Shh Gene Regulates the Proliferation and Apoptosis of Dermal Papilla Cells to Affect Its Differential Expression in Secondary Hair Follicle Growth Cycle of Cashmere Goats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Sample Collection
2.2. In Vitro Culture of DPCs
2.3. Real-Time Quantitative PCR (RT-qPCR)
2.4. Western Blot
2.5. Immunohistochemistry
2.6. Transformation and Extraction of Expression Vectors and Recombinant Products
2.7. DPCs Transfection and Viability Detection
2.8. Apoptosis Detection of DPCs
2.9. Data Statistical Analysis
3. Results
3.1. Expression of Shh Gene in Secondary Hair Follicle of Cashmere Goats
3.1.1. Expression of Shh Gene in Secondary Hair Follicle Cycle Growth Skins
3.1.2. Expression of Shh in Secondary Hair Follicle Cycle Growth Skins of Cashmere Goats
3.1.3. Expression Site of Shh in Secondary Hair Follicles
3.2. Effect of Shh Gene Overexpression on Proliferation and Apoptosis of Secondary Hair Follicle DPCs in Cashmere Goats
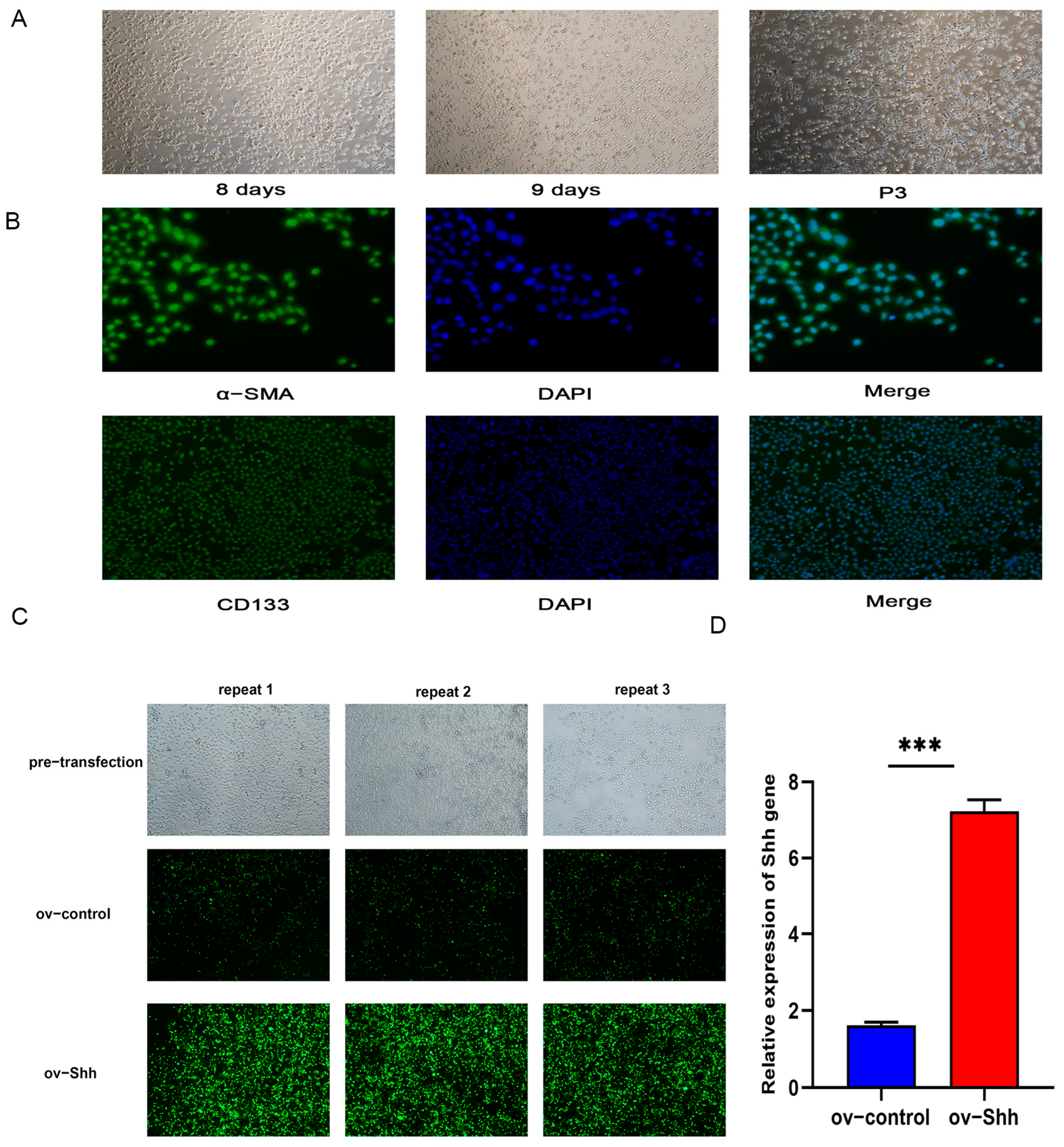
3.2.1. DPCs Cultivation in vitro and Identification
3.2.2. Construction of Shh Gene Overexpression Vector and DPCs Transfection
3.2.3. Detection of DPCs’ Proliferation and Apoptosis
3.3. Effect of Interfering with Shh Gene on the Proliferation and Apoptosis of Secondary DPCs in Cashmere Goats
3.3.1. Construction of Shh Gene Interference Vector and DPCs Transfection
3.3.2. Proliferation and Apoptosis Detection of DPCs
3.4. Effect of Shh Gene on the Expression of Hedgehog-Signaling-Pathway-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, H.R.; Wu, L.J.; Liu, F. Sheep race will witness the road of high quality development of Inner Mongolia white goat (Albas type). Livest. Ind. 2023, 10, 43–48. (In Chinese) [Google Scholar]
- Li, J.Q. Study on Breeding Methods in Inner Mongolia Cashmere Goats. Ph.D. Dissertation, China Agricultural University, Beijing, China, 2005. (In Chinese). [Google Scholar]
- Sun, L.M.; Jiang, H.Z.; Li, J.Y.; Chen, Y.; Guo, D. Analysis of Secondary Follicles Structure during Anagen of Liaoning Cashmere Goats. J. Domest. Anim. Ecol. 2015, 36, 15–18. (In Chinese) [Google Scholar]
- Kumamoto, T.; Shalhevet, D.; Matsue, H.; Mummert, M.E.; Ward, B.R.; Jester, J.V.; Takashima, A. Hair follicles serve as local reservoirs of skin mast cell precursors. Blood 2003, 102, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Yin, J.; Li, J.Q.; Li, C.Q. Study on Hair Follicle Structure and Morphogenesis of the Inner Mongolian Arbas Cashmere Goat. Sci. Agric. Sin. 2007, 05, 1017–1023. (In Chinese) [Google Scholar]
- Feng, Y. Advances in Mammalian Hair Follicle Genesis and Its Regulation. Chin. J. Anim. Sci. 2022, 58, 26–32. (In Chinese) [Google Scholar]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef]
- Müller-Röver, S.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Fuchs, E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 2006, 22, 339–373. [Google Scholar] [CrossRef] [PubMed]
