Assessing Movements between Freshwater and Saltwater by Brown Trout (Salmo trutta L.) Based on Otolith Microchemistry
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
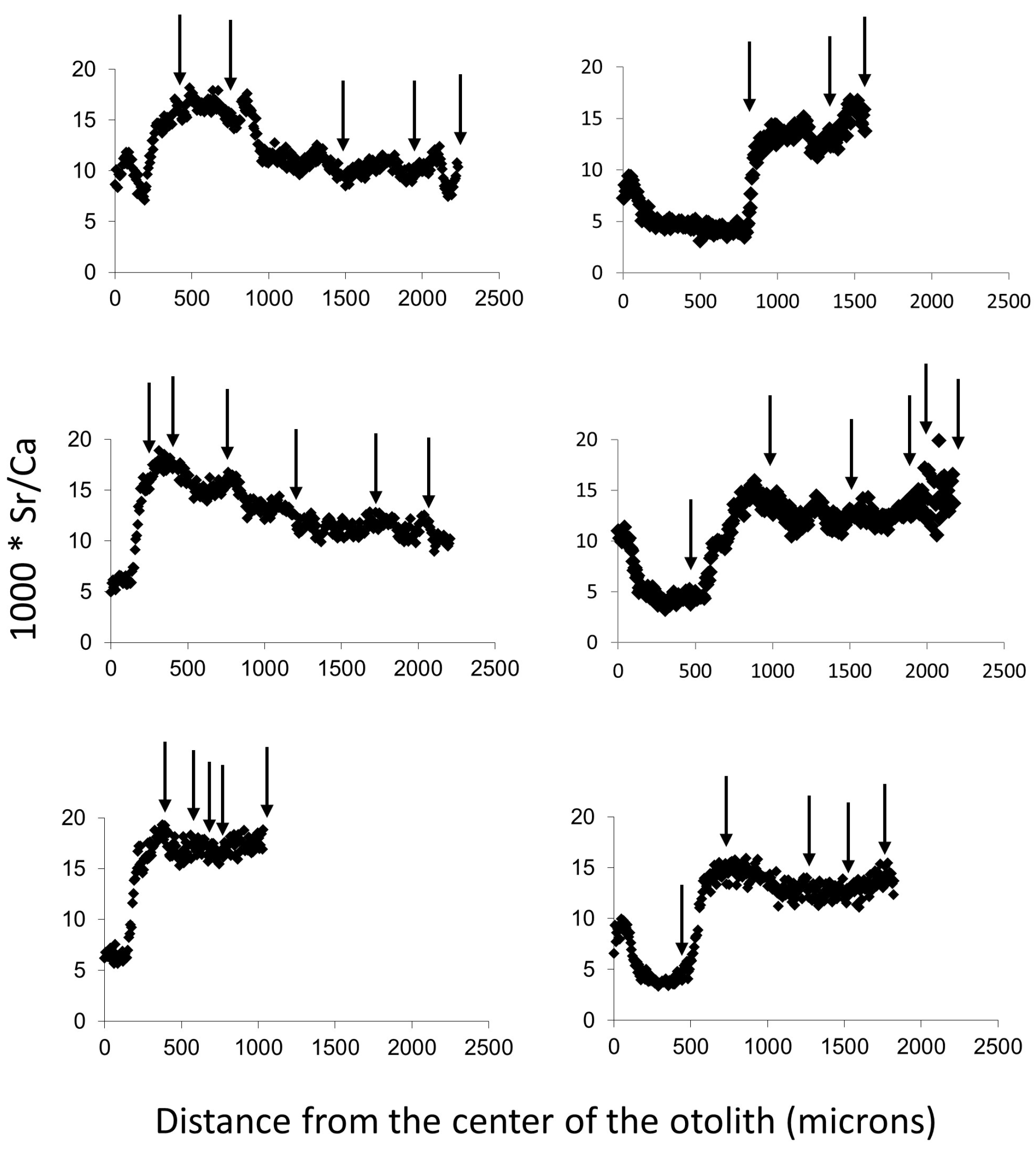
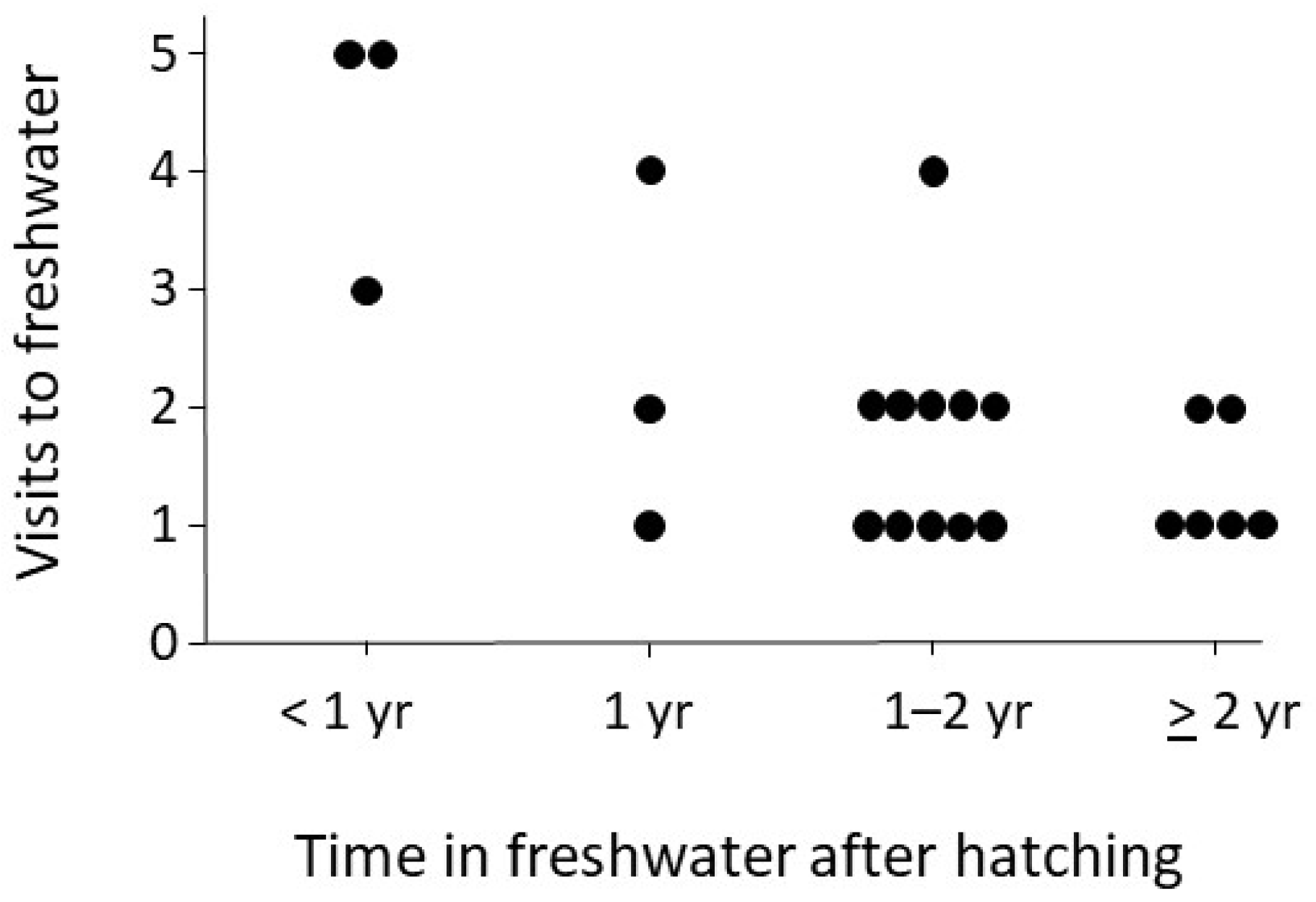
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drenner, S.M.; Clark, T.D.; Whitney, C.K.; Martins, E.G.; Cooke, S.J.; Hinch, S.G. A synthesis of tagging studies examining the behaviour and survival of anadromous salmonids in marine environments. PLoS ONE 2012, 7, e31311. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Thorstad, E.; Aarestrup, K. Overlooked aspects of the Salmo salar and Salmo trutta lifecycles. Rev. Fish Biol. Fish. 2019, 29, 749–766. [Google Scholar] [CrossRef]
- Strøm, J.; Jensen, J.; Nikolopoulos, A.; Nordi, E.; Bjørn, P.; Bøhn, T. Sea trout Salmo trutta in the subarctic: Home-bound but large variation in migratory behaviour between and within populations. J. Fish Biol. 2021, 99, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, J.J.; Ter Hofstede, R.; Winter, H.V. Sea growth of anadromous brown trout (Salmo trutta). J. Sea Res. 2007, 58, 163–165. [Google Scholar] [CrossRef]
- Hoar, W.S. Smolt transformation: Evolution, behavior, and physiology. J. Fish. Board Can. 1976, 33, 1233–1252. [Google Scholar] [CrossRef]
- Jonsson, B.; L’Abee-Lund, J.J. Latitudinal clines in life history variables of anadromous brown trout in Europe. J. Fish Biol. 1993, 43 (Suppl. A), 1–16. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Naturally and hatchery produced European trout Salmo trutta: Do their marine survival and dispersal differ? J. Coast. Conserv. 2014, 18, 79–87. [Google Scholar] [CrossRef]
- Alm, G. The sea-trout population of the Åva stream. Inst. Freshw. Res. Drottningholm Rep. 1950, 31, 26–56. [Google Scholar]
- Jonsson, B. Life history patterns of freshwater resident and sea-run migrant brown trout in Norway. Trans. Am. Fish. Soc. 1985, 114, 182–194. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Ecology of Atlantic Salmon and Brown Trout: Habitat as a Template for Life Histories; Fish and Fisheries Series 33; Springer: Dordrecht, The Netherlands, 2011; xxii + 708p. [Google Scholar]
