Immunohistochemical Expression of PTEN in Canine Gliomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Histology and Morphological Diagnosis
2.3. Immunohistochemistry
3. Results
3.1. Clinicopathological Features
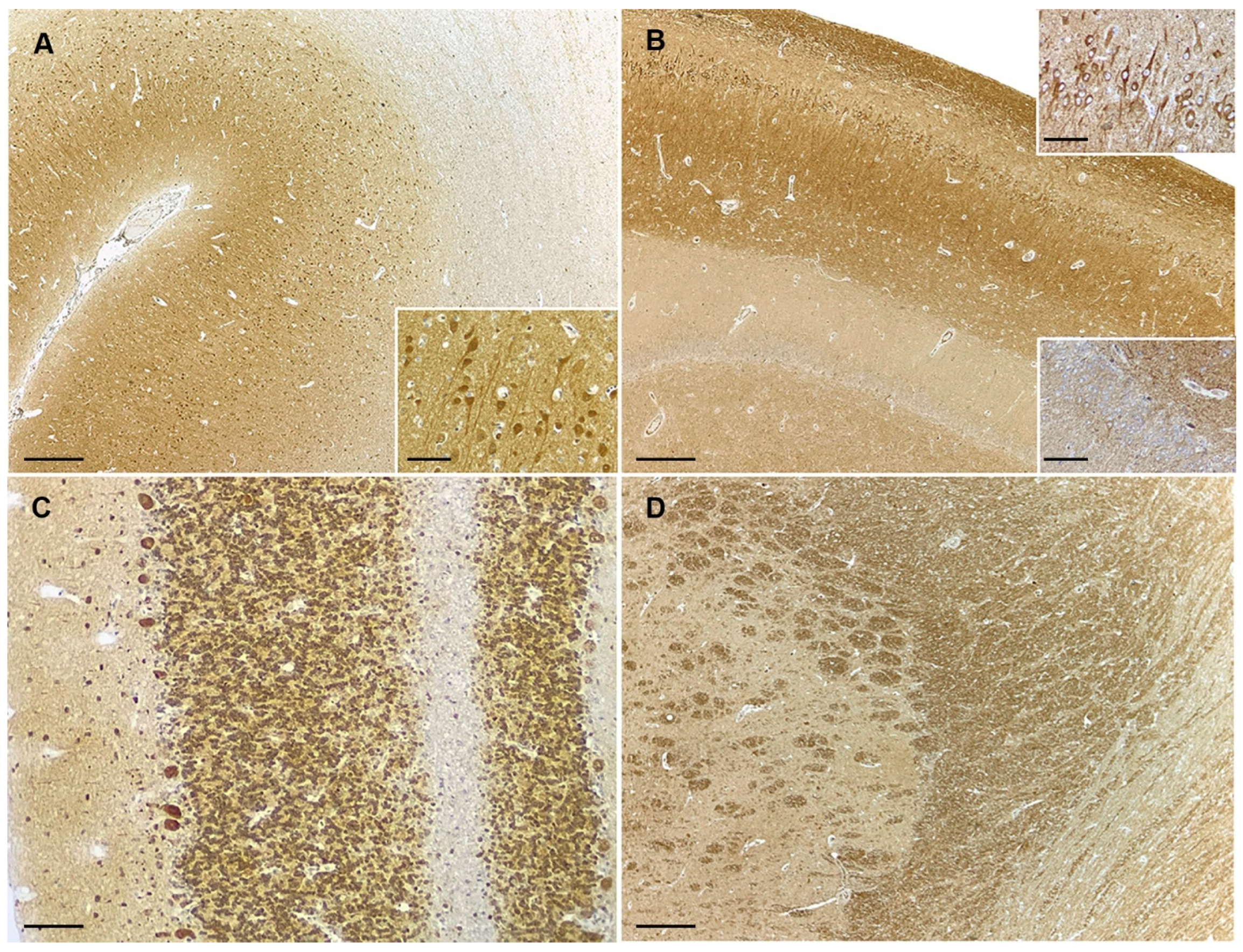
3.2. PTEN Immunohistochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jose-Lopez, R.; Gutierrez-Quintana, R.; de la Fuente, C.; Manzanilla, E.G.; Sunol, A.; Pi Castro, D.; Anor, S.; Sanchez-Masian, D.; Fernandez-Flores, F.; Ricci, E.; et al. Clinical features, diagnosis, and survival analysis of dogs with glioma. J. Vet. Intern. Med. 2021, 35, 1902–1917. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.M.; Shofer, F.S.; Van Winkle, T.J.; Massicotte, C. Canine intracranial primary neoplasia: 173 cases (1986–2003). J. Vet. Intern. Med. 2006, 20, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Song, R.B.; Vite, C.H.; Bradley, C.W.; Cross, J.R. Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, age, and body weight. J. Vet. Intern. Med. 2013, 27, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.W.; Miller, A.D.; Miller, C.R.; Porter, B.; Aldape, K.; Beck, J.; Brat, D.; Cornax, I.; Corps, K.; Frank, C.; et al. A Revised Diagnostic Classification of Canine Glioma: Towards Validation of the Canine Glioma Patient as a Naturally Occurring Preclinical Model for Human Glioma. J. Neuropathol. Exp. Neurol. 2018, 77, 1039–1054. [Google Scholar] [CrossRef]
- Krane, G.A.; Shockley, K.R.; Malarkey, D.E.; Miller, A.D.; Miller, C.R.; Tokarz, D.A.; Jensen, H.L.; Janardhan, K.S.; Breen, M.; Mariani, C.L. Inter-pathologist agreement on diagnosis, classification and grading of canine glioma. Vet. Comp. Oncol. 2022, 20, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Herranz, C.; Fernandez, F.; Martin-Ibanez, R.; Blasco, E.; Crespo, E.; De la Fuente, C.; Anor, S.; Rabanal, R.M.; Canals, J.M.; Pumarola, M. Spontaneously Arising Canine Glioma as a Potential Model for Human Glioma. J. Comp. Pathol. 2016, 154, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R.T.; Ahmed, A.U.; Yanke, A.B.; Cohen-Gadol, A.A.; Dey, M. Dogs are man’s best friend: In sickness and in health. Neuro-Oncol. 2017, 19, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.D.; Miller, C.R.; Rossmeisl, J.H. Canine Primary Intracranial Cancer: A Clinicopathologic and Comparative Review of Glioma, Meningioma, and Choroid Plexus Tumors. Front. Oncol. 2019, 9, 1151. [Google Scholar] [CrossRef]
- Mitchell, D.; Chintala, S.; Fetcko, K.; Henriquez, M.; Tewari, B.N.; Ahmed, A.; Bentley, R.T.; Dey, M. Common Molecular Alterations in Canine Oligodendroglioma and Human Malignant Gliomas and Potential Novel Therapeutic Targets. Front. Oncol. 2019, 9, 780. [Google Scholar] [CrossRef]
- Boudreau, C.E.; York, D.; Higgins, R.J.; LeCouteur, R.A.; Dickinson, P.J. Molecular signalling pathways in canine gliomas. Vet. Comp. Oncol. 2017, 15, 133–150. [Google Scholar] [CrossRef]
