The Characteristics of Milk Fatty Acid Profile Predicted by Fourier-Transform Mid-Infrared Spectroscopy (FT-MIRS) in Chinese Holstein Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Milk Samples
2.2. Milk Characteristics
2.3. Prediction of Fatty Acid (FA) Group Content
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Milk Composition in Chinese Holstein Cows
3.2. Effects of Fixed Factors on Milk FA Groups
3.3. Alteration Tendency of FA Group Contents
3.3.1. The Alteration Tendency of FA Group Contents at Various DIM
3.3.2. The Alteration Tendency of FA Group Content at Different SCS
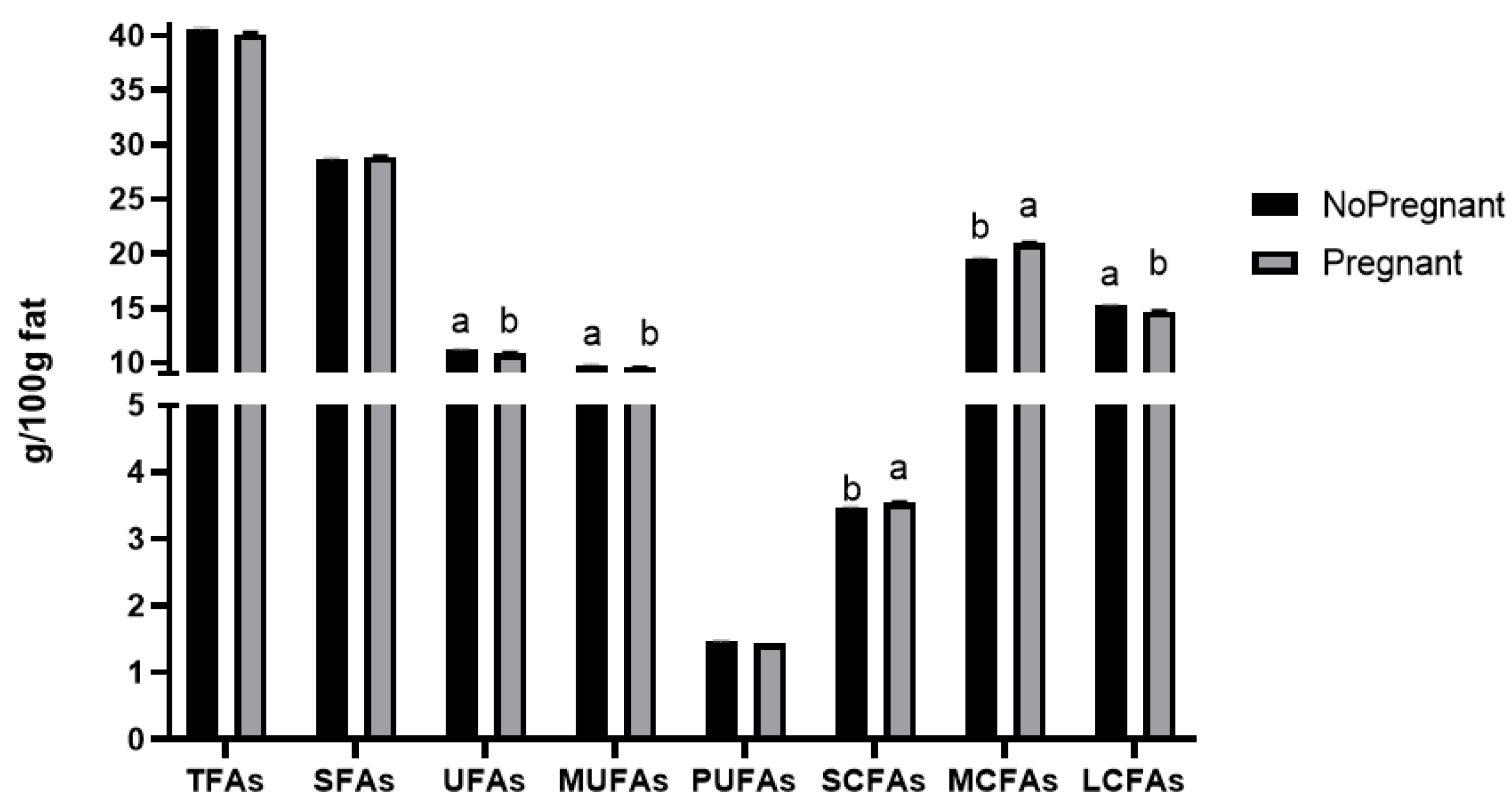
3.3.3. The Alteration Tendency of FA Group Content under Different Pregnancy States
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClearn, B.; Delaby, L.; Gilliland, T.J.; Galvin, N.; Guy, C.; Dineen, M.; Coughlan, F.; McCarthy, B. The effect of Holstein-Friesian, Jersey × Holstein-Friesian, and Norwegian Red × (Jersey × Holstein-Friesian) cows on dry matter intake and production efficiencies in pasture-based systems. J. Dairy Sci. 2022, 105, 242–254. [Google Scholar] [CrossRef]
- Ramji, D.P.; Davies, T.S. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015, 26, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Heileson, J.L. Dietary saturated fat and heart disease: A narrative review. Nutr. Rev. 2020, 78, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Tsai, T.H.; Chuang, L.T.; Li, Y.Y.; Zouboulis, C.C.; Tsai, P.J. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. J. Dermatol. Sci. 2014, 73, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, C.; Xu, Q.; Zhang, Y.; Li, H.; Li, F.; Liu, Y.; Guo, C. Caprylic acid suppresses inflammation via TLR4/NF-κB signaling and improves atherosclerosis in ApoE-deficient mice. Nutr. Metab. 2019, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Ghosh, A.; Ray, M.; Sarkar, S. Lauric acid modulates cancer-associated microRNA expression and inhibits the growth of the cancer cell. Anticancer Agents Med. Chem. 2020, 20, 834–844. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Tamminga, S.; Dewhurst, R.J.; van Vuuren, A.; De Brabander, D.; Demeyer, D. Milk odd and branched-chain fatty acids in relation to the rumen fermentation pattern. J. Dairy Sci. 2006, 89, 3954–3956. [Google Scholar] [CrossRef]
- Van Haelst, Y.N.; Beeckman, A.; Van Knegsel, A.T.; Fievez, V. Short communication: Elevated concentrations of oleic acid and long-chain fatty acids in milk fat of multiparous subclinical ketotic cows. J. Dairy Sci. 2008, 91, 4683–4686. [Google Scholar] [CrossRef]