- Landergren, P. Factors affecting early migration of sea trout Salmo trutta parr to brackish water. Fish. Res. 2004, 67, 283–294. [Google Scholar] [CrossRef]
- Limburg, K.; Landergren, P.; Westin, L.; Elfman, M.; Kristiansson, P. Flexible modes of anadromy in Baltic sea trout: Making the most of marginal spawning streams. J. Fish Biol. 2001, 59, 682–695. [Google Scholar] [CrossRef]
- Taal, I.; Rohtla, M.; Saks, L.; Kesler, M.; Jürgens, K.; Svirgsden, R.; Matetski, L.; Verliin, A.; Paiste, P.; Vetemaa, M. Parr dispersal between streams via a marine environment: A novel mechanism behind straying for anadromous brown trout? Ecol. Freshw. Fish 2017, 27, 209–215. [Google Scholar] [CrossRef]
- Kalish, J. Use of otolith microchemistry to distinguish the progeny of sympatric anadromous and nonanadromous salmonids. Fish. Bull. 1990, 88, 657–666. [Google Scholar]
- Gillanders, B.M. Otolith chemistry to determine movements of diadromous and freshwater fish. Aquat. Living Resour. 2005, 18, 291–300. [Google Scholar] [CrossRef]
- Elsdon, T.S.; Wells, B.K.; Campana, S.E.; Gillanders, B.M.; Jones, C.M.; Limburg, K.E.; Secor, D.H.; Thorrold, S.R.; Walther, B.D. Otolith chemistry to describe movements and life-history parameters of fishes: Hypotheses, assumptions, limitations and inferences. In Oceanography and Marine Biology: An Annual Review; Gibson, R.N., Atkinson, R.J.A., Gordon, J.D.M., Eds.; Taylor & Francis: Abingdon, UK, 2008; Volume 46, pp. 297–330. [Google Scholar] [CrossRef]
- Brown, R.J.; Severin, K.P. Otolith chemistry analyses indicate that water Sr: Ca is the primary factor influencing otolith Sr: Ca for freshwater and diadromous fish but not for marine fish. Can. J. Fish. Aquat. Sci. 2009, 66, 1790–1808. [Google Scholar] [CrossRef]
- Engstedt, O.; Stenroth, P.; Larsson, P.; Ljunggren, L.; Elfman, M. Assessment of natal origin of pike (Esox lucius L.) in the Baltic Sea using Sr:Ca in otoliths. Environ. Biol. Fishes 2010, 89, 547–555. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, T.; Liu, H.; Chen, X.; Yang, J. Otolith microchemistry reveals life history and habitat use of Coilia nasus from the Dayang River of China. Fishes 2022, 7, 306. [Google Scholar] [CrossRef]
- Zimmerman, C.E. Relationship of otolith strontium-to-calcium ratios and salinity: Experimental validation for juvenile salmonids. Can. J. Fish. Aquat. Sci. 2005, 62, 88–97. [Google Scholar] [CrossRef]