- Filley, A.; Henriquez, M.; Bhowmik, T.; Tewari, B.N.; Rao, X.; Wan, J.; Miller, M.A.; Liu, Y.; Bentley, R.T.; Dey, M. Immunologic and gene expression profiles of spontaneous canine oligodendrogliomas. J. Neurooncol. 2018, 137, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Hollander, M.C.; Blumenthal, G.M.; Dennis, P.A. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer 2011, 11, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, J.; He, L.; Stiles, B.L. PTEN: Tumor Suppressor and Metabolic Regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin. Cancer Biol. 2019, 59, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Luongo, F.; Colonna, F.; Calapa, F.; Vitale, S.; Fiori, M.E.; De Maria, R. PTEN Tumor-Suppressor: The Dam of Stemness in Cancer. Cancers 2019, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Etemad, S.; Rezaei, S.; Ziaolhagh, S.; Rajabi, R.; Rahmanian, P.; Abdi, S.; Koohpar, Z.K.; Rafiei, R.; Raei, B.; et al. Progress in targeting PTEN/PI3K/Akt axis in glioblastoma therapy: Revisiting molecular interactions. Biomed. Pharmacother. 2023, 158, 114204. [Google Scholar] [CrossRef] [PubMed]
- Koul, D. PTEN signaling pathways in glioblastoma. Cancer Biol. Ther. 2008, 7, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Endersby, R.; Baker, S.J. PTEN signaling in brain: Neuropathology and tumorigenesis. Oncogene 2008, 27, 5416–5430. [Google Scholar] [CrossRef]
- Dickinson, P.J.; York, D.; Higgins, R.J.; LeCouteur, R.A.; Joshi, N.; Bannasch, D. Chromosomal Aberrations in Canine Gliomas Define Candidate Genes and Common Pathways in Dogs and Humans. J. Neuropathol. Exp. Neurol. 2016, 75, 700–710. [Google Scholar] [CrossRef]
- Higgins, R.J.; Dickinson, P.J.; LeCouteur, R.A.; Bollen, A.W.; Wang, H.; Wang, H.; Corely, L.J.; Moore, L.M.; Zang, W.; Fuller, G.N. Spontaneous canine gliomas: Overexpression of EGFR, PDGFRalpha and IGFBP2 demonstrated by tissue microarray immunophenotyping. J. Neurooncol. 2010, 98, 49–55. [Google Scholar] [CrossRef]
- Dickinson, P.J.; Sturges, B.K.; Higgins, R.J.; Roberts, B.N.; Leutenegger, C.M.; Bollen, A.W.; LeCouteur, R.A. Vascular endothelial growth factor mRNA expression and peritumoral edema in canine primary central nervous system tumors. Vet. Pathol. 2008, 45, 131–139. [Google Scholar] [CrossRef]
- Dickinson, P.J.; Roberts, B.N.; Higgins, R.J.; Leutenegger, C.M.; Bollen, A.W.; Kass, P.H.; LeCouteur, R.A. Expression of receptor tyrosine kinases VEGFR-1 (FLT-1), VEGFR-2 (KDR), EGFR-1, PDGFRalpha and c-Met in canine primary brain tumours. Vet. Comp. Oncol. 2006, 4, 132–140. [Google Scholar] [CrossRef]
- Ide, T.; Uchida, K.; Kikuta, F.; Suzuki, K.; Nakayama, H. Immunohistochemical characterization of canine neuroepithelial tumors. Vet. Pathol. 2010, 47, 741–750. [Google Scholar] [CrossRef]
