Effects of Different Photoperiods on the Transcriptome of the Ovary and Small White Follicles in Zhedong White Geese
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experimental Design and Sample Collection
2.2. RNA Extraction, Library Construction, and Sequencing
2.3. RNA-seq Data Analysis
2.4. Enrichment Analysis and Protein–Protein Interaction Network Analysis
2.5. Weighted Gene Co-Expression Network Analysis
2.6. Quantitative RT-PCR Validation of mRNA Expression
3. Results
3.1. Summary of RNA-seq Data
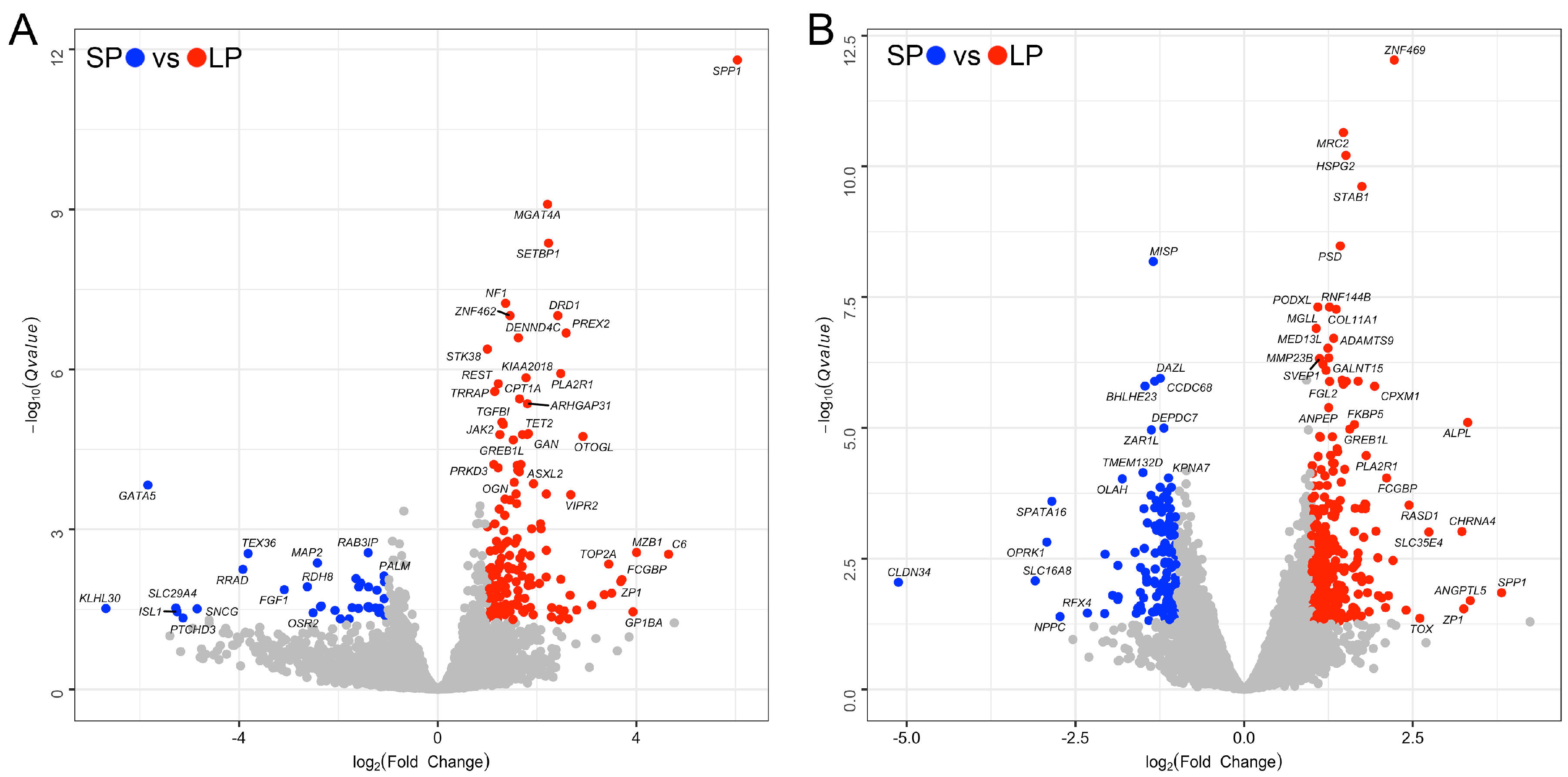
3.2. Identification of Differentially Expressed Genes
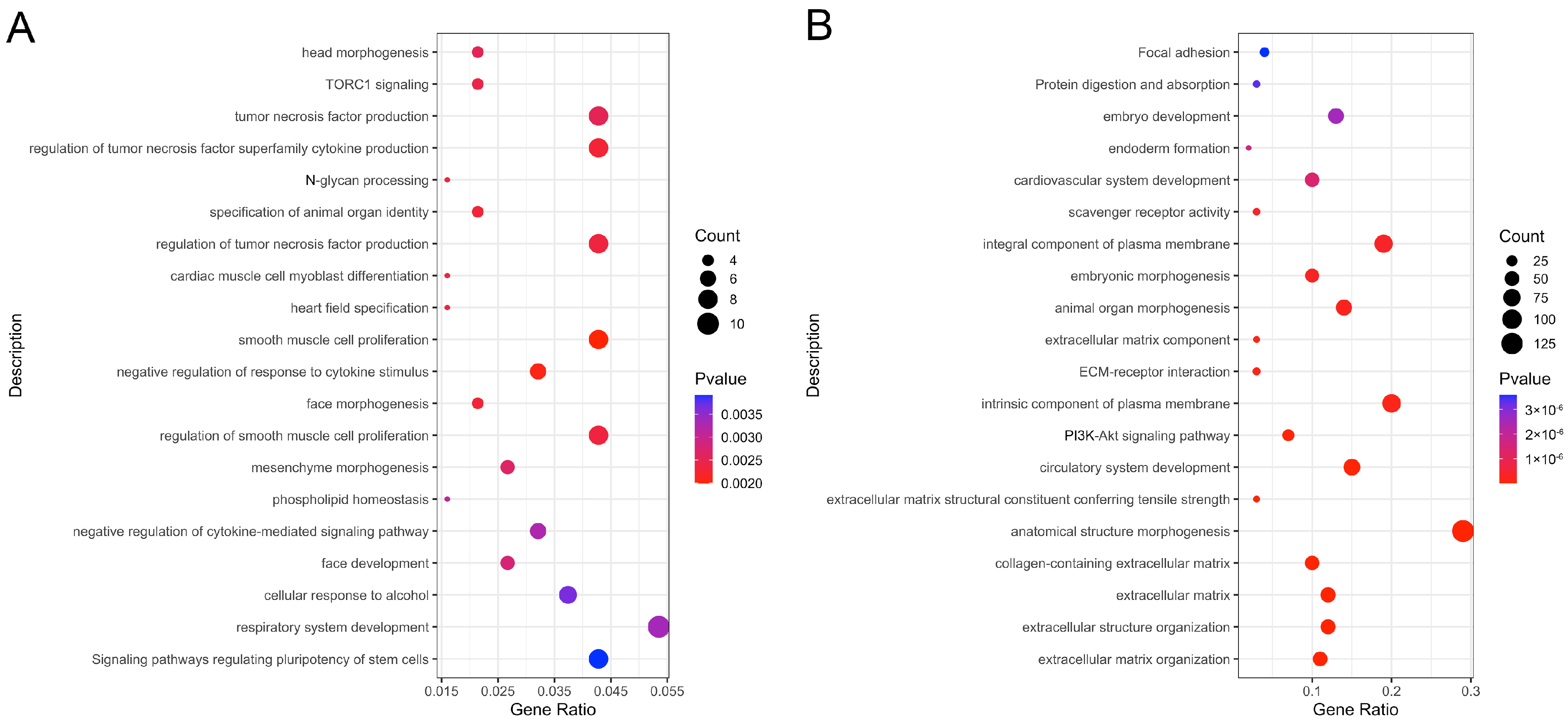
3.3. Enrichment Analysis of Pathways
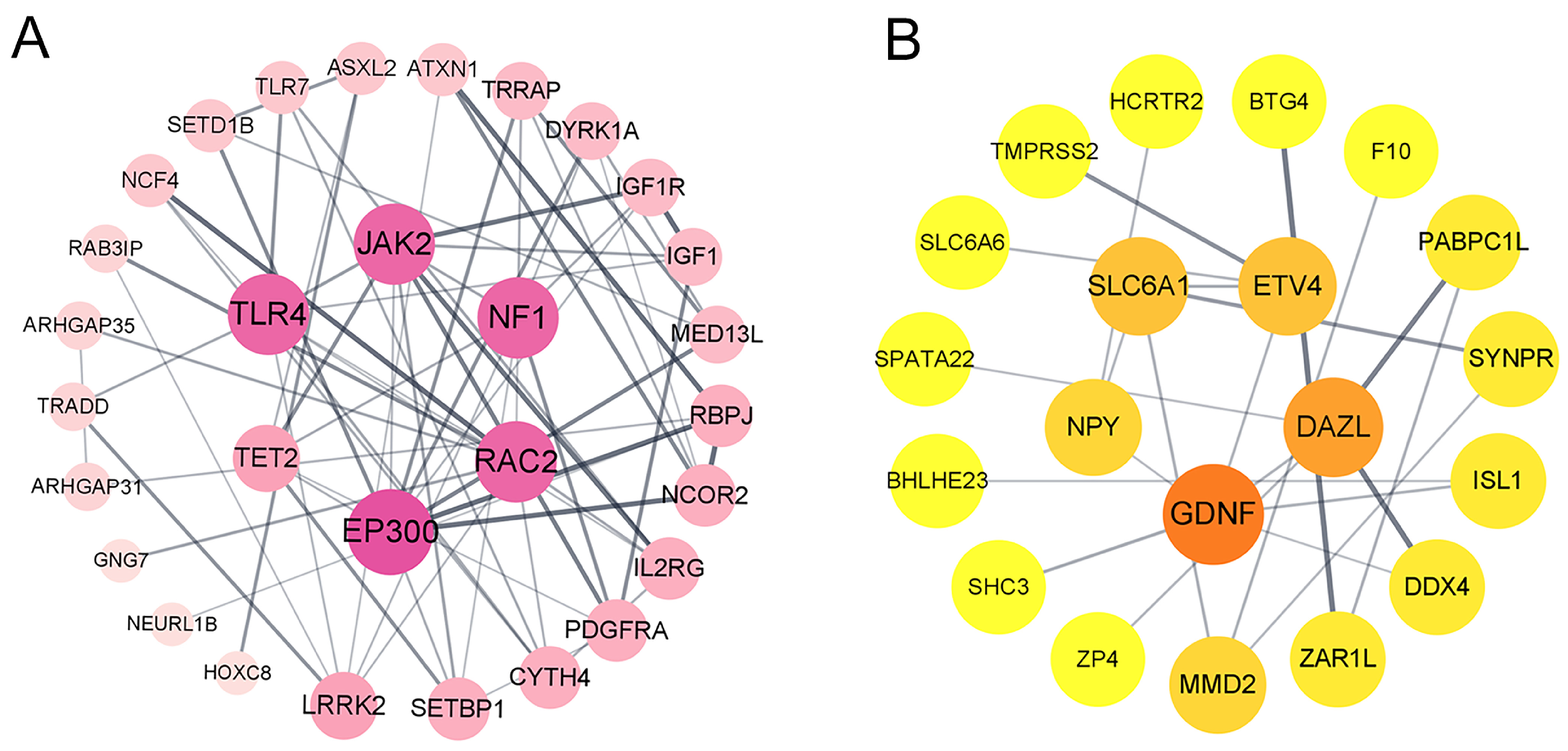
3.4. Protein–Protein Interaction Network
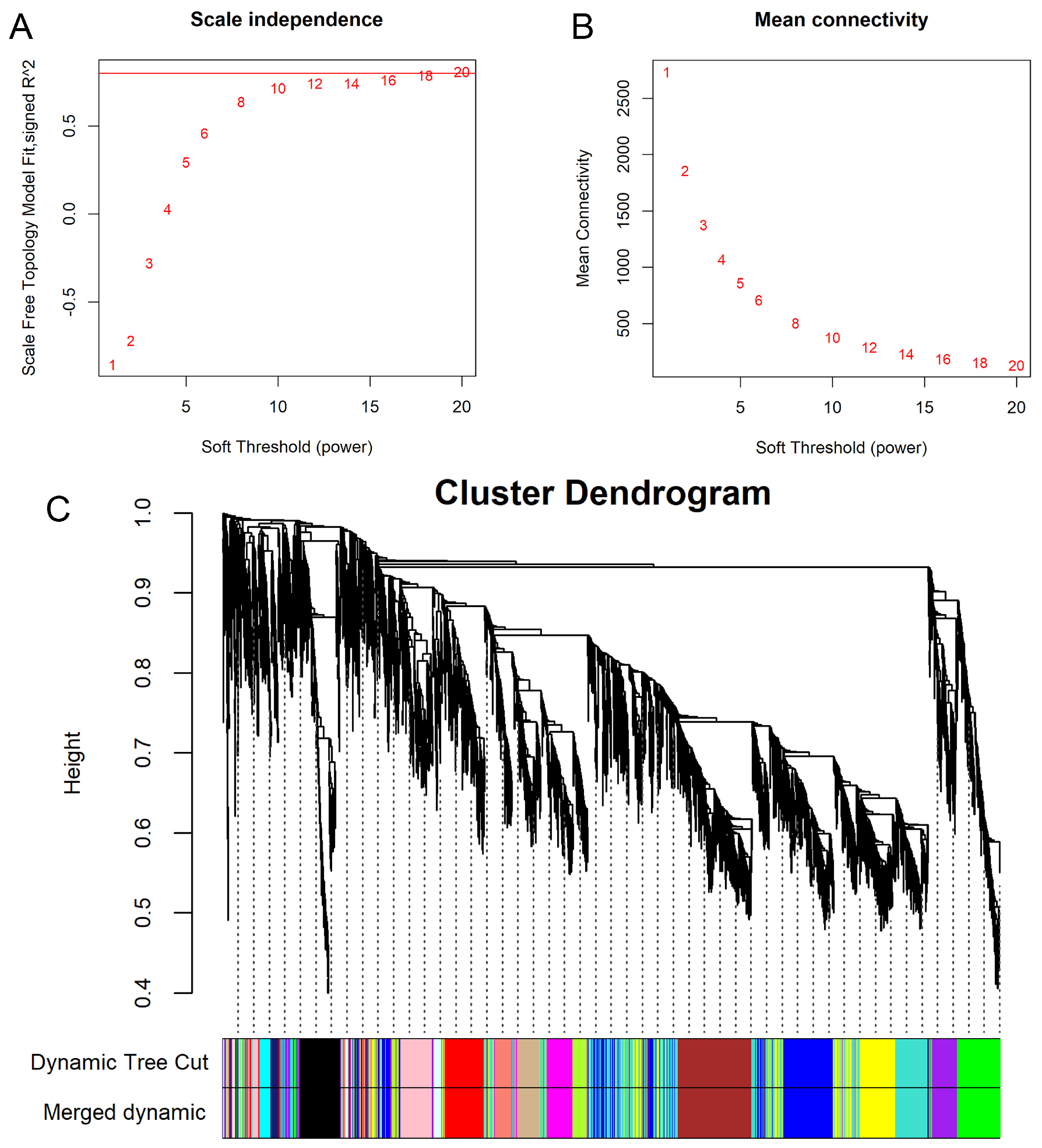
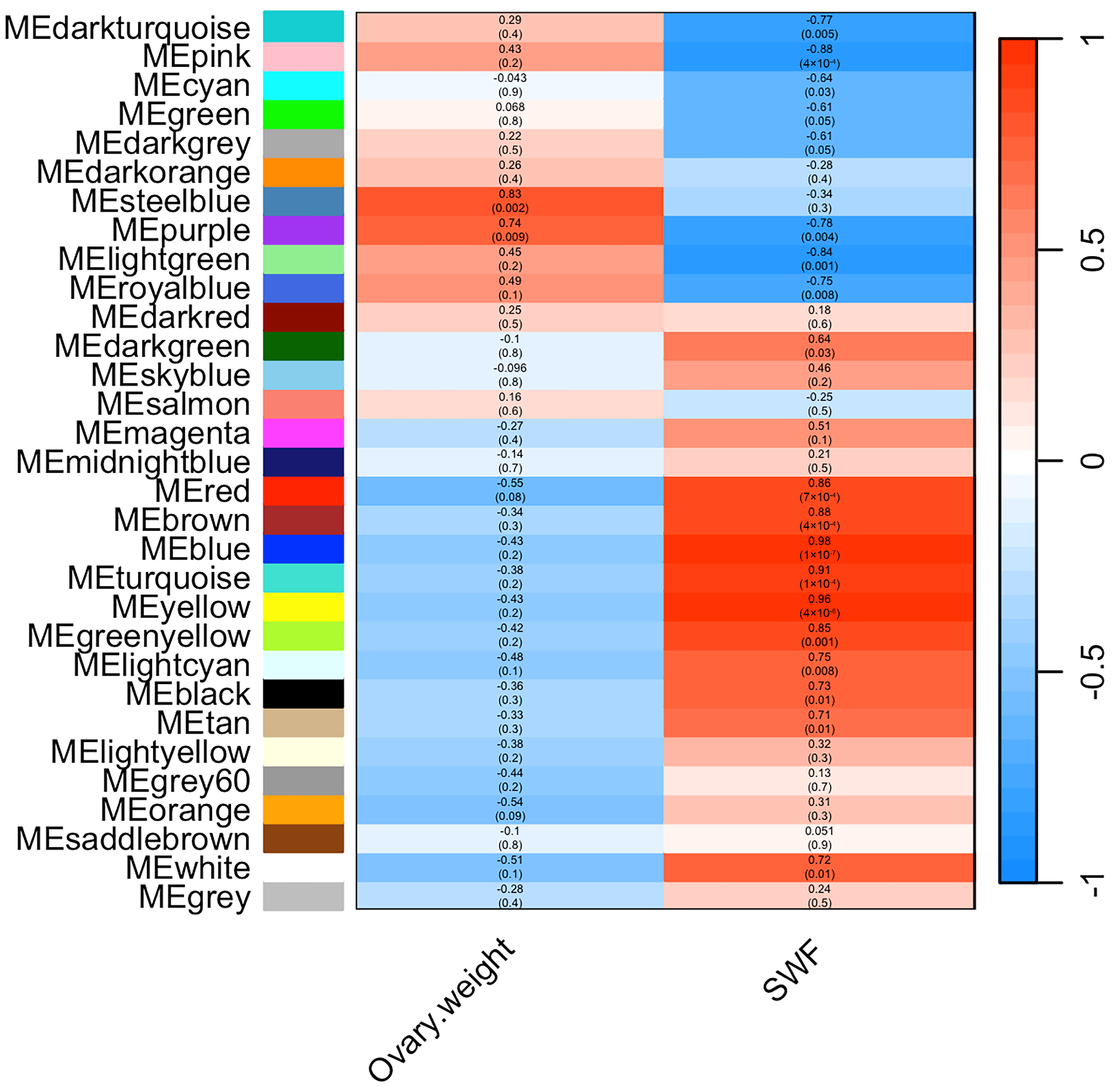
3.5. Weighted Gene Co-Expression Network Construction and Module Detection
3.6. Validation of the RNA-seq Data by Quantitative RT–PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bao, Q.; Gu, W.; Song, L.; Weng, K.; Cao, Z.; Zhang, Y.; Zhang, Y.; Ji, T.; Xu, Q.; Chen, G. The Photoperiod-Driven Cyclical Secretion of Pineal Melatonin Regulates Seasonal Reproduction in Geese (Anser cygnoides). Int. J. Mol. Sci. 2023, 24, 11998. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.D.; Backhouse, D.; Gous, R.M. Constant photoperiods and sexual maturity in broiler breeder pullets. Br. Poult. Sci. 2004, 45, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Gongruttananun, N. Influence of red light on reproductive performance, eggshell ultrastructure, and eye morphology in Thai-native hens. Poult. Sci. 2011, 90, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.; Joseph, N.; Osborne, V.; Bédécarrats, G. Red light is necessary to activate the reproductive axis in chickens independently of the retina of the eye. Poult. Sci. 2014, 93, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Renema, R.A.; Robinson, F.E. Effects of light intensity from photostimulation in four strains of commercial egg layers: 1. Ovarian morphology and carcass parameters. Poult. Sci. 2001, 80, 1112–1120. [Google Scholar] [CrossRef]