- Thorrold, S.; Jones, C.; Campana, S.; McLaren, J.; Lam, J. Trace element signatures in otoliths record natal river of juvenile American shad (Alosa sapidissima). Limnol. Oceanogr. 1998, 43, 1826–1835. [Google Scholar] [CrossRef]
- Gabrielsen, S.E.; Lennox, R.J.; Wiers, T.; Barlaup, B.T. Saltwater spawning of sea-run brown trout. Environ. Biol. Fishes 2021, 104, 1207–1213. [Google Scholar] [CrossRef]
- Leonardsson, K.; Hudd, R.; Veneranta, L.; Huhmarniemi, A.; Jokikokko, E. Optimal time and sample allocation for unicohort fish larvae, sea-spawning whitefish (Coregonus lavaretus s. l.) as a case study. ICES J. Mar. Sci. 2016, 73, 374–383. [Google Scholar] [CrossRef]
- Kissinger, B.C.; Gantner, N.; Anderson, W.G.; Gills, D.M.; Halden, N.M.; Harwood, L.A.; Reist, J.D. Brackish-water residence and semianadromy in Arctic lake trout (Salvelinus namaicush) inferred from otolith microchemistry. J. Great Lakes Res. 2016, 42, 267–275. [Google Scholar] [CrossRef]
- Heard, W.R. Life history of pink salmon (Oncorhynchus gorbuscha). In Pacific Salmon Life Histories; Groot, C., Margolis, L., Eds.; University of British Columbia Press: Vancouver, BC, Canada, 1991; pp. 119–230.gorbuscha. [Google Scholar]
- Salo, E.O. Life history of chum salmon (Oncorhynchus keta). In Pacific Salmon Life Histories; Groot, C., Margolis, L., Eds.; University of British Columbia Press: Vancouver, BC, Canada, 1991; pp. 231–310. [Google Scholar]
- Svärdson, G. Lax och öring i Em. [Salmon and trout in Em]; Sötvattenslaboratoriet: Drottningholm, Sweden, 1967; Volume 7, pp. 1–47. (In Swedish) [Google Scholar]
- Calles, E.O.; Greenberg, L.A. Evaluation of nature-like fishways for re-establishing connectivity in fragmented salmonid populations in the River Emån. Riv. Res. Appl. 2005, 21, 951–960. [Google Scholar] [CrossRef]
- Halldén, A.; Johansson, P.; Nydén, T. Fiskevårdsplan Emån 2000; Länsstyrelsen i Jönköpings län: Jönköping, Sweden, 2000; p. 30. [Google Scholar]
- Johansson, S.A.; Johansson, T.B. Analytical application of particle induced X-ray emission. Nucl. Instrum. Methods 1976, 137, 473–516. [Google Scholar] [CrossRef]
- Malmqvist, K.G. Particle-induced X-ray emission—A quantitative technique suitable for microanalysis. In Microbeam and Nanobeam Analysis; Springer: Vienna, Austria, 1996; pp. 117–133. [Google Scholar]