- Bergamaschi, M.; Cipolat-Gotet, C.; Stocco, G.; Valorz, C.; Bazzoli, I.; Sturaro, E.; Ramanzin, M.; Bittante, G. Cheesemaking in highland pastures: Milk technological properties, cream, cheese and ricotta yields, milk nutrients recovery, and products composition. J. Dairy Sci. 2016, 99, 9631–9646. [Google Scholar] [CrossRef]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef]
- Gross, J.; van Dorland, H.A.; Bruckmaier, R.M.; Schwarz, F.J. Milk fatty acid profile related to energy balance in dairy cows. J. Dairy Res. 2011, 78, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Luke, T.D.W.; Rochfort, S.; Wales, W.J.; Bonfatti, V.; Marett, L.; Pryce, J.E. Metabolic profiling of early-lactation dairy cows using milk mid-infrared spectra. J. Dairy Sci. 2019, 102, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.G.; Schenkel, F.S.; Fleming, A.; Koeck, A.; Malchiodi, F.; Jamrozik, J.; Johnston, J.; Sargolzaei, M.; Miglior, F. Genetic analysis of groups of mid-infrared predicted fatty acids in milk. J. Dairy Sci. 2017, 100, 4731–4744. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Alvarado, H.; Vazquez, A.I.; de Los Campos, G.; Tempelman, R.J.; Gabai, G.; Cecchinato, A.; Bittante, G. Changes in milk characteristics and fatty acid profile during the estrous cycle in dairy cows. J. Dairy Sci. 2018, 101, 9135–9153. [Google Scholar] [CrossRef]
- Tiplady, K.M.; Lopdell, T.J.; Littlejohn, M.D.; Garrick, D.J. The evolving role of Fourier-transform mid-infrared spectroscopy in genetic improvement of dairy cattle. J. Anim. Sci. Biotechnol. 2020, 11, 39. [Google Scholar] [CrossRef]
- Wiggans, G.R.; Shook, G.E. A lactation measure of somatic cell count. J. Dairy Sci. 1987, 70, 2666–2672. [Google Scholar] [CrossRef]
- Murphy, S.C.; Martin, N.H.; Barbano, D.M.; Wiedmann, M. Influence of raw milk quality on processed dairy products: How do raw milk quality test results relate to product quality and yield? J. Dairy Sci. 2016, 99, 10128–10149. [Google Scholar] [CrossRef]
- Kocsis, R.; Süle, J.; Nagy, P.; Gál, J.; Tardy, E.; Császár, G.; Rácz, B. Annual and seasonal trends in cow’s milk quality determined by FT-MIR spectroscopy in Hungary between 2011 and 2020. Acta Vet. Hung. 2022, 29, 207–214. [Google Scholar] [CrossRef]
- Bokharaeian, M.; Toghdory, A.; Ghoorchi, T.; Ghassemi Nejad, J.; Esfahani, I.J. Quantitative associations between season, month, and temperature-humidity index with milk yield, composition, somatic cell counts, and microbial load: Aa comprehensive study across ten dairy farms over an Aannual cycle. Animals 2023, 13, 3205. [Google Scholar] [CrossRef]
