The Relationship between Canine Behavioral Disorders and Gut Microbiome and Future Therapeutic Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
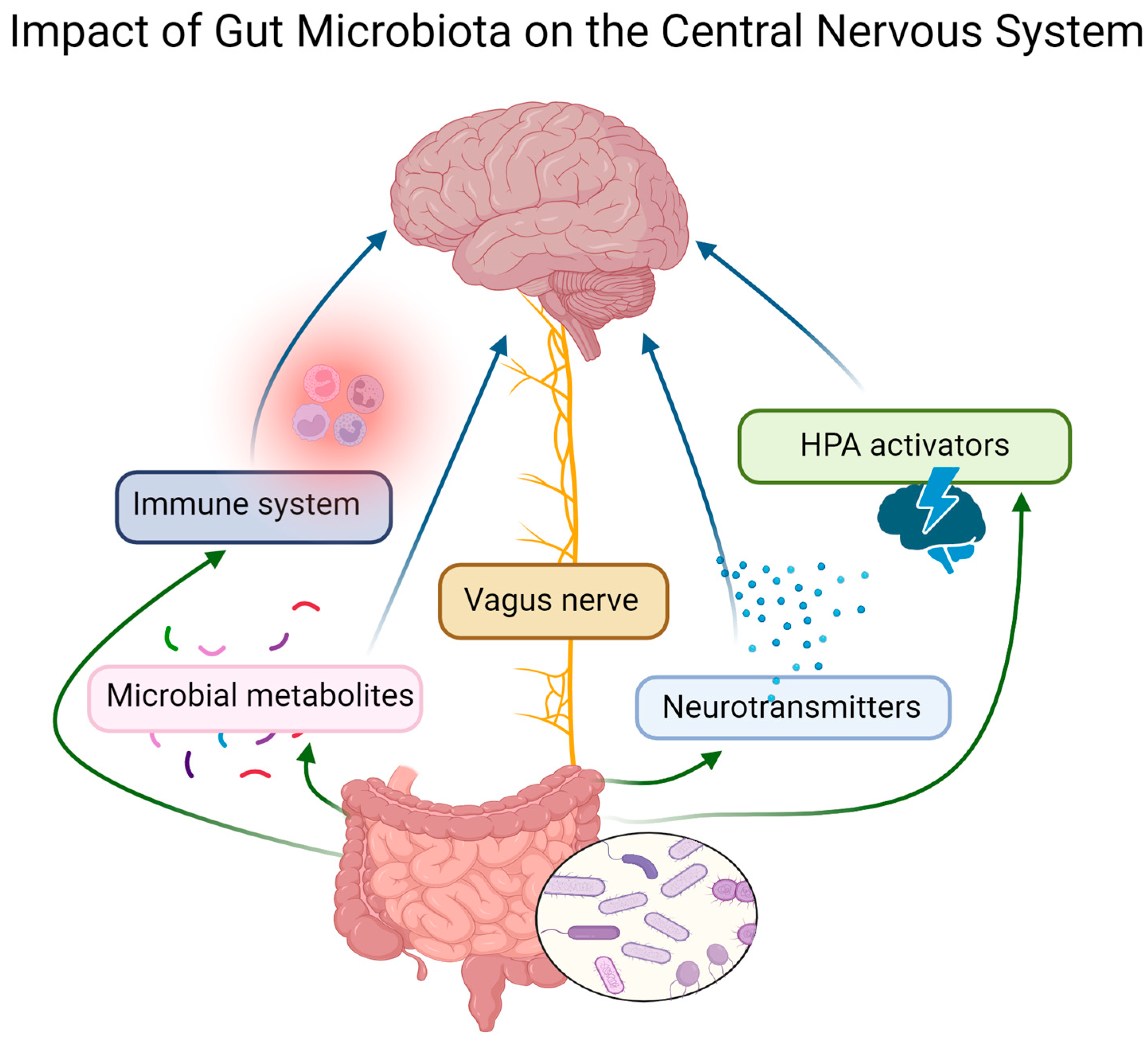
2. Gut–Brain Connection
2.1. Canine Gut Microbiome and Dysbiosis
2.2. Gut–Brain Axis
2.3. Gut Microbiome and Neurotransmitters
| Neurotransmitter | Effect on Animal Behavior | Gut Microbiota Regulation | Canine Behavioral Disorder |
|---|---|---|---|
| Serotonin | Regulates mood, sleep, cognition, social interactions, and anxiety [77] |
| Significantly lower serotonin serum level in aggressive dogs [86,87,88] |
| Dopamine | Regulates reward-related behavior and motivation [90,91,92,93] |
| |
| Gamma-aminobutyric acid (GABA) | Regulates mood and anxiety and prevents inappropriate emotional and behavioral responses [102,103,104,105] | Various bacteria produce/influence GABA in human gut microbiota, and manipulation of gut microbiota may impact GABA levels [109] | Reduced activity and urinary cortisol level in dogs after orally administered GABA [115] |
2.4. Main Microbial Metabolites—Short-Chain Fatty Acids (SCFAs)
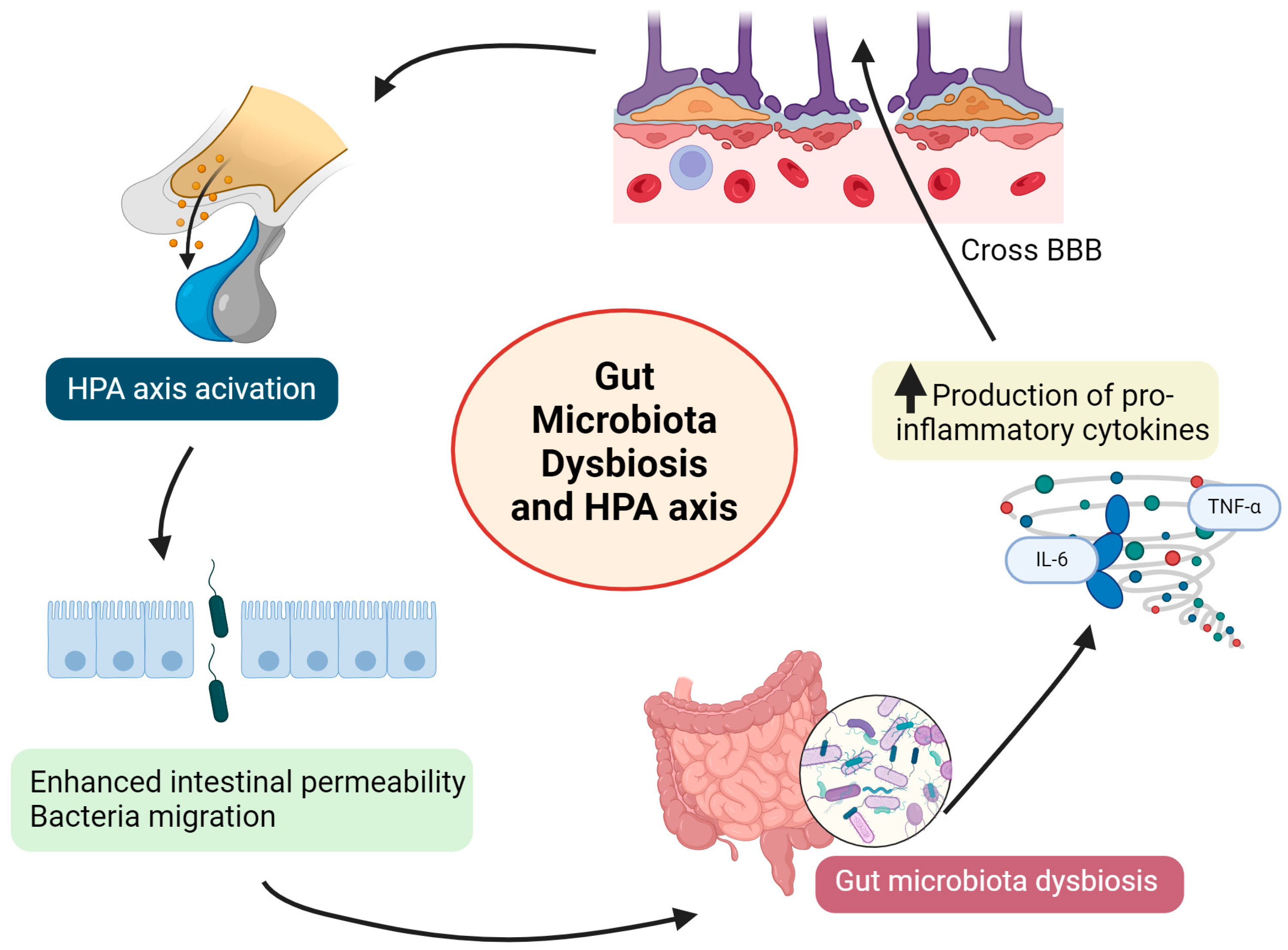
2.5. Gut Dysbiosis and Inflammation
2.6. Gut Dysbiosis and Hypothalamic–Pituitary–Adrenal (HPA) Axis
3. Fecal Microbiota Transplantation (FMT)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campbell, W.E. The prevalence of behavioural problems in American dogs. Mod. Vet. Pract. 1986, 67, 28–31. [Google Scholar]
- Dinwoodie, I.R.; Dwyer, B.; Zottola, V.; Gleason, D.; Dodman, N.H. Demographics and comorbidity of behavior problems in dogs. J. Vet. Behav. Clin. Appl. Res. 2019, 32, 62–71. [Google Scholar] [CrossRef]
- Salonen, M.; Sulkama, S.; Mikkola, S.; Puurunen, J.; Hakanen, E.; Tiira, K.; Araujo, C.; Lohi, H. Prevalence, comorbidity, and breed differences in canine anxiety in 13,700 Finnish pet dogs. Sci. Rep. 2020, 10, 2962. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Langford, F.; Kiddie, J. Risk factors for aggressive behaviour in domestic dogs (Canis familiaris), as reported by owners in mainland China. Appl. Anim. Behav. Sci. 2021, 234, 105211. [Google Scholar] [CrossRef]
- Yamada, R.; Kuze-Arata, S.; Kiyokawa, Y.; Takeuchi, Y. Prevalence of 25 canine behavioral problems and relevant factors of each behavior in Japan. J. Vet. Med. Sci. 2019, 81, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Mcconnell, A.R.; Paige Lloyd, E.; Humphrey, B.T. We Are Family: Viewing Pets as Family Members Improves Wellbeing. Anthrozoös 2019, 32, 459–470. [Google Scholar] [CrossRef]