- Campana, S. Chemistry and composition of fish otoliths: Pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 1999, 188, 263–297. [Google Scholar] [CrossRef]
- Jonsson, B. Comparison of scales and otoliths for age determination in brown trout, Salmo trutta L. Nor. J. Zool. 1976, 24, 295–301. [Google Scholar]
- L’Abee-Lund, J.H.; Jonsson, B.; Jensen, A.J.; Sættem, L.M.; Heggberget, T.G.; Johnsen, B.O.; Næsje, T.F. Latitudinal variation in life-history characteristics of sea-run migrant brown trout Salmo trutta. J. Anim. Ecol. 1989, 58, 525–542. [Google Scholar] [CrossRef]
- Titus, R.G.; Mosegaard, H.H. Smolting at age 1 and its adaptive significance for migratory trout, Salmo trutta L., in a small Baltic-coast stream. J. Fish Biol. 1989, 35, 351–353. [Google Scholar] [CrossRef]
- Järvi, T.; Holmgren, K.; Rubin, J.F.; Petersson, E.; Lundberg, S.; Glimsäter, C. Newly-emerged Salmo trutta fry that migrate to the sea-an alternative choice of feeding habitat? Nord. J. Freshw. Res. 1996, 5, 52–62. [Google Scholar]
- Elliott, J.M. Quantitative Ecology and the Brown Trout; Oxford University Press: Oxford, UK, 1994; 286p. [Google Scholar]
- Gross, M.R.; Coleman, R.M.; McDowall, R.M. Aquatic productivity and the evolution of diadromous fish migration. Science 1988, 239, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Altinokand, I.; Grizzle, M. Effects of brackish water on growth, feed conversion and energy absorption efficiency by juvenile euryhaline and freshwater stenohaline fishes. J. Fish Biol. 2001, 59, 1142–1152. [Google Scholar] [CrossRef]
- Forseth, T.; Nesje, T.F.; Jonsson, B.; Hårsaker, K. Juvenile migration in brown trout: A consequence of energetic state. J. Anim. Ecol. 1999, 68, 783–793. [Google Scholar] [CrossRef]
- Morinville, G.; Rasmussen, J.B. Early juvenile bioenergetic differences between anadromous and resident brook trout (Salvelinus fontinalis). Can. J. Fish. Aquat. Sci. 2003, 60, 401–410. [Google Scholar] [CrossRef]
- Økland, F.; Jonsson, B.; Jensen, A.J.; Hansen, L.P. Is there a threshold size regulating smolt size in brown trout and Atlantic salmon? J. Fish Biol. 2001, 42, 541–550. [Google Scholar] [CrossRef]