- Sharp, P.J. Photoperiodic control of reproduction in the domestic hen. Poult. Sci. 1993, 72, 897–905. [Google Scholar] [CrossRef]
- Wang, C.M.; Chen, L.R.; Lee, S.R.; Jea, Y.S.; Kao, J.Y. Supplementary artificial light to increase egg production of geese under natural lighting conditions. Anim. Reprod. Sci. 2009, 113, 317–321. [Google Scholar] [CrossRef]
- Guo, B.; Zhao, S.; Shao, X.; Ding, W.; Shi, Z.; Tang, Z. Analyses of mathematical models for Yangzhou geese egg-laying curves. Anim. Reprod. Sci. 2019, 203, 10–24. [Google Scholar] [CrossRef]
- Lin, X.; Liu, X.; Guo, C.; Liu, M.; Mi, Y.; Zhang, C. Promotion of the prehierarchical follicle growth by postovulatory follicles involving PGE(2) -EP2 signaling in chickens. J. Cell Physiol. 2018, 233, 8984–8995. [Google Scholar] [CrossRef]
- Johnson, P.A. Follicle selection in the avian ovary. Reprod. Domest. Anim. 2012, 47 (Suppl. 4), 283–287. [Google Scholar] [CrossRef]
- Johnson, A.L. Ovarian follicle selection and granulosa cell differentiation. Poult. Sci. 2015, 94, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Renema, R.A.; Robinson, F.E.; Oosterhoff, H.H.; Feddes, J.J.; Wilson, J.L. Effects of photostimulatory light intensity on ovarian morphology and carcass traits at sexual maturity in modern and antique egg-type pullets. Poult. Sci. 2001, 80, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.T.; Hou, N.N.; Wang, X.J.; Jiao, H.C.; Zhao, J.P.; Song, Z.G.; Lin, H. Increased hepatic yolk precursor synthesis, secretion and facilitated uptake by follicles are involved in the rejuvenation of reproductive performance of molted hens (Gallus gallus domesticus). Gen. Comp. Endocrinol. 