- Charles, N. Post-Human Families? Dog-Human Relations in the Domestic Sphere. Sociol. Res. Online 2016, 21, 83–94. [Google Scholar] [CrossRef]
- Barker, S.B.; Barker, R.T. The human-canine bond: Closer than family ties? J. Ment. Health Couns. 1988, 10, 46–56. [Google Scholar]
- Ratschen, E.; Shoesmith, E.; Shahab, L.; Silva, K.; Kale, D.; Toner, P.; Reeve, C.; Mills, D.S. Human-animal relationships and interactions during the COVID-19 lockdown phase in the UK: Investigating links with mental health and loneliness. PLoS ONE 2020, 15, e0239397. [Google Scholar] [CrossRef]
- Bussolari, C.; Currin-McCulloch, J.; Packman, W.; Kogan, L.; Erdman, P. “I Couldn’t Have Asked for a Better Quarantine Partner!”: Experiences with Companion Dogs during COVID-19. Animals 2021, 11, 330. [Google Scholar] [CrossRef]
- Kogan, L.R.; Currin-McCulloch, J.; Bussolari, C.; Packman, W.; Erdman, P. The Psychosocial Influence of Companion Animals on Positive and Negative Affect during the COVID-19 Pandemic. Animals 2021, 11, 2084. [Google Scholar] [CrossRef] [PubMed]
- Fatjó, J.; Ruiz-de-la-Torre, J.; Manteca, X. The epidemiology of behavioural problems in dogs and cats: A survey of veterinary practitioners. Anim. Welf. 2006, 15, 179–185. [Google Scholar] [CrossRef]
- Cannas, S.; Talamonti, Z.; Mazzola, S.; Minero, M.; Picciolini, A.; Palestrini, C. Factors associated with dog behavioral problems referred to a behavior clinic. J. Vet. Behav. Clin. Appl. Res. 2018, 24, 42–47. [Google Scholar] [CrossRef]
- Normando, S.; Di Raimondo, G.; Bellaio, E. An investigation using different data gathering methods into the prevalence of behavior problems in shelter dogs—A pilot study. J. Vet. Behav. Clin. Appl. Res. 2019, 30, 1–8. [Google Scholar] [CrossRef]
- Eagan, B.H.; Gordon, E.; Protopopova, A. Reasons for Guardian-Relinquishment of Dogs to Shelters: Animal and Regional Predictors in British Columbia, Canada. Front. Vet. Sci. 2022, 9, 857634. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Slater, M.; Garrison, L.; Drain, N.; Dolan, E.; Scarlett, J.M.; Zawistowski, S.L. Large Dog Relinquishment to Two Municipal Facilities in New York City and Washington, D.C.: Identifying Targets for Intervention. Animals 2014, 4, 409–433. [Google Scholar] [CrossRef] [PubMed]
- Shore, E.R. Returning a recently adopted companion animal: Adopters’ reasons for and reactions to the failed adoption experience. J. Appl. Anim. Welf. Sci. 2005, 8, 187–198. [Google Scholar] [CrossRef]