- Koenig, A.; Bianco, S.R.; Fosmire, S.; Wojcieszyn, J.; Modiano, J.F. Expression and significance of p53, rb, p21/waf-1, p16/ink-4a, and PTEN tumor suppressors in canine melanoma. Vet. Pathol. 2002, 39, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, E.B.; Thomas, R.; Fosmire, S.P.; Lamerato-Kozicki, A.R.; Bianco, S.R.; Wojcieszyn, J.W.; Breen, M.; Helfand, S.C.; Modiano, J.F. Mutations of phosphatase and tensin homolog deleted from chromosome 10 in canine hemangiosarcoma. Vet. Pathol. 2005, 42, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.A.; Forest, T.; Smith, C. Tumor suppressor PTEN is mutated in canine osteosarcoma cell lines and tumors. Vet. Pathol. 2002, 39, 372–378. [Google Scholar] [CrossRef]
- Russell, D.S.; Jaworski, L.; Kisseberth, W.C. Immunohistochemical detection of p53, PTEN, Rb, and p16 in canine osteosarcoma using tissue microarray. J. Vet. Diagn. Investig. 2018, 30, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Calderon, L.G.; Fonseca-Alves, C.E.; Kobayashi, P.E.; Carvalho, M.; Drigo, S.A.; de Oliveira Vasconcelos, R.; Laufer-Amorim, R. Alterations in PTEN, MDM2, TP53 and AR protein and gene expression are associated with canine prostate carcinogenesis. Res. Vet. Sci. 2016, 106, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.W.; Lin, D.G.; Wang, J.Q.; Li, C.Y.; Deng, G.Z. Expression and significance of PTEN and VEGF in canine mammary gland tumours. Vet. Res. Commun. 2008, 32, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Ressel, L.; Millanta, F.; Caleri, E.; Innocenti, V.M.; Poli, A. Reduced PTEN protein expression and its prognostic implications in canine and feline mammary tumors. Vet. Pathol. 2009, 46, 860–868. [Google Scholar] [CrossRef]
- Asproni, P.; Millanta, F.; Ressel, L.; Podesta, F.; Parisi, F.; Vannozzi, I.; Poli, A. An Immunohistochemical Study of the PTEN/AKT Pathway Involvement in Canine and Feline Mammary Tumors. Animals 2021, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Seung, B.J.; Cho, S.H.; Lim, H.Y.; Bae, M.K.; Sur, J.H. Dysregulation of PI3K/Akt/PTEN Pathway in Canine Mammary Tumor. Animals 2021, 11, 2079. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.B.; Anderson, K.J.; Boudreau, C.E.; Martinez-Ledesma, E.; Kocakavuk, E.; Johnson, K.C.; Barthel, F.P.; Varn, F.S.; Kassab, C.; Ling, X.; et al. Comparative Molecular Life History of Spontaneous Canine and Human Gliomas. Cancer Cell 2020, 37, 243–257.e7. [Google Scholar] [CrossRef] [PubMed]
- Pi Castro, D.; Jose-Lopez, R.; Fernandez Flores, F.; Rabanal Prados, R.M.; Mandara, M.T.; Arus, C.; Pumarola Batlle, M. Expression of FOXP3 in Canine Gliomas: Immunohistochemical Study of Tumor-Infiltrating Regulatory Lymphocytes. J. Neuropathol. Exp. Neurol. 2020, 79, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Wiencke, J.K.; Zheng, S.; Jelluma, N.; Tihan, T.; Vandenberg, S.; Tamguney, T.; Baumber, R.; Parsons, R.; Lamborn, K.R.; Berger, M.S.; et al. Methylation of the PTEN promoter defines low-grade gliomas and secondary glioblastoma. Neuro-Oncol. 2007, 9, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Cecener, G.; Tunca, B.; Egeli, U.; Bekar, A.; Guler, G.; Vatan, O.; Tolunay, S. Investigation of MMAC/PTEN gene mutations and protein expression in low grade gliomas. Cell Mol. Neurobiol. 2009, 29, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Carico, C.; Nuno, M.; Mukherjee, D.; Elramsisy, A.; Dantis, J.; Hu, J.; Rudnick, J.; Yu, J.S.; Black, K.L.; Bannykh, S.I.; et al. Loss of PTEN is not associated with poor survival in newly diagnosed glioblastoma patients of the temozolomide era. PLoS ONE 2012, 7, e33684. [Google Scholar] [CrossRef] [PubMed]