- Turini, L.; Conte, G.; Bonelli, F.; Serra, A.; Sgorbini, M.; Mele, M. Multivariate factor analysis of milk fatty acid composition in relation to the somatic cell count of single udder quarters. J. Dairy Sci. 2020, 103, 7392–7406. [Google Scholar] [CrossRef]
- Henao-Velásquez, A.F.; Múnera-Bedoya, O.D.; Herrera, A.C.; Agudelo-Trujillo, J.H.; Cerón-Muñoz, M.F. Lactose and milk urea nitrogen: Fluctuations during lactation in Holstein cows. Rev. Bras. Zootec. 2014, 43, 479–484. [Google Scholar] [CrossRef]
- Ma, L.; Luo, H.; Brito, L.F.; Chang, Y.; Chen, Z.; Lou, W.; Zhang, F.; Wang, L.; Guo, G.; Wang, Y. Estimation of genetic parameters and single-step genome-wide association studies for milk urea nitrogen in Holstein cattle. J. Dairy Sci. 2023, 106, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Harper, M.; Oh, J.; Giallongo, F.; Lopes, J.C.; Cudoc, G.; Clay, J.; Ward, R.; Chase, L.E. Short communication: Variability in milk urea nitrogen and dairy total mixed ration composition in the northeastern United States. J. Dairy Sci. 2018, 101, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Stoop, W.M.; Schennink, A.; Visker, M.H.; Mullaart, E.; van Arendonk, J.A.; Bovenhuis, H. Genome-wide scan for bovine milk-fat composition. I. Quantitative trait loci for short- and medium-chain fatty acids. J. Dairy Sci. 2009, 92, 4664–4675. [Google Scholar] [CrossRef]
- Sauer, F.D.; Fellner, V.; Kinsman, R.; Kramer, J.K.; Jackson, H.A.; Lee, A.J.; Chen, S. Methane output and lactation response in Holstein cattle with monensin or unsaturated fat added to the diet. J. Anim. Sci. 1998, 76, 906–914. [Google Scholar] [CrossRef]
- Bilal, G.; Cue, R.I.; Mustafa, A.F.; Hayes, J.F. Short communication: Genetic parameters of individual fatty acids in milk of Canadian Holsteins. J. Dairy Sci. 2014, 97, 1150–1156. [Google Scholar] [CrossRef]
- Whetstine, M.E.C.; Drake, M.A. Flavor characteristics of goat milk and other minor species milk products. In Handbook of Milk of Non-Bovine Mammals; Blackwell Publishing: Oxford, UK, 2006; pp. 107–120. [Google Scholar] [CrossRef]
- Regado, M.A.; Cristóvão, B.M.; Moutinho, C.G.; Balcão, V.M.; Aires-Barros, R.; Ferreira, J.P.M.; Xavier Malcata, F. Flavour development via lipolysis of milkfats: Changes in free fatty acid pool. Int. J. Food Sci. Technol. 2007, 42, 961–968. [Google Scholar] [CrossRef]
- Tsubura, A.; Yuri, T.; Yoshizawa, K.; Uehara, N.; Takada, H. Role of fatty acids in malignancy and visual impairment: Epidemiological evidence and experimental studies. Histol. Histopathol. 2009, 24, 223–234. [Google Scholar] [CrossRef]
- Shramko, V.S.; Polonskaya, Y.V.; Kashtanova, E.V.; Stakhneva, E.M.; Ragino, Y.I. The short overview on the relevance of fatty acids for human cardiovascular disorders. Biomolecules 2020, 10, 1127. [Google Scholar] [CrossRef]