- Kwan, J.Y.; Bain, M.J. Owner attachment and problem behaviors related to relinquishment and training techniques of dogs. J. Appl. Anim. Welf. Sci. 2013, 16, 168–183. [Google Scholar] [CrossRef]
- Scarlett, J.M.; Salman, M.D.; New, J.G.; Kass, P.H. The role of veterinary practitioners in reducing dog and cat relinquishments and euthanasias. J. Am. Vet. Med. Assoc. 2002, 3, 306–311. [Google Scholar] [CrossRef]
- Kass, P.H.; New, J.C., Jr.; Scarlett, J.M.; Salman, M.D. Understanding animal companion surplus in the United States: Relinquishment of nonadoptables to animal shelters for euthanasia. J. Appl. Anim. Welf. Sci. 2001, 4, 237–248. [Google Scholar] [CrossRef]
- Siracusa, C.; Provoost, L.R.; Reisner, I. Dog- and owner-related risk factors for consideration of euthanasia or rehoming before a referral behavioral consultation and for euthanizing or rehoming the dog after the consultation. J. Vet. Behav. Clin. Appl. Res. 2017, 22, 46–56. [Google Scholar] [CrossRef]
- Barcelos, A.M.; Kargas, N.; Assheton, P.; Maltby, J.; Hall, S.; Mills, D.S. Dog owner mental health is associated with dog behavioural problems, dog care and dog-facilitated social interaction: A prospective cohort study. Sci. Rep. 2023, 13, 21734. [Google Scholar] [CrossRef]
- Barcelos, A.M.; Kargas, N.; Maltby, J.; Hall, S.; Mills, D.S. A framework for understanding how activities associated with dog ownership relate to human well-being. Sci. Rep. 2020, 10, 11363. [Google Scholar] [CrossRef] [PubMed]
- Enders-Slegers, M.; Hediger, K. Pet ownership and human–animal interaction in an aging population: Rewards and challenges. Anthrozoös 2019, 32, 255–265. [Google Scholar] [CrossRef]
- Kuntz, K.; Ballantyne, K.C.; Cousins, E.; Spitznagel, M.B. Assessment of caregiver burden in owners of dogs with behavioral problems and factors related to its presence. J. Vet. Behav. 2023, 64–65, 41–46. [Google Scholar] [CrossRef]
- Shabelansky, A.; Dowling-Guyer, S. Characteristics of Excitable Dog Behavior Based on Owners’ Report from a Self-Selected Study. Animals 2016, 6, 22. [Google Scholar] [CrossRef]
- De Keuster, T.; Lamoureux, J.; Kahn, A. Epidemiology of dog bites: A Belgian experience of canine behaviour and public health concerns. Vet. J. 2006, 172, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Ishaya, N.; Habib, T.; Van Rooyen, C.; Steinberg, W.J. Profile of dog bite injuries in patients presenting at Kimberley Hospital Complex’s emergency and gateway centres, 2015 to 2017. Afr. J. Prim Health Care Fam. Med. 2020, 12, a2301. [Google Scholar] [CrossRef]