- Baeza, N.; Weller, M.; Yonekawa, Y.; Kleihues, P.; Ohgaki, H. PTEN methylation and expression in glioblastomas. Acta Neuropathol. 2003, 106, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Idoate, M.A.; Soria, E.; Lozano, M.D.; Sola, J.J.; Panizo, A.; de Alava, E.; Manrique, M.; Pardo-Mindan, F.J. PTEN protein expression correlates with PTEN gene molecular changes but not with VEGF expression in astrocytomas. Diagn. Mol. Pathol. 2003, 12, 160–165. [Google Scholar] [CrossRef]
- Idoate, M.A.; Echeveste, J.; Diez-Valle, R.; Lozano, M.D.; Aristu, J. Biological and clinical significance of the intratumour heterogeneity of PTEN protein expression and the corresponding molecular abnormalities of the PTEN gene in glioblastomas. Neuropathol. Appl. Neurobiol. 2014, 40, 736–746. [Google Scholar] [CrossRef]
- Sano, T.; Lin, H.; Chen, X.; Langford, L.A.; Koul, D.; Bondy, M.L.; Hess, K.R.; Myers, J.N.; Hong, Y.K.; Yung, W.K.; et al. Differential expression of MMAC/PTEN in glioblastoma multiforme: Relationship to localization and prognosis. Cancer Res. 1999, 59, 1820–1824. [Google Scholar] [PubMed]
- Fraser, M.M.; Bayazitov, I.T.; Zakharenko, S.S.; Baker, S.J. Phosphatase and tensin homolog, deleted on chromosome 10 deficiency in brain causes defects in synaptic structure, transmission and plasticity, and myelination abnormalities. Neuroscience 2008, 151, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.Y.; Chen, X.S.; Zhong, S.C.; Luo, X.; Yao, Z.X. Differential expression of PTEN in normal adult rat brain and upregulation of PTEN and p-Akt in the ischemic cerebral cortex. Anat. Rec. 2009, 292, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Teugels, E.; Sadones, J.; De Brakeleer, S.; Duerinck, J.; Du Four, S.; Michotte, A.; De Greve, J.; Neyns, B. Correlation of EGFR, IDH1 and PTEN status with the outcome of patients with recurrent glioblastoma treated in a phase II clinical trial with the EGFR-blocking monoclonal antibody cetuximab. Int. J. Oncol. 2012, 41, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Ballester, L.Y.; Fuller, G.N.; Powell, S.Z.; Sulman, E.P.; Patel, K.P.; Luthra, R.; Routbort, M.J. Retrospective Analysis of Molecular and Immunohistochemical Characterization of 381 Primary Brain Tumors. J. Neuropathol. Exp. Neurol. 2017, 76, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Fults, D.; Pedone, C. Immunocytochemical mapping of the phosphatase and tensin homolog (PTEN/MMAC1) tumor suppressor protein in human gliomas. Neuro-Oncol. 2000, 2, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, A.; Golanov, A.; Sycheva, R. Immunohistochemical markers for prognosis of anaplastic astrocytomas. J. Neurooncol. 