2013, 194, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, T.; Akosman, M.S.; Yilmaz, O.; Ulutas, E.; Bulbul, A. Supplementary dietary nitric oxide donor (sodium nitroprusside) or inhibitor (NG-nitro-L-arginine methyl ester) depressed growth performance and ovarian primordial and primary follicles in Japanese quail (Coturnix coturnix japonica) in a dose-dependent manner. Br. Poult. Sci. 2015, 56, 113–120. [Google Scholar] [CrossRef]
- Long, L.; Wu, S.G.; Yuan, F.; Zhang, H.J.; Wang, J.; Qi, G.H. Effects of dietary octacosanol supplementation on laying performance, egg quality, serum hormone levels, and expression of genes related to the reproductive axis in laying hens. Poult. Sci. 2017, 96, 894–903. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Zhao, W.; Yuan, T.; Fu, Y.; Niu, D.; Chen, W.; Chen, L.; Lu, L. Seasonal differences in the transcriptome profile of the Zhedong white goose (Anser cygnoides) pituitary gland. Poult. Sci. 2021, 100, 1154–1166. [Google Scholar] [CrossRef]
- Du, L.; Gu, T.; Zhang, Y.; Huang, Z.; Wu, N.; Zhao, W.; Chang, G.; Xu, Q.; Chen, G. Transcriptome profiling to identify key mediators of granulosa cell proliferation upon FSH stimulation in the goose (Anser cygnoides). Br. Poult. Sci. 2018, 59, 416–421. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Zhang, Y.; Yao, Y.; Zhao, W.; Xu, Q.; Chen, G. Characterization of ovarian morphology and reproductive hormones in Zhedong white geese (Anser cygnoides domesticus) during the reproductive cycle. J. Anim. Physiol. Anim. Nutr. 2021, 105, 938–945. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic. Acids. Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Tilly, J.L.; Kowalski, K.I.; Johnson, A.L. Stage of ovarian follicular development associated with the initiation of steroidogenic competence in avian granulosa cells. Biol. Reprod. 1991, 44, 305–314. [Google Scholar] [CrossRef]
