
| ALT (U/L) | 73.12 a | 62.18 c | 65.48 c | 67.01 bc | 69.09 b | 3.24 |
| AST (U/L) | 111.25 a | 87.5 b | 76.25 c | 78.5 c | 85.91 b | 3.78 |
| Creatinine (mg/dL) | 0.60 a | 0.40 bc | 0.47 b | 0.41 bc | 0.33 c | 0.23 |
| Urea (mg/dL) | 16.2 a | 10.15 b | 12.1 b | 11.75 bc | 12.0 b | 4.75 |
| Total protein (g/dL) | 1.38 b | 3.29 a | 1.33 b | 1.54 b | 3.09 a | 0.31 |
| Albumin (g/dL) | 0.80 c | 1.35 ab | 0.84 c | 1.08 b | 1.54 a | 0.28 |
| Globulin (g/dL) | 0.58 b | 1.94 a | 0.49 b | 0.46 b | 1.55 a | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mounes, H.A.M.; Abd-El Azeem, Z.M.A.; Abd El-Bary, D.A.; Al-Sagheer, A.A.; Abd-Elhakim, Y.M.; Hassan, B.A.; Sadek, S.S.; Ahmed, K.M. Effect of Substituting Soybean Meal in Oreochromis niloticus Diets with Pumpkin (Cucurbita maxima) Seed Cake on Water Quality, Growth, Antioxidant Capacity, Immunity, and Carcass Composition. Animals 2024, 14, 195. https://doi.org/10.3390/ani14020195
Mounes HAM, Abd-El Azeem ZMA, Abd El-Bary DA, Al-Sagheer AA, Abd-Elhakim YM, Hassan BA, Sadek SS, Ahmed KM. Effect of Substituting Soybean Meal in Oreochromis niloticus Diets with Pumpkin (Cucurbita maxima) Seed Cake on Water Quality, Growth, Antioxidant Capacity, Immunity, and Carcass Composition. Animals. 2024; 14(2):195. https://doi.org/10.3390/ani14020195
Chicago/Turabian StyleMounes, Hossam A. M., Zeinab M. A. Abd-El Azeem, Dena. A. Abd El-Bary, Adham A. Al-Sagheer, Yasmina M. Abd-Elhakim, Bayan A. Hassan, Sherif S. Sadek, and Kareem. M. Ahmed. 2024. "Effect of Substituting Soybean Meal in Oreochromis niloticus Diets with Pumpkin (Cucurbita maxima) Seed Cake on Water Quality, Growth, Antioxidant Capacity, Immunity, and Carcass Composition" Animals 14, no. 2: 195. https://doi.org/10.3390/ani14020195
APA StyleMounes, H. A. M., Abd-El Azeem, Z. M. A., Abd El-Bary, D. A., Al-Sagheer, A. A., Abd-Elhakim, Y. M., Hassan, B. A., Sadek, S. S., & Ahmed, K. M. (2024). Effect of Substituting Soybean Meal in Oreochromis niloticus Diets with Pumpkin (Cucurbita maxima) Seed Cake on Water Quality, Growth, Antioxidant Capacity, Immunity, and Carcass Composition. Animals, 14(2), 195. https://doi.org/10.3390/ani14020195