2002, 58, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Thorarinsdottir, H.K.; Santi, M.; McCarter, R.; Rushing, E.J.; Cornelison, R.; Jales, A.; MacDonald, T.J. Protein expression of platelet-derived growth factor receptor correlates with malignant histology and PTEN with survival in childhood gliomas. Clin. Cancer Res. 2008, 14, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Serra, H.; Chivite, I.; Angulo-Urarte, A.; Soler, A.; Sutherland, J.D.; Arruabarrena-Aristorena, A.; Ragab, A.; Lim, R.; Malumbres, M.; Fruttiger, M.; et al. PTEN mediates Notch-dependent stalk cell arrest in angiogenesis. Nat. Commun. 2015, 6, 7935. [Google Scholar] [CrossRef]
- Rodriguez, S.; Huynh-Do, U. The Role of PTEN in Tumor Angiogenesis. J. Oncol. 2012, 2012, 141236. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Luzzi, S. Immune Landscape in PTEN-Related Glioma Microenvironment: A Bioinformatic Analysis. Brain Sci. 2022, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Vidotto, T.; Melo, C.M.; Castelli, E.; Koti, M.; Dos Reis, R.B.; Squire, J.A. Emerging role of PTEN loss in evasion of the immune response to tumours. Br. J. Cancer 2020, 122, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Cretella, D.; Digiacomo, G.; Giovannetti, E.; Cavazzoni, A. PTEN Alterations as a Potential Mechanism for Tumor Cell Escape from PD-1/PD-L1 Inhibition. Cancers 2019, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Chan, L.C.; Li, C.W.; Hsu, J.L.; Hung, M.C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Q.; Liu, F.; Qiu, X.Y.; Chen, X.Q. The Prognostic and Therapeutic Value of PD-L1 in Glioma. Front. Pharmacol. 2018, 9, 1503. [Google Scholar] [CrossRef] [PubMed]
- Krane, G.A.; O’Dea, C.A.; Malarkey, D.E.; Miller, A.D.; Miller, C.R.; Tokarz, D.A.; Jensen, H.L.; Janardhan, K.S.; Shockley, K.R.; Flagler, N.; et al. Immunohistochemical evaluation of immune cell infiltration in canine gliomas. Vet. Pathol. 2021, 58, 952–963. [Google Scholar] [CrossRef]
- José-López, R. Characterizing Canine Glioma as a Naturally Occurring Model for Immune Evasion in Human Glioma; Universitat Autònoma de Barcelona: Barcelona, Spain, 2021. [Google Scholar]
- Litak, J.; Mazurek, M.; Grochowski, C.; Kamieniak, P.; Rolinski, J. PD-L1/PD-1 Axis in Glioblastoma Multiforme. Int. J. Mol. Sci. 2019, 20, 5347. [Google Scholar] [CrossRef]



| Case | Breed | Sex | Age (Years) | Tumor Location | Diagnosis |
|---|---|---|---|---|---|
| 1 | Boxer | M | 8.0 | Diencephalic | HA |
| 2 | Boxer | FN | 4.8 | Infratentorial (mesencephalon) | HA |
| 3 | Boxer | M | 9.0 | Infratentorial (cerebellum) | HA |
| 4 | French bulldog | FN | 10.0 | Hemispheric (parietal) | HA |
| 5 | Boxer | F | 8.3 | Hemispheric (temporal) | HA |
| 6 | Staffordshire bull terrier | M | 11.7 | Hemispheric (fronto-olfactory) | HA |
| 7 | Boxer | MN | 8.4 | Infratentorial (mesencephalon) | HA |
| 8 | Boxer | M | 9.8 | Hemispheric (fronto-olfactory) | HA |
| 9 | Polish Tatra Sheepdog | FN | 7.0 | Hemispheric (parietal) | HA |
| 10 | French bulldog | MN | 8.0 | Hemispheric (temporal) | HA |
| 11 | German Shepherd | M | 5.0 | Hemispheric (occipital) | HA |