- Peus, D.; Pittelkow, M.R. Growth factors in hair organ development and the hair growth cycle. Dermatol. Clin. 1996, 14, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Gorojankina, T. Hedgehog signaling pathway: A novel model and molecular mechanisms of signal transduction. Cell Mol. Life Sci. 2016, 73, 1317–1332. [Google Scholar] [CrossRef] [PubMed]
- Ruch, J.M.; Kim, E.J. Hedgehog signaling pathway and cancer therapeutics: Progress to date. Drugs 2013, 73, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ren, X.R.; Nelson, C.D.; Barak, L.S.; Chen, J.K.; Beachy, P.A.; de Sauvage, F.; Lefkowitz, R.J. Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science 2004, 306, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Arensdorf, A.M.; Marada, S.; Ogden, S.K. Smoothened Regulation: A Tale of Two Signals. Trends Pharmacol. Sci. 2016, 37, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Robbins, D.J.; Fei, D.L.; Riobo, N.A. The Hedgehog signal transduction network. Sci. Signal. 2012, 5, re6. [Google Scholar] [CrossRef] [PubMed]
- Kieran, M.W. Targeted treatment for sonic hedgehog-dependent medulloblastoma. Neuro Oncol. 2014, 16, 1037–1047. [Google Scholar] [CrossRef]
- Girardi, D.; Barrichello, A.; Fernandes, G.; Pereira, A. Targeting the Hedgehog Pathway in Cancer: Current Evidence and Future Perspectives. Cells 2019, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, Y.; Sun, B.; McMahon, A.P.; Wang, Y. Hedgehog Signaling: From Basic Biology to Cancer Therapy. Cell Chem. Biol. 2017, 24, 252–280. [Google Scholar] [CrossRef] [PubMed]
- Tibes, R.; Mesa, R.A. Targeting hedgehog signaling in myelofibrosis and other hematologic malignancies. J. Hematol. Oncol. 2014, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Beke, F.; O’Brien, L.M.; Kapeni, C.; Chen, H.C.; Carbonaro, V.; Kim, A.B.; Kishore, K.; Adolph, T.E.; Skjoedt, M.O.; et al. Cell-autonomous Hedgehog signaling controls Th17 polarization and pathogenicity. Nat. Commun. 2022, 13, 4075. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.; Yanez, D.C.; Ross, S.; Lau, C.I.; Papaioannou, E.; Li, J.; Saldaña, J.I.; Crompton, T. Gli3 in fetal thymic epithelial cells promotes thymocyte positive selection and differentiation by repression of Shh. Development 2018, 145, dev146910. [Google Scholar] [CrossRef]
- Barbarulo, A.; Lau, C.I.; Mengrelis, K.; Ross, S.; Solanki, A.; Saldaña, J.I.; Crompton, T. Hedgehog Signalling in the Embryonic Mouse Thymus. J. Dev. Biol. 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Outram, S.V.; Hager-Theodorides, A.L.; Shah, D.K.; Rowbotham, N.J.; Drakopoulou, E.; Ross, S.E.; Lanske, B.; Dessens, J.T.; Crompton, T. Indian hedgehog (Ihh) both promotes and restricts thymocyte differentiation. Blood 2009, 113, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, X.; Zeng, X.; Jin, R.; Wang, S.; Jiang, H.; Tang, Y.; Chen, G.; Wei, J.; Chen, T.; et al. Gut microbiota dysbiosis promotes the development of epithelial ovarian cancer via regulating Hedgehog signaling pathway. Gut Microbes 2023, 15, 2221093. [Google Scholar] [CrossRef] [PubMed]