- Werner, E.E.; Gilliam, J.F. The ontogenetic niche and species interactions in size structured populations. Annu. Rev. Ecol. Syst. 1984, 15, 393–425. [Google Scholar] [CrossRef]
- Jensen, A.J.; Finstad, B.; Fiske, P. The cost of anadromy: Marine and freshwater mortality rates in anadromous Arctic char and brown trout in the Arctic region of Norway. Can. J. Fish. Aquat. Sci. 2019, 76, 2408–2417. [Google Scholar] [CrossRef]
- Phillip, C.C.; Moore, J.M.; Buoro, M.; Hayes, S.A.; Garza, J.C.; Pearce, D.E. Shifting thresholds: Rapid evolution of migratory life histories in steelhead/rainbow trout, Oncorhynchus mykiss. J. Hered. 2016, 107, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Durtsche, R.D.; Jonsson, B.; Greenberg, L.A. Thermal conditions during embryogenesis influence metabolic rates of juvenile brown trout Salmo trutta. Ecosphere 2021, 12, e03374. [Google Scholar] [CrossRef]
- Greenberg, L.; Filipsson, K.; Bergman, E.; Jonsson, B. Egg incubation temperature has carry-over effects on the swimming activity of juvenile brown trout Salmo trutta. Behav. Ecol. Sociobiol. 2023, 77, 114. [Google Scholar] [CrossRef]
- Landergren, P.; Vallin, L. Spawning of sea trout, Salmo trutta L., in brackish waters- lost effort or successful strategy. Fish. Res. 1998, 35, 229–236. [Google Scholar] [CrossRef]
- Huntsman, A.G. Migration of salmon parr. J. Fish. Board Can. 1945, 6, 399–402. [Google Scholar] [CrossRef]
- Taal, I.; Rohtla, M.; Saks, L.; Svirgsden, R.; Kesler, M.; Matetski, L.; Vetemaa, M. Evidence of Atlantic salmon Salmo salar fry movement between fresh water and a brackish environment. J. Fish Biol. 2017, 91, 695–703. [Google Scholar] [CrossRef]
- Birt, T.P.; Green, J.M.; Davidson, W.S. Smolt status of downstream migrating Atlantic salmon (Salmo salar) parr. Can. J. Fish. Aquat. Sci. 1980, 47, 1136–1139. [Google Scholar] [CrossRef]
- Gallagher, Z.S.; Bystriansky, J.S.; Farrell, A.P.; Brauner, C.J. A novel pattern of smoltification in the most anadromous salmonid: Pink salmon (Oncorhynchus gorbuscha). Can. J. Fish. Aquat. Sci. 2013, 70, 349–357. [Google Scholar] [CrossRef]
- Kuroki, M.; Tamate, T.; Morita, K. An additional life-history tactic of masu salmon: Migration of parr to coastal habitats. Ecol. Freshw. Fish 2020, 29, 495–501. [Google Scholar] [CrossRef]