| 12 | French bulldog | F | 7.0 | Infratentorial (mesencephalon) | HA |
| 13 | Boxer | FN | 8.0 | Hemispheric (temporal) | HA |
| 14 | French bulldog | F | 2.0 | Diencephalic | HO |
| 15 | Yorkshire terrier | F | 13.0 | Hemispheric (fronto-olfactory) | HO |
| 16 | French bulldog | F | 8.2 | Diencephalic | HO |
| 17 | Dogue de Bordeaux | M | 3.7 | Hemispheric (parietal) | HO |
| 18 | Border Terrier | MN | 5.0 | Diencephalic | HO |
| 19 | West Highland white terrier | MN | 9.3 | Hemispheric (parietal) | HO |
| 20 | Staffordshire bull terrier | MN | 7.5 | Diencephalic | HO |
| 21 | Boxer | FN | 8.6 | Hemispheric (fronto-olfactory) | HO |
| 22 | German Shepherd | FN | 7.5 | Hemispheric (temporal) | HO |
| 23 | French bulldog | F | 3.2 | Hemispheric (fronto-olfactory) | HO |
| 24 | Boxer | F | 6.3 | Hemispheric (fronto-olfactory) | HO |
| 25 | French bulldog | F | 9.0 | Hemispheric (temporal) | HO |
| 26 | French bulldog | F | 7.5 | Hemispheric (fronto-olfactory) | HO |
| 27 | Boxer | F | 7.7 | Hemispheric (temporal) | HO |
| 28 | French bulldog | M | 10.0 | Hemispheric (temporo-parietal) | HO |
| 29 | French bulldog. | F | 6.0 | Hemispheric (temporal) | HO |
| 30 | French bulldog | MN | 9.0 | Hemispheric (temporal) | HO |
| 31 | French bulldog | F | 9.0 | Hemispheric (fronto-olfactory) | HO |
| 32 | Boxer | F | 7.5 | Diencephalic | LO |
| 33 | French bulldog | F | 10.2 | Hemispheric (temporal) | HO |
| 34 | French bulldog | M | 8.0 | Hemispheric (temporal) | HU |
| 35 | French bulldog | F | 9.0 | Hemispheric (frontal) | HU |
| 36 | Mongrel | F | 6.5 | Diencephalic | HU |
| 37 | French bulldog | M | 10.4 | Diencephalic | HU |
| PTEN IHC | HO | HA | HU | LO | TOTAL |
|---|---|---|---|---|---|
| Expression | |||||
| High | 12/19 (63.2%) | 4/13 (30.8%) | 2/4 (50%)) | 1/1 (100%) | 19/37 (52.6%) |
| Reduced | 3/19 (15.8%) | 2/13 (15.4%) | 0/4 (0%) | 0/1 (0%) | 5/37 (13.5%) |
| Highly reduced | 4/19 (21%) | 7/13 (53.8%) | 2/4 (50%) | 0/1 (0%) | 13/37 (35.1%) |
| Pattern | |||||
| Diffuse positive | 10/19 (52.6%) | 4/13 (30.8%) | 0/4 (0%) | 1/1 (100%) | 15/37 (40.5%) |
| Heterogeneous | 8/19 (42.1%) | 6/13 (46.1%) | 3/4 (75%) | 0/1 (0%) | 17/37 (46%) |
| Diffuse negative | 1/19 (5.3%) | 3/13 (23.1%) | 1/4 (25%) | 0/1 (0%) | 5/37 (13.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molín, J.; José-López, R.; Ramírez, G.A.; Pumarola, M. Immunohistochemical Expression of PTEN in Canine Gliomas. Animals 2024, 14, 2115. https://doi.org/10.3390/ani14142115
Molín J, José-López R, Ramírez GA, Pumarola M. Immunohistochemical Expression of PTEN in Canine Gliomas. Animals. 2024; 14(14):2115. https://doi.org/10.3390/ani14142115
Chicago/Turabian StyleMolín, Jéssica, Roberto José-López, Gustavo A. Ramírez, and Martí Pumarola. 2024. "Immunohistochemical Expression of PTEN in Canine Gliomas" Animals 14, no. 14: 2115. https://doi.org/10.3390/ani14142115
APA StyleMolín, J., José-López, R., Ramírez, G. A., & Pumarola, M. (2024). Immunohistochemical Expression of PTEN in Canine Gliomas. Animals, 14(14), 2115. https://doi.org/10.3390/ani14142115