- Onagbesan, O.M.; Bruggeman, V.; Van As, P.; Tona, K.; Williams, J.; Decuypere, E. BMPs and BMPRs in chicken ovary and effects of BMP-4 and -7 on granulosa cell proliferation and progesterone production in vitro. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E973–E983. [Google Scholar] [CrossRef]
- Macias-Silva, M.; Hoodless, P.A.; Tang, S.J.; Buchwald, M.; Wrana, J.L. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J. Biol. Chem. 1998, 273, 25628–25636. [Google Scholar] [CrossRef]
- Miyazono, K.; Kusanagi, K.; Inoue, H. Divergence and convergence of TGF-beta/BMP signaling. J. Cell Physiol. 2001, 187, 265–276. [Google Scholar] [CrossRef]
- Vitt, U.A.; Mazerbourg, S.; Klein, C.; Hsueh, A.J. Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol. Reprod. 2002, 67, 473–480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adashi, E.Y.; Resnick, C.E.; Brodie, A.M.; Svoboda, M.E.; Van Wyk, J.J. Somatomedin-C-mediated potentiation of follicle-stimulating hormone-induced aromatase activity of cultured rat granulosa cells. Endocrinology 1985, 117, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Adashi, E.Y.; Resnick, C.E.; Ricciarelli, E.; Hurwitz, A.; Kokia, E.; Tedeschi, C.; Botero, L.; Hernandez, E.R.; Rosenfeld, R.G.; Carlsson-Skwirut, C.; et al. Granulosa cell-derived insulin-like growth factor (IGF) binding proteins are inhibitory to IGF-I hormonal action. Evidence derived from the use of a truncated IGF-I analogue. J. Clin. Investig. 1992, 90, 1593–1599. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Onagbesan, O.M.; Peddie, M.J. Effects of insulin-like growth factor I and interactions with transforming growth factor alpha and LH on proliferation of chicken granulosa cells and production of progesterone in culture. J. Reprod. Fertil. 1995, 104, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Onagbesan, O.M.; Vleugels, B.; Buys, N.; Bruggeman, V.; Safi, M.; Decuypere, E. Insulin-like growth factors in the regulation of avian ovarian functions. Domest. Anim. Endocrinol. 