- Tomata, Y.; Wang, Y.; Hägg, S.; Jylhävä, J. Fatty acids and frailty: A mendelian randomization study. Nutrients 2021, 13, 3539. [Google Scholar] [CrossRef]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: IV. Particular saturated fatty acids in the diet. Metabolism 1965, 14, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The relationship of saturated fats and coronary heart disease: Fa(c)t or fiction? A commentary. Ther. Adv. Cardiovasc. Dis. 2018, 12, 33–37. [Google Scholar] [CrossRef] [PubMed]
- The American Heart Association’s Diet and Lifestyle Recommendations. Available online: http://www.heart.org/HEARTORG/HealthyLiving/HealthyEating/Nutrition/The-American-HeartAssociations-Diet-and-Lifestyle-eecommendations_UCM_305855_Article.jsp#.XKItpetKjOQ (accessed on 20 May 2019).
- Healthy Diet—World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 20 May 2019).
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Polyunsaturated fatty acids and inflammation. IUBMB Life 2015, 67, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Nam, M.; Son, H.Y.; Hyun, K.; Jang, S.Y.; Kim, J.W.; Kim, M.W.; Jung, Y.; Jang, E.; Yoon, S.-J.; et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 32433–32442. [Google Scholar] [CrossRef]
- Haycock, P.C.; Borges, M.C.; Burrows, K.; Lemaitre, R.N.; Burgess, S.; Khankari, N.K.; Tsilidise, K.K.; Gaunta, T.R.; Hemania, G.; Zheng, J.; et al. Fatty acids in cancer mendelian randomization collaboration. The association between genetically elevated polyunsaturated fatty acids and risk of cancer. EBioMedicine 2023, 91, 104510. [Google Scholar] [CrossRef] [PubMed]
- Stocco, G.; Summer, A.; Cipolat-Gotet, C.; Zanini, L.; Vairani, D.; Dadousis, C.; Zecconi, A. Differential somatic cell count as a novel indicator of milk quality in dairy cows. Animals 2020, 10, 753. [Google Scholar] [CrossRef]
- Pegolo, S.; Toscano, A.; Bisutti, V.; Vanzin, A.; Giannuzzi, D.; Gallo, L.; Tagliapietra, F.; Ruegg, P.; Gianesella, M.; Mele, M.; et al. Associations between subclinical intramammary infection and milk fatty acid profile at the quarter level in Holstein cattle. Animal 2023, 17, 100978. [Google Scholar] [CrossRef]
- Chandler, T.L.; Pralle, R.S.; Dórea, J.R.R.; Poock, S.E.; Oetzel, G.R.; Fourdraine, R.H.; White, H.M. Predicting hyperketonemia by logistic and linear regression using test-day milk and performance variables in early-lactation Holstein and Jersey cows. J. Dairy Sci. 2018, 101, 2476–2491. [Google Scholar] [CrossRef]