- Kaye, A.E.; Belz, J.M.; Kirschner, R.E. Pediatric dog bite injuries: A 5-year review of the experience at the Children’s Hospital of Philadelphia. Plast. Reconstr. Surg. 2009, 124, 551–558. [Google Scholar] [CrossRef]
- Wormald, D.; Lawrence, A.J.; Carter, G.; Fisher, A.D. Reduced heart rate variability in pet dogs affected by anxiety-related behaviour problems. Physiol. Behav. 2017, 168, 122–127. [Google Scholar] [CrossRef]
- Luño, I.; Palacio, J.; García-Belenguer, S.; González-Martínez, Á.; Rosado, B. Perception of Canine Welfare Concerns among Veterinary Students, Practitioners, and Behavior Specialists in Spain. J. Vet. Med. Educ. 2017, 44, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Malkani, R.; Paramasivam, S.; Wolfensohn, S. A Multidimensional Evaluation of the Factors in the Animal Welfare Assessment Grid (AWAG) That Are Associated with, and Predictive of, Behaviour Disorders in Dogs. Animals 2024, 14, 528. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.L.; Hemsworth, P.H. The validity of physiological and behavioural measures of animal welfare. Appl. Anim. Behav. Sci. 1990, 25, 177–187. [Google Scholar] [CrossRef]
- Protopopova, A. Effects of sheltering on physiology, immune function, behavior, and the welfare of dogs. Physiol. Behav. 2016, 159, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Dinwoodie, I.R.; Zottola, V.; Dodman, N.H. An investigation into the effectiveness of various professionals and behavior modification programs, with or without medication, for the treatment of canine aggression. J. Vet. Behav. 2021, 43, 46–53. [Google Scholar] [CrossRef]
- Dodman, N.H.; Smith, A.; Holmes, D. Comparison of the efficacy of remote consultations and personal consultations for the treatment of dogs which are aggressive towards their owners. Vet. Rec. 2005, 156, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Sig Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Honneffer, J.B.; Steiner, J.M.; Lidbury, J.A.; Suchodolski, J.S. Variation of the microbiota and metabolome along the canine gastrointestinal tract. Metabolomics 2017, 13, 26. [Google Scholar] [CrossRef]
- Song, S.J.; Lauber, C.; Costello, E.K.; Lozupone, C.A.; Humphrey, G.; Berg-Lyons, D.; Caporaso, J.G.; Knights, D.; Clemente, J.C.; Nakielny, S.; et al. Cohabiting family members share microbiota with one another and with their dogs. eLife 2013, 2, e00458. [Google Scholar] [CrossRef]