1999, 17, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Chabrolle, C.; Crochet, S.; Tesseraud, S.; Dupont, J. IGF-1 receptor signaling pathways and effects of AMPK activation on IGF-1-induced progesterone secretion in hen granulosa cells. Domest. Anim. Endocrinol. 2008, 34, 204–216. [Google Scholar] [CrossRef]
- Nakamura, I.; Kusakabe, M.; Swanson, P.; Young, G. Regulation of sex steroid production and mRNAs encoding gonadotropin receptors and steroidogenic proteins by gonadotropins, cyclic AMP and insulin-like growth factor-I in ovarian follicles of rainbow trout (Oncorhynchus mykiss) at two stages of vitellogenesis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 201, 132–140. [Google Scholar] [CrossRef]
- Onagbesan, O.M.; Decuypere, E.; Leenstra, F.; Ehlhardt, D.A. Differential effects of amount of feeding on cell proliferation and progesterone production in response to gonadotrophins and insulin-like growth factor I by ovarian granulosa cells of broiler breeder chickens selected for fatness or leanness. J. Reprod. Fertil. 1999, 116, 73–85. [Google Scholar] [CrossRef][Green Version]
- Onagbesan, O.M.; Mast, J.; Goddeeris, B.; Decuypere, E. Effect of TNF-alpha on LH and IGF-I modulated chicken granulosa cell proliferation and progesterone production during follicular development. J. Reprod. Fertil. 2000, 120, 433–442. [Google Scholar] [CrossRef]
- Lovell, T.M.; Gladwell, R.T.; Groome, N.P.; Knight, P.G. Modulatory effects of gonadotrophins and insulin-like growth factor on the secretion of inhibin A and progesterone by granulosa cells from chicken preovulatory (F1-F3) follicles. Reproduction 2002, 123, 291–300. [Google Scholar] [CrossRef]