- Samková, E.; Špička, J.; Pešek, M.; Pelikánová, T.; Hanuš, O. Animal factors affecting fatty acid composition of cow milk fat:a review. Anim. Sci. 2012, 42, 83–100. [Google Scholar] [CrossRef]
- Bastin, C.; Berry, D.P.; Soyeurt, H.; Gengler, N. Genetic correlations of days open with production traits and contents in milk of major fatty acids predicted by mid-infrared spectrometry. J. Dairy Sci. 2012, 95, 6113–6121. [Google Scholar] [CrossRef]
- Castro, M.M.D.; Matson, R.D.; Santschi, D.E.; Marcondes, M.I.; DeVries, T.J. Association of housing and management practices with milk yield, milk composition, and fatty acid profile, predicted using Fourier transform mid-infrared spectroscopy, in farms with automated milking systems. J. Dairy Sci. 2022, 105, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Ferlay, A.; Mansbridge, R.M.; Doreau, M. Ruminant milk fat plasticity: Nutritional control of saturated, polyunsaturated, trans and conjugated fatty acids. Ann. De Zootech. 2000, 49, 181–205. [Google Scholar] [CrossRef]
- Gómez-Miranda, A.; Colín-Navarro, V.; López-González, F.; Morales-Almaráz, E.; Estrada-Flores, J.G.; Arriaga-Jordán, C.M. The effect of feeding whole-crop barley or black oat silage in the fatty acid content of milk from cows in small-scale dairy systems in the highlands of Mexico. Trop Anim. Health Prod. 2021, 53, 399. [Google Scholar] [CrossRef]
- Pacheco-Pappenheim, S.; Yener, S.; Nichols, K.; Dijkstra, J.; Hettinga, K.; van Valenberg, H.J.F. Feeding hydrogenated palm fatty acids and rumen-protected protein to lactating Holstein-Friesian dairy cows modifies milk fat triacylglycerol composition and structure, and solid fat content. J. Dairy Sci. 2022, 105, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Castellani, F.; Vitali, A.; Bernardi, N.; Marone, E.; Palazzo, F.; Grotta, L.; Martino, G. Dietary supplementation with dried olive pomace in dairy cows modifies the composition of fatty acids and the aromatic profile in milk and related cheese. J. Dairy Sci. 2017, 100, 8658–8669. [Google Scholar] [CrossRef]
- Xu, W.; Vervoort, J.; Saccenti, E.; Kemp, B.; van Hoeij, R.J.; van Knegsel, A.T.M. Relationship between energy balance and metabolic profiles in plasma and milk of dairy cows in early lactation. J. Dairy Sci. 2020, 103, 4795–4805. [Google Scholar] [CrossRef]
- Kay, J.K.; Weber, W.J.; Moore, C.E.; Bauman, D.E.; Hansen, L.B.; Chester-Jones, H.; Crooker, B.A.; Baumgard, L.H. Effects of week of lactation and genetic selection for milk yield on milk fatty acid composition in Holstein cows. J. Dairy Sci. 2005, 88, 3886–3893. [Google Scholar] [CrossRef]