- Kjelson, M.A.; Raquel, P.F.; Fishes, F.W. Life history of fall-run juvenile chinook salmon, Oncorhynchus tshawytscha, in the Sacramento-San Joaquin estuary. In Estuarine Comparisons; Kennedy, V.S., Ed.; Academic Press: New York, NY, USA, 1982; pp. 393–411. [Google Scholar]
- Bond, M.H.; Hayes, S.A.; Hanson, C.V.; MacFarlane, R.B. Marine survival of steelhead (Oncorhynchus mykiss) enhanced by a seasonally closed estuary. Can. J. Fish. Aquat. Sci. 2008, 65, 2242–2252. [Google Scholar] [CrossRef]
- Simmons, R.K.; Quinn, T.P.; Seeb, L.W.; Schindler, D.E.; Hilborn, R. Role of estuarine rearing for sockeye salmon in Alaska (USA). Mar. Ecol. Prog. Ser. 2013, 481, 211–223. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, X.; Dong, S.; Wang, S.; Yang, J.; Zhou, Y. Comparison of salinity adaptation in terms of growth, body composition, and energy budget in juveniles f rainbow and steelhead trout. J. Ocean Univ. China 2019, 18, 509–518. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N.; Ruud-Hansen, J. Downstream displacement and life history variables of Arctic charr (Salvelinus alpinus) in a Norwegian river. Physiol. Ecol. Jpn. 1989, 1, 93–105. [Google Scholar]
- Calles, E.O.; Greenberg, L.A. Connectivity is a two-way street- the need for a holistic approach to fish passage problems in regulated rivers. Riv. Res. Appl. 2009, 25, 1268–1286. [Google Scholar] [CrossRef]
- Jonsson, N.; Jonsson, B.; Hansen, L.P. Marine survival and growth of sea ranched and wild Atlantic salmon. J. Appl. Ecol. 2003, 40, 900–911. [Google Scholar] [CrossRef]
- Koski, K.V. The fate of coho salmon nomads: The story. of an estuarine-rearing strategy promoting resilience. Ecol. Soc. 2009, 14, 4. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, M.; Jonsson, N. Water level influences migratory patterns of anadromous brown trout in small streams. Ecol. Freshw. Fish 2018, 27, 1066–1075. [Google Scholar] [CrossRef]
- Degerman, E.; Leonardsson, K.; Lundqvist, K. Coastal migrations, temporary use of neighbouring rivers, and growth of sea trout (Salmo trutta) from nine northern Baltic Sea rivers. ICES J. Mar. Sci. 2012, 69, 971–980. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N.; Hansen, L.P. Atlantic salmon straying from the River Imsa. J. Fish Biol. 2003, 62, 641–657. [Google Scholar] [CrossRef]