- Nitta, H.; Osawa, Y.; Bahr, J.M. Immunolocalization of steroidogenic cells in small follicles of the chicken ovary: Anatomical arrangement and location of steroidogenic cells change during follicular development. Domest. Anim. Endocrinol. 1991, 8, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Marrone, B.L.; Hertelendy, F. Steroid metabolism by avian ovarian cells during follicular maturation. Biol. Reprod. 1983, 29, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Ma, Y.; Zhao, D.; Mi, Y.; Zhang, C. Transcriptome profiling analysis of underlying regulation of growing follicle development in the chicken. Poult. Sci. 2020, 99, 2861–2872. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-H.; Chen, M.-J. The effect of steroid hormones on ovarian follicle development. Vitam. Horm. 2018, 107, 155–175. [Google Scholar]
- Zi, X.D.; Lu, J.Y.; Zhou, H.; Ma, L.; Xia, W.; Xiong, X.R.; Lan, D.L.; Wu, X.H. Comparative analysis of ovarian transcriptomes between prolific and non-prolific goat breeds via high-throughput sequencing. Reprod. Domest. Anim. 2018, 53, 344–351. [Google Scholar] [CrossRef]
- Ling, Y.H.; Quan, Q.; Xiang, H.; Zhu, L.; Chu, M.X.; Zhang, X.R.; Han, C.Y. Expression profiles of differentially expressed genes affecting fecundity in goat ovarian tissues. Genet. Mol. Res. 2015, 14, 18743–18752. [Google Scholar] [CrossRef]
- Chen, P.R.; Shin, S.; Choi, Y.M.; Kim, E.; Han, J.Y.; Lee, K. Overexpression of G0/G1 Switch Gene 2 in Adipose Tissue of Transgenic Quail Inhibits Lipolysis Associated with Egg Laying. Int. J. Mol. Sci. 2016, 17, 384. [Google Scholar] [CrossRef]
- Yang, S.; Suh, Y.; Choi, Y.M.; Shin, S.; Han, J.Y.; Lee, K. Loss of fat with increased adipose triglyceride lipase-mediated lipolysis in adipose tissue during laying stages in quail. Lipids 2013, 48, 13–21. [Google Scholar] [CrossRef]
- Ahn, J.; Oh, S.A.; Suh, Y.; Moeller, S.J.; Lee, K. Porcine G(0)/G(1) switch gene 2 (G0S2) expression is regulated during adipogenesis and short-term in-vivo nutritional interventions. Lipids 2013, 48, 209–218. [Google Scholar] [CrossRef]