- Van, Q.C.D.; Knapp, E.; Hornick, J.L.; Dufrasne, I. Influence of Days in Milk and Parity on Milk and Blood Fatty Acid Concentrations, Blood Metabolites and Hormones in Early Lactation Holstein Cows. Animals 2020, 10, 2081. [Google Scholar] [CrossRef]
- Pecka-Kiełb, E.; Vasil, M.; Zachwieja, A.; Zawadzki, W.; Elečko, J.; Zigo, F.; Illek, J.; Farkašová, Z. An effect of mammary gland infection caused by Streptococcus uberis on composition and physicochemical changes of cows’ milk. Pol. J. Vet. Sci. 2016, 19, 49–55. [Google Scholar] [CrossRef]
- Ma, Y.; Ryan, C.; Barbano, D.M.; Galton, D.M.; Rudan, M.A.; Boor, K.J. Effects of somatic cell count on quality and shelf-life of pasteurized fluid milk. J. Dairy Sci. 2000, 83, 264–274. [Google Scholar] [CrossRef]
- Bastin, C.; Soyeurt, H.; Gengler, N. Genetic parameters of milk production traits and fatty acid contents in milk for Holstein cows in parity 1–3. J. Anim. Breed Genet. 2013, 130, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Stádník, L.; Ducháček, J.; Beran, J.; Toušová, R.; Ptáček, M. Relationships between milk fatty acids composition in early lactation and subsequent reproductive performance in Czech Fleckvieh cows. Anim. Reprod. Sci. 2015, 155, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Ntallaris, T.; Båge, R.; Karlsson, J.; Holtenius, K. Milk fatty acids as indicators of delayed commencement of luteal activity in dairy cows in early lactation. Reprod. Domest. Anim. 2023, 58, 500–510. [Google Scholar] [CrossRef] [PubMed]
| Trait | Included Fatty Acids |
|---|---|
| TFAs | SFAs, UFAs, SCFAs, MCFAs, LCFAs |
| SFAs | C4:0, C6:0, C8:0, C10:0, C11:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, C21:0, C22:0, C23:0, C24:0 |
| UFAs | MUFAs, PUFAs |
| MUFAs | C14:1 cis-9, C15:1 cis-10, C16:1 cis-9, C17:1 cis-10, C18:1 cis-9, C18:1 trans-11, C20:1 cis-11, C22:1 cis-13, C24:1 cis-15 |
| PUFAs | C18:2 cis-9,12, C18:2 trans-9,12, C18:3 cis-6,9,12, C18:3 cis-9,12,15, C20:2 cis-11,14, C20:3 cis-8,11,14, C20:3 cis-11,14,17, C20:4 cis-5,8,11,14, C20:5 cis-5,8,11,14,17, C22:2 cis-13,16, C22:6 cis-4,7,10,13,16,19 |
| SCFAs | C4–C10 |
| MCFAs | C11–C16 |
| LCFAs | C17–C24 |
| Model | Rcv2 | RMSEcv |
|---|---|---|
| TFAs | 0.6991 | 9.2437 |
| SFAs | 0.7096 | 6.5024 |
| UFAs | 0.6505 | 3.1345 |
| MUFAs | 0.6475 | 2.7831 |
| PUFAs | 0.6798 | 0.4021 |
| SCFAs | 0.7239 | 0.9693 |
| MCFAs | 0.6643 | 5.1758 |
| LCFAs | 0.6192 | 4.1199 |
| Fatty Acids | Mean ± SD | % of TFAs | Milk Composition | Mean ± SD |
|---|---|---|---|---|
| TFAs (g/100 g of fat) | 40.26 ± 13.75 | Fat (%) | 3.94 ± 0.82 | |
| SFAs (g/100 g of fat) | 28.78 ± 9.77 | 71.48 | Protein (%) | 3.32 ± 0.38 |
| UFAs (g/100 g of fat) | 10.97 ± 4.37 | 27.25 | Lactose (%) | 5.14 ± 0.23 |