- Wipler, J.; Čermáková, Z.; Hanzálek, T.; Horáková, H.; Žemličková, H. Sharing bacterial microbiota between owners and their pets (dogs, cats). Klin. Mikrobiol. Infekc. Lek. 2017, 23, 48–57. [Google Scholar]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Bien, J.; Palagani, V.; Bozko, P. The intestinal microbiota dysbiosis and Clostridium difficile infection: Is there a relationship with inflammatory bowel disease? Therap Adv. Gastroenterol. 2013, 6, 53–68. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Dowd, S.E.; Wilke, V.; Steiner, J.M.; Jergens, A.E. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e39333. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, S.E.; Kim, H.B.; Isaacson, R.E.; Seo, K.W.; Song, K.H. Association of obesity with serum leptin, adiponectin, and serotonin and gut microflora in beagle dogs. J. Vet. Intern. Med. 2015, 29, 43–50. [Google Scholar] [CrossRef]
- Isaiah, A.; Parambeth, J.C.; Steiner, J.M.; Lidbury, J.A.; Suchodolski, J.S. The fecal microbiome of dogs with exocrine pancreatic insufficiency. Anaerobe 2017, 45, 50–58. [Google Scholar] [CrossRef]
- Li, Q.; Larouche-Lebel, É.; Loughran, K.A.; Huh, T.P.; Suchodolski, J.S.; Oyama, M.A. Metabolomics Analysis Reveals Deranged Energy Metabolism and Amino Acid Metabolic Reprogramming in Dogs with Myxomatous Mitral Valve Disease. J. Am. Heart Assoc. 2021, 10, e018923. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Murphy, D.L.; Andrew, A.M.; Wichems, C.H.; Li, Q.; Tohda, M.; Greenberg, B. Brain serotonin neurotransmission: An overview and update with an emphasis on serotonin subsystem heterogeneity, multiple receptors, interactions with other neurotransmitter systems, and consequent implications for understanding the actions of serotonergic drugs. J. Clin. Psychiatry 1998, 59 (Suppl. S15), 4–12. [Google Scholar]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocrinol. 2018, 51, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Suchodolski, J.S. The Gut Microbiome of Dogs and Cats, and the Influence of Diet. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 605–621. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, G.; Surette, M.; Moayyedi, P. The gut microbiota and psychiatric illness. J. Psychiatry Neurosci. 2017, 42, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Shin, G.E.; Cheong, Y.; Shin, J.H.; Shin, D.M.; Chun, W.Y. Experiencing social exclusion changes gut microbiota composition. Transl. Psychiatry 2022, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Malan-Müller, S.; Valles-Colomer, M.; Palomo, T.; Leza, J.C. The gut-microbiota-brain axis in a Spanish population in the aftermath of the COVID-19 pandemic: Microbiota composition linked to anxiety, trauma, and depression profiles. Gut Microbes 2023, 15, 2162306. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Chen, B.; Duan, Z.; Xia, Z.; Ding, Y.; Chen, T.; Liu, H.; Wang, B.; Yang, B.; Wang, X.; et al. Depression and anxiety in patients with active ulcerative colitis: Crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes 2021, 13, 1987779. [Google Scholar] [CrossRef]
- Li, S.; Zhuo, M.; Huang, X.; Huang, Y.; Zhou, J.; Xiong, D.; Li, J.; Liu, Y.; Pan, Z.; Li, H.; et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ 2020, 8, e9574. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, L.Y.; Zhang, Z.; Zhou, Y.Y.; Jiang, H.Y.; Ruan, B. Analysis of gut mycobiota in first-episode, drug-naïve Chinese patients with schizophrenia: A pilot study. Behav. Brain Res. 2020, 379, 112374. [Google Scholar] [CrossRef]
- Gao, F.; Guo, R.; Ma, Q.; Li, Y.; Wang, W.; Fan, Y.; Ju, Y.; Zhao, B.; Gao, Y.; Qian, L.; et al. Stressful events induce long-term gut microbiota dysbiosis and associated post-traumatic stress symptoms in healthcare workers fighting against COVID-19. J. Affect. Disord. 2022, 303, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Malan-Muller, S.; Valles-Colomer, M.; Foxx, C.L.; Vieira-Silva, S.; van den Heuvel, L.L.; Raes, J.; Seedat, S.; Lowry, C.A.; Hemmings, S.M.J. Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. Eur. Neuropsychopharmacol. 2022, 56, 24–38. [Google Scholar] [CrossRef]
- Dressman, J.B. Comparison of canine and human gastrointestinal physiology. Pharm. Res. 1986, 3, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.H.; Ruffman, T. Emotional contagion: Dogs and humans show a similar physiological response to human infant crying. Behav. Processes. 2014, 108, 155–165. [Google Scholar] [CrossRef]
- Kujala, M.V.; Kujala, J.; Carlson, S.; Hari, R. Dog experts’ brains distinguish socially relevant body postures similarly in dogs and humans. PLoS ONE 2012, 7, e39145. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.P.; Kultima, J.R.; Costea, P.I.; Fournier, C.; Pan, Y.; Czarnecki-Maulden, G.; Hayward, M.R.; Forslund, S.K.; Schmidt, T.S.B.; Descombes, P.; et al. Similarity of the dog and human gut microbiomes in gene content and response to diet. Microbiome 2018, 6, 72. [Google Scholar] [CrossRef]