- Razumilava, N.; Gumucio, D.L.; Samuelson, L.C.; Shah, Y.M.; Nusrat, A.; Merchant, J.L. Indian Hedgehog Suppresses Intestinal Inflammation. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, J.; Zeng, X.; Nie, M.; Luan, J.; Wang, Y.; Ju, D.; Yin, K. Hedgehog signaling in gastrointestinal carcinogenesis and the gastrointestinal tumor microenvironment. Acta Pharm. Sin. B 2021, 11, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Braune, J.; Weyer, U.; Matz-Soja, M.; Hobusch, C.; Kern, M.; Kunath, A.; Klöting, N.; Kralisch, S.; Blüher, M.; Gebhardt, R.; et al. Hedgehog signalling in myeloid cells impacts on body weight, adipose tissue inflammation and glucose metabolism. Diabetologia 2017, 60, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cho, J.S.; Kim, J.T.; Moon, J.H.; Zhou, Y.; Lee, S.B.; Park, H.J.; Lee, H.J. Caudatin suppresses adipogenesis in 3T3-L1 adipocytes and reduces body weight gain in high-fat diet-fed mice through activation of hedgehog signaling. Phytomedicine 2021, 92, 153715. [Google Scholar] [CrossRef] [PubMed]
- Waas, B.; Carpenter, B.S.; Franks, N.E.; Merchant, O.Q.; Verhey, K.J.; Allen, B.L. Dual and opposing roles for the kinesin-2 motor, KIF17, in Hedgehog-dependent cerebellar development. Sci. Adv. 2024, 10, eade1650. [Google Scholar] [CrossRef] [PubMed]
- Palma, V.; Lim, D.A.; Dahmane, N.; Sánchez, P.; Brionne, T.C.; Herzberg, C.D.; Gitton, Y.; Carleton, A.; Alvarez-Buylla, A.; Ruiz i Altaba, A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 2005, 132, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yam, P.T.; Schlienger, S.; Cai, E.; Zhang, J.; Chen, W.J.; Torres Gutierrez, O.; Jimenez Amilburu, V.; Ramamurthy, V.; Ting, A.Y.; et al. Numb positively regulates Hedgehog signaling at the ciliary pocket. Nat. Commun. 2024, 15, 3365. [Google Scholar] [CrossRef] [PubMed]
- Hor, C.H.H.; Lo, J.C.W.; Cham, A.L.S.; Leong, W.Y.; Goh, E.L.K. Multifaceted Functions of Rab23 on Primary Cilium-Mediated and Hedgehog Signaling-Mediated Cerebellar Granule Cell Proliferation. J. Neurosci. 2021, 41, 6850–6863. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.A. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr. Biol. 1999, 9, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.J.; Speranza, G.; Dansky Ullmann, C. Targeting embryonic signaling pathways in cancer therapy. Expert. Opin. Ther. Targets 2012, 16, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.M.; Li, Z.X.; Zhang, D.Y.; Yang, Y.C.; Fu, S.A.; Zhang, Z.Q.; Yang, R.H.; Xiong, K. A systematic summary of survival and death signalling during the life of hair follicle stem cells. Stem Cell Res. Ther. 2021, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Tanaka, N. Roles of the Hedgehog Signaling Pathway in Epidermal and Hair Follicle Development, Homeostasis, and Cancer. J. Dev. Biol. 2017, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.; Mok, K.W.; Rendl, M. An updated classification of hair follicle morphogenesis. Exp. Dermatol. 2019, 28, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Cao, W.; Ma, X.; Ye, X.; Qin, C.; Li, B.; Liu, W.; Lu, Q.; Wu, C.; Fu, X. Metabolomics reveals metabolites associated with hair follicle cycle in cashmere goats. BMC Vet. Res. 2024, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamaguchi, Y.; Villacorte, M.; Mihara, K.; Akiyama, M.; Shimizu, H.; Taketo, M.M.; Nakagata, N.; Tsukiyama, T.; Yamaguchi, T.P.; et al. Embryonic hair follicle fate change by augmented beta-catenin through Shh and Bmp signaling. Development 2009, 136, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Woo, W.M.; Zhen, H.H.; Oro, A.E. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes. Dev. 2012, 26, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- DiBaise, M.; Tarleton, S.M. Hair, Nails, and Skin: Differentiating Cutaneous Manifestations of Micronutrient Deficiency. Nutr. Clin. Pr. 2019, 34, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zou, Z.; Chang, H.; Shen, Q.; Liu, L.; Xing, D. Photobiomodulation therapy for hair regeneration: A synergetic activation of β-CATENIN in hair follicle stem cells by ROS and paracrine WNTs. Stem Cell Rep. 2021, 16, 1568–1583. [Google Scholar] [CrossRef] [PubMed]
- Taghiabadi, E.; Nilforoushzadeh, M.A.; Aghdami, N. Maintaining Hair Inductivity in Human Dermal Papilla Cells: A Review of Effective Methods. Ski. Pharmacol. Physiol. 2020, 33, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, Z.; Lutz, H.; Huang, K.; Su, T.; Cores, J.; Dinh, P.C.; Cheng, K. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Sci. Adv. 2020, 6, eaba1685. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.A.; Armstrong, R.C. Postnatal Sonic hedgehog (Shh) responsive cells give rise to oligodendrocyte lineage cells during myelination and in adulthood contribute to remyelination. Exp. Neurol. 2018, 299, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Snedecor, E.R.; Choi, Y.J.; Yang, N.; Zhang, X.; Xu, Y.; Han, Y.; Jones, E.C.; Shroyer, K.R.; Clark, R.A.; et al. Gorab Is Required for Dermal Condensate Cells to Respond to Hedgehog Signals during Hair Follicle Morphogenesis. J. Investig. Dermatol. 2016, 136, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Leopold, P.L.; Crystal, R.G. Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog. J. Clin. Investig. 1999, 104, 855–864. [Google Scholar] [CrossRef]
- Cui, C.Y.; Kunisada, M.; Childress, V.; Michel, M.; Schlessinger, D. Shh is required for Tabby hair follicle development. Cell Cycle 2011, 10, 3379–3386. [Google Scholar] [CrossRef]
- Wang, L.C.; Liu, Z.Y.; Gambardella, L.; Delacour, A.; Shapiro, R.; Yang, J.; Sizing, I.; Rayhorn, P.; Garber, E.A.; Benjamin, C.D.; et al. Regular articles: Conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J. Investig. Dermatol. 2000, 114, 901–908. [Google Scholar] [CrossRef]
- Shwartz, Y.; Gonzalez-Celeiro, M.; Chen, C.L.; Pasolli, H.A.; Sheu, S.H.; Fan, S.M.; Shamsi, F.; Assaad, S.; Lin, E.T.; Zhang, B.; et al. Cell Types Promoting Goosebumps Form a Niche to Regulate Hair Follicle Stem Cells. Cell 2020, 182, 578–593.e519. [Google Scholar] [CrossRef] [PubMed]