| MUFAs (g/100 g of fat) | 9.59 ± 3.94 | 23.82 | SNF (%) | 9.10 ± 0.43 |
| PUFAs (g/100 g of fat) | 1.46 ± 0.55 | 3.63 | TS (%) | 13.07 ± 1.07 |
| SCFAs (g/100 g of fat) | 3.55 ± 1.47 | 8.82 | Urea (mg/100 mL) | 12.93 ± 3.17 |
| MCFAs (g/100 g of fat) | 20.76 ± 7.42 | 51.56 | Milk yield (kg/day) | 35.63 ± 10.77 |
| LCFAs (g/100 g of fat) | 14.85 ± 5.78 | 36.88 | SCS (units) | 3.42 ± 3.81 |
| Fatty Acid Groups | Parities | DIM | Pregnancy States | Calving Seasons | SCS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | Pr > F | F-Value | Pr > F | F-Value | Pr > F | F-Value | Pr > F | F-Value | Pr > F | |
| TFAs | 0.09 | 0.91 | 12.3 | <0.01 | 2.98 | 0.08 | 1.68 | 0.17 | 1.38 | 0.21 |
| SFAs | 0.13 | 0.88 | 19.10 | <0.01 | 1.06 | 0.30 | 1.58 | 0.19 | 0.92 | 0.49 |
| UFAs | 0.29 | 0.75 | 9.56 | <0.01 | 17.24 | <0.01 | 1.46 | 0.22 | 2.3 | 0.02 |
| MUFAs | 0.36 | 0.70 | 9.36 | <0.01 | 18.79 | <0.01 | 1.46 | 0.22 | 3.46 | 0.00 |
| PUFAs | 0.54 | 0.58 | 18.43 | <0.01 | 2.38 | 0.12 | 1.40 | 0.24 | 2.82 | 0.01 |
| SCFAs | 1.57 | 0.21 | 11.41 | <0.01 | 8.45 | 0.00 | 1.26 | 0.29 | 4.34 | <0.01 |
| MCFAs | 0.77 | 0.47 | 41.46 | <0.01 | 107.62 | <0.01 | 1.11 | 0.34 | 11.75 | <0.01 |
| LCFAs | 1.40 | 0.25 | 24.08 | <0.01 | 32.53 | <0.01 | 1.61 | 0.19 | 12.71 | <0.01 |
| DIM | TFAs | SFAs | UFAs | MUFAs | PUFAs | SCFAs | MCFAs | LCFAs |
|---|---|---|---|---|---|---|---|---|
| 5–35 | 39.33 ± 0.22 c | 28.26 ± 0.16 b | 10.53 ± 0.07 c | 9.17 ± 0.06 c | 1.44 ± 0.01 bc | 3.43 ± 0.02 b | 20.32 ± 0.12 bc | 14.9 ± 0.09 b |
| 36–65 | 39.99 ± 0.21 b | 28.52 ± 0.15 b | 10.84 ± 0.07 b | 9.47 ± 0.06 b | 1.45 ± 0.01 bc | 3.48 ± 0.02 b | 20.22 ± 0.12 bc | 15.2 ± 0.09 ab |
| 66–95 | 40.28 ± 0.18 b | 28.62 ± 0.13 b | 10.99 ± 0.06 b | 9.6 ± 0.05 b | 1.46 ± 0.01 bc | 3.44 ± 0.02 b | 19.77 ± 0.09 c | 15.38 ± 0.07 a |
| 96–125 | 41.36 ± 0.21 a | 29.42 ± 0.15 a | 11.36 ± 0.07 a | 9.92 ± 0.06 a | 1.52 ± 0.01 a | 3.57 ± 0.02 ab | 20.36 ± 0.11 bc | 15.29 ± 0.09 ab |
| 126–155 | 39.53 ± 0.21 c | 27.76 ± 0.15 b | 10.98 ± 0.07 b | 9.62 ± 0.06 b | 1.42 ± 0.01 c | 3.41 ± 0.02 b | 19.08 ± 0.11 d | 15.19 ± 0.09 ab |
| 156–185 | 40.62 ± 0.21 b | 28.93 ± 0.15 b | 11.24 ± 0.07 a | 9.85 ± 0.06 a | 1.45 ± 0.01 bc | 3.52 ± 0.02 ab | 20.57 ± 0.11 b | 14.83 ± 0.09 b |
| 186–215 | 40.62 ± 0.21 b | 28.85 ± 0.15 b | 11.17 ± 0.07 ab | 9.77 ± 0.06 ab | 1.48 ± 0.01 b | 3.52 ± 0.02 ab | 20.04 ± 0.11 c | 15.03 ± 0.09 b |
| 216–245 | 41.09 ± 0.21 a | 29.25 ± 0.15 a | 11.24 ± 0.07 a | 9.84 ± 0.06 a | 1.48 ± 0.01 b | 3.53 ± 0.02 ab | 20.54 ± 0.11 b | 14.96 ± 0.09 b |
| 246–275 | 40.93 ± 0.21 ab | 29.17 ± 0.15 ab | 11.24 ± 0.07 a | 9.84 ± 0.06 a | 1.47 ± 0.01 bc | 3.55 ± 0.02 ab | 20.61 ± 0.12 b | 14.83 ± 0.09 b |