- Mizukami, K.; Uchiyama, J.; Igarashi, H.; Murakami, H.; Osumi, T.; Shima, A.; Ishiahra, G.; Nasukawa, T.; Une, Y.; Sakaguchi, M. Age-related analysis of the gut microbiome in a purebred dog colony. FEMS Microbiol. Lett. 2019, 366, fnz095. [Google Scholar] [CrossRef]
- Masuoka, H.; Shimada, K.; Kiyosue-Yasuda, T.; Kiyosue, M.; Oishi, Y.; Kimura, S.; Yamada, A.; Hirayama, K. Transition of the intestinal microbiota of dogs with age. Biosci. Microbiota Food Health 2017, 36, 27–31. [Google Scholar] [CrossRef]
- Kubinyi, E.; Bel Rhali, S.; Sándor, S.; Szabó, A.; Felföldi, T. Gut Microbiome Composition is Associated with Age and Memory Performance in Pet Dogs. Animals 2020, 10, 1488. [Google Scholar] [CrossRef]
- Mondo, E.; Barone, M.; Soverini, M.; D’Amico, F.; Cocchi, M.; Petrulli, C.; Mattioli, M.; Marliani, G.; Candela, M.; Accorsi, P.A. Gut microbiome structure and adrenocortical activity in dogs with aggressive and phobic behavioral disorders. Heliyon 2020, 6, e03311. [Google Scholar] [CrossRef] [PubMed]
- Kirchoff, N.S.; Udell, M.A.R.; Sharpton, T.J. The gut microbiome correlates with conspecific aggression in a small population of rescued dogs (Canis familiaris). PeerJ 2019, 7, e6103. [Google Scholar] [CrossRef] [PubMed]
- Craddock, H.A.; Godneva, A.; Rothschild, D.; Motro, Y.; Grinstein, D.; Lotem-Michaeli, Y.; Narkiss, T.; Segal, E.; Moran-Gilad, J. Phenotypic correlates of the working dog microbiome. NPJ Biofilms Microbiomes 2022, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Pellowe, S.D.; Zhang, A.; Bignell, A.R.D.; Peña-Castillo, L.; Walsh, C.J. Gut microbiome composition is related to anxiety and aggression score in companion dogs. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef]
- Sbrini, G.; Hanswijk, S.I.; Brivio, P.; Middelman, A.; Bader, M.; Fumagalli, F.; Alenina, N.; Homberg, J.R.; Calabrese, F. Peripheral Serotonin Deficiency Affects Anxiety-like Behavior and the Molecular Response to an Acute Challenge in Rats. Int. J. Mol. Sci. 2022, 23, 4941. [Google Scholar] [CrossRef]
- Nanthakumaran, S.; Sridharan, S.; Somagutta, M.R.; Arnold, A.A.; May, V.; Pagad, S.; Malik, B.H. The Gut-Brain Axis and Its Role in Depression. Cureus. 2020, 12, e10280. [Google Scholar] [CrossRef]
- Sjögren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Bäckhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Ding, C.; Zhao, W.; Xu, L.; Tian, H.; Gong, J.; Zhu, M.; Li, J.; Li, N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 2017, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Keszthelyi, D.; Troost, F.J.; Jonkers, D.M.; Kruimel, J