- Mill, P.; Mo, R.; Fu, H.; Grachtchouk, M.; Kim, P.C.; Dlugosz, A.A.; Hui, C.C. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003, 17, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A.; Paus, R. Molecular biology of hair morphogenesis: Development and cycling. J. Exp. Zool. B Mol. Dev. Evol. 2003, 298, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Swan, R.Z.; Grachtchouk, M.; Bolinger, M.; Litingtung, Y.; Robertson, E.K.; Cooper, M.K.; Gaffield, W.; Westphal, H.; Beachy, P.A.; et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev. Biol. 1999, 205, 1–9. [Google Scholar] [CrossRef] [PubMed]
- St-Jacques, B.; Dassule, H.R.; Karavanova, I.; Botchkarev, V.A.; Li, J.; Danielian, P.S.; McMahon, J.A.; Lewis, P.M.; Paus, R.; McMahon, A.P. Sonic hedgehog signaling is essential for hair development. Curr. Biol. 1998, 8, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Bartz, S.R.; Schelter, J.; Kobayashi, S.V.; Burchard, J.; Mao, M.; Li, B.; Cavet, G.; Linsley, P.S. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003, 21, 635–637. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer Sequence (5′-3′) |
|---|---|
| Shh | F: AGCCTACAAGCAGTTTATCCC R: GGTCCGCTCCAGTGTTTTC |
| PCNA | F: AGAGGAGGAAGCTGTTACCAT R: GACAGTGGAGTGGCTTTTGT |
| Ki67 | F: TGTTGCCAAAATAGCTGCTG R: GTACCGTTTCACTGCTGGAT |
| Ptch | F: AGGCAGCGGTAGTAGTAGTG R: GTAGCGGGTATTGTCCGTG |
| Smo | F: CAACCCTCTGGGTCTTCCCTACT R: CGCTTCGTCTTCTGGCTGCTC |
| Gli2 | F: GGTAGCTGGCTGATCCGAATTG R: TACACTGCGGCTCTGAACACT |
| Gene | Name | Sequence 5′→3′ | Size (bp) |
|---|---|---|---|
| siRNA-Shh | a | GATCCCCTCGAGTTTTTGGAT | 21 |
| b | AGCTATCCAAAAACTCGAGGG | 21 | |
| siRNA-NC | a | GCACATCCACTGCTCAGTGAA | 21 |
| b | AGCTATCCAAAAACTCGAGGG | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, Y.; Chang, J.; Zhang, R.; Liu, Z.; Liang, J.; Wang, D.; Feng, J.; Zhao, W.; Xiao, H. Shh Gene Regulates the Proliferation and Apoptosis of Dermal Papilla Cells to Affect Its Differential Expression in Secondary Hair Follicle Growth Cycle of Cashmere Goats. Animals 2024, 14, 2049. https://doi.org/10.3390/ani14142049
Zhang J, Liu Y, Chang J, Zhang R, Liu Z, Liang J, Wang D, Feng J, Zhao W, Xiao H. Shh Gene Regulates the Proliferation and Apoptosis of Dermal Papilla Cells to Affect Its Differential Expression in Secondary Hair Follicle Growth Cycle of Cashmere Goats. Animals. 2024; 14(14):2049. https://doi.org/10.3390/ani14142049
Chicago/Turabian StyleZhang, Junjie, Yujing Liu, Jiale Chang, Ru Zhang, Zhaomin Liu, Jiayue Liang, Dong Wang, Juan Feng, Wei Zhao, and Hongmei Xiao. 2024. "Shh Gene Regulates the Proliferation and Apoptosis of Dermal Papilla Cells to Affect Its Differential Expression in Secondary Hair Follicle Growth Cycle of Cashmere Goats" Animals 14, no. 14: 2049. https://doi.org/10.3390/ani14142049
APA StyleZhang, J., Liu, Y., Chang, J., Zhang, R., Liu, Z., Liang, J., Wang, D., Feng, J., Zhao, W., & Xiao, H. (2024). Shh Gene Regulates the Proliferation and Apoptosis of Dermal Papilla Cells to Affect Its Differential Expression in Secondary Hair Follicle Growth Cycle of Cashmere Goats. Animals, 14(14), 2049. https://doi.org/10.3390/ani14142049