- Lu, X.; Yang, X.; Liu, J. Differential control of ATGL-mediated lipid droplet degradation by CGI-58 and G0S2. Cell Cycle 2010, 9, 2719–2725. [Google Scholar] [CrossRef]
- Oh, S.A.; Suh, Y.; Pang, M.G.; Lee, K. Cloning of avian G(0)/G(1) switch gene 2 genes and developmental and nutritional regulation of G(0)/G(1) switch gene 2 in chicken adipose tissue. J. Anim. Sci. 2011, 89, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Park, J.; Lee, J.H.; Park, J.W.; Park, B.C. Disruption of G(0)/G(1) switch gene 2 (G0S2) reduced abdominal fat deposition and altered fatty acid composition in chicken. FASEB J. 2019, 33, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Gillan, L.; Matei, D.; Fishman, D.A.; Gerbin, C.S.; Karlan, B.Y.; Chang, D.D. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002, 62, 5358–5364. [Google Scholar] [PubMed]
- Jing, Y.; Shan, X.; Mu, F.; Qin, N.; Zhu, H.; Liu, D.; Yuan, S.; Xu, R. Associations of the Novel Polymorphisms of Periostin and Platelet-Derived Growth Factor Receptor-Like Genes with Egg Production Traits in Local Chinese Dagu Hens. Anim. Biotechnol. 2016, 27, 208–216. [Google Scholar] [CrossRef]
- Nie, X.; Shen, C.; Tan, J.; Wu, Z.; Wang, W.; Chen, Y.; Dai, Y.; Yang, X.; Ye, S.; Chen, J.; et al. Periostin: A Potential Therapeutic Target for Pulmonary Hypertension? Circ. Res. 2020, 127, 1138–1152. [Google Scholar] [CrossRef]
- Xiao, Z.M.; Wang, X.Y.; Wang, A.M. Periostin induces chemoresistance in colon cancer cells through activation of the PI3K/Akt/survivin pathway. Biotechnol. Appl. Biochem. 2015, 62, 401–406. [Google Scholar] [CrossRef]
- Otoukesh, B.; Abbasi, M.; Gorgani, H.O.; Farahini, H.; Moghtadaei, M.; Boddouhi, B.; Kaghazian, P.; Hosseinzadeh, S.; Alaee, A. MicroRNAs signatures, bioinformatics analysis of miRNAs, miRNA mimics and antagonists, and miRNA therapeutics in osteosarcoma. Cancer. Cell Int. 2020, 20, 254. [Google Scholar] [CrossRef]