| Captured at | Capture Date | Sex | Age (yr) | Length (cm) | Weight (kg) |
|---|---|---|---|---|---|
| Em | 2007-09-17 | male | 5+ | 93 | 9.7 |
| Em | 2008-09-15 | male | 4+ | 84 | 6.0 |
| Em | xxxx-03-05 | female? | 4 | 64 | 2.2 |
| Em | xxxx-03-21 | female | 5 | 68 | 2.6 |
| Em | 2008-08-23 | female | 3+ | 72 | 3.8 |
| Em | 2008-08-24 | female | 3+ | unknown | 2.8 |
| Em | 2007-09-10 | male | 4+ | 58 | 2.1 |
| Em | xxxx-04-26 | female | 4 | 66 | 3.0 |
| Em | 2008-03-15 | female | 5 | 61 | 2.6 |
| Em | unknown | male? | 4+ | unknown | c. 2–3 |
| Em | 2008-04-20 | male | 4 | 62 | 2.4 |
| Em | 2008-03-18 | female | 6 | 73 | 3.7 |
| Em | 2008-09-21 | male | 5+ | unknown | 5.5 |
| Em | unknown | female? | 4+ | unknown | c. 2–3 |
| Em | 2008-03-17 | female | 5 | unknown | 3.6 |
| Em | 2008-09-24 | female? | 3+ | 47 | 1.2 |
| Em | 2008-08-01 | female | 6+ | 85 | 8.6 |
| Emsfors | 2008-03-20 | male? | 3 | 55 | 1.8 |
| Emsfors | 2008-04-06 | male? | 5 | 68 | 3.7 |
| Emsfors | 2008-04-13 | male? | 4 | 61 | 2.0 |
| Emsfors | Spring 2008 | male? | 5 | unknown | c. 3–5 |
| Emsfors | Spring 2008 | female? | 6 | unknown | c. 3–5 |
| Emsfors | Spring 2008 | female? | 3 | unknown | c. 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersson, M.; Jonsson, B.; Calles, O.; Greenberg, L. Assessing Movements between Freshwater and Saltwater by Brown Trout (Salmo trutta L.) Based on Otolith Microchemistry. Animals 2024, 14, 2116. https://doi.org/10.3390/ani14142116
Andersson M, Jonsson B, Calles O, Greenberg L. Assessing Movements between Freshwater and Saltwater by Brown Trout (Salmo trutta L.) Based on Otolith Microchemistry. Animals. 2024; 14(14):2116. https://doi.org/10.3390/ani14142116
Chicago/Turabian StyleAndersson, Magdalena, Bror Jonsson, Olle Calles, and Larry Greenberg. 2024. "Assessing Movements between Freshwater and Saltwater by Brown Trout (Salmo trutta L.) Based on Otolith Microchemistry" Animals 14, no. 14: 2116. https://doi.org/10.3390/ani14142116
APA StyleAndersson, M., Jonsson, B., Calles, O., & Greenberg, L. (2024). Assessing Movements between Freshwater and Saltwater by Brown Trout (Salmo trutta L.) Based on Otolith Microchemistry. Animals, 14(14), 2116. https://doi.org/10.3390/ani14142116