| 276–305 | 40.08 ± 0.21 b | 28.94 ± 0.15 b | 10.85 ± 0.07 b | 9.47 ± 0.06 b | 1.44 ± 0.01 c | 3.59 ± 0.02 a | 21.17 ± 0.11 a | 14.1 ± 0.09 c |
| SCS | TFAs | SFAs | UFAs | MUFAs | PUFAs | SCFAs | MCFAs | LCFAs |
|---|---|---|---|---|---|---|---|---|
| ≤−1 | 40.63 ± 0.6 | 28.99 ± 0.43 | 11.04 ± 0.19 ab | 9.66 ± 0.17 ab | 1.47 ± 0.02 a | 3.4 ± 0.06 b | 19.47 ± 0.32 c | 15.36 ± 0.25 a |
| −1 < SCS ≤ 0 | 40.27 ± 0.24 | 28.7 ± 0.17 | 11.04 ± 0.08 ab | 9.64 ± 0.07 ab | 1.47 ± 0.01 ab | 3.42 ± 0.03 b | 19.66 ± 0.13 c | 15.01 ± 0.10 a |
| >0, ≤1 | 40.32 ± 0.13 | 28.78 ± 0.09 | 11.03 ± 0.04 ab | 9.63 ± 0.04 ab | 1.47 ± 0.01 a | 3.53 ± 0.01 a | 20.38 ± 0.07 b | 15.14 ± 0.05 a |
| >1, ≤2 | 40.28 ± 0.12 | 28.73 ± 0.08 | 11.00 ± 0.04 ab | 9.60 ± 0.03 b | 1.46 ± 0.00 a | 3.55 ± 0.01 a | 20.56 ± 0.06 ab | 15.13 ± 0.05 a |
| >2, ≤3 | 40.23 ± 0.12 | 28.72 ± 0.09 | 10.95 ± 0.04 b | 9.56 ± 0.03 b | 1.46 ± 0.00 ab | 3.55 ± 0.01 a | 20.65 ± 0.06 a | 15.02 ± 0.05 a |
| >3, ≤4 | 40.32 ± 0.13 | 28.69 ± 0.09 | 11.07 ± 0.04 ab | 9.68 ± 0.04 ab | 1.46 ± 0.01 ab | 3.55 ± 0.01 a | 20.65 ± 0.07 ab | 14.86 ± 0.05 a |
| >4, ≤5 | 40.75 ± 0.15 | 28.96 ± 0.11 | 11.17 ± 0.05 a | 9.78 ± 0.04 a | 1.47 ± 0.01 ab | 3.54 ± 0.02 a | 20.58 ± 0.08 ab | 14.76 ± 0.06 ab |
| >5 | 40.28 ± 0.18 | 28.62 ± 0.13 | 11.05 ± 0.06 ab | 9.71 ± 0.05 ab | 1.44 ± 0.01 b | 3.51 ± 0.02 a | 20.19 ± 0.10 b | 14.48 ± 0.08 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Wang, H.; Fan, Y.; Zhou, Z.; Li, Y.; Liang, S.; Ma, Y.; Zhang, S. The Characteristics of Milk Fatty Acid Profile Predicted by Fourier-Transform Mid-Infrared Spectroscopy (FT-MIRS) in Chinese Holstein Cows. Animals 2024, 14, 2785. https://doi.org/10.3390/ani14192785
Li C, Wang H, Fan Y, Zhou Z, Li Y, Liang S, Ma Y, Zhang S. The Characteristics of Milk Fatty Acid Profile Predicted by Fourier-Transform Mid-Infrared Spectroscopy (FT-MIRS) in Chinese Holstein Cows. Animals. 2024; 14(19):2785. https://doi.org/10.3390/ani14192785
Chicago/Turabian StyleLi, Chunfang, Haitong Wang, Yikai Fan, Zengpo Zhou, Yuanbao Li, Shengchao Liang, Yabin Ma, and Shujun Zhang. 2024. "The Characteristics of Milk Fatty Acid Profile Predicted by Fourier-Transform Mid-Infrared Spectroscopy (FT-MIRS) in Chinese Holstein Cows" Animals 14, no. 19: 2785. https://doi.org/10.3390/ani14192785
APA StyleLi, C., Wang, H., Fan, Y., Zhou, Z., Li, Y., Liang, S., Ma, Y., & Zhang, S. (2024). The Characteristics of Milk Fatty Acid Profile Predicted by Fourier-Transform Mid-Infrared Spectroscopy (FT-MIRS) in Chinese Holstein Cows. Animals, 14(19), 2785. https://doi.org/10.3390/ani14192785