- Smith, S.K. Angiogenesis and reproduction. Br. J. Obstet. Gynaecol. 2001, 108, 777–783. [Google Scholar] [CrossRef]
- Chen, X.; Sun, X.; Chimbaka, I.M.; Qin, N.; Xu, X.; Liswaniso, S.; Xu, R.; Gonzalez, J.M. Transcriptome Analysis of Ovarian Follicles Reveals Potential Pivotal Genes Associated with Increased and Decreased Rates of Chicken Egg Production. Front. Genet. 2021, 12, 622751. [Google Scholar] [CrossRef]
- Conconi, M.T.; Spinazzi, R.; Nussdorfer, G.G. Endogenous ligands of PACAP/VIP receptors in the autocrine-paracrine regulation of the adrenal gland. Int. Rev. Cytol. 2006, 249, 1–51. [Google Scholar] [CrossRef]
- Sun, X.; Chen, X.; Zhao, J.; Ma, C.; Yan, C.; Liswaniso, S.; Xu, R.; Qin, N. Transcriptome comparative analysis of ovarian follicles reveals the key genes and signaling pathways implicated in hen egg production. BMC Genom. 2021, 22, 899. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Fei, M.; Zhou, X.; He, K.; Yang, S.; Zhao, A. Effects of Different Photoperiods on the Transcriptome of the Ovary and Small White Follicles in Zhedong White Geese. Animals 2024, 14, 2747. https://doi.org/10.3390/ani14182747
Huang T, Fei M, Zhou X, He K, Yang S, Zhao A. Effects of Different Photoperiods on the Transcriptome of the Ovary and Small White Follicles in Zhedong White Geese. Animals. 2024; 14(18):2747. https://doi.org/10.3390/ani14182747
Chicago/Turabian StyleHuang, Tao, Meina Fei, Xiaolong Zhou, Ke He, Songbai Yang, and Ayong Zhao. 2024. "Effects of Different Photoperiods on the Transcriptome of the Ovary and Small White Follicles in Zhedong White Geese" Animals 14, no. 18: 2747. https://doi.org/10.3390/ani14182747
APA StyleHuang, T., Fei, M., Zhou, X., He, K., Yang, S., & Zhao, A. (2024). Effects of Different Photoperiods on the Transcriptome of the Ovary and Small White Follicles in Zhedong White Geese. Animals, 14(18), 2747. https://doi.org/10.3390/ani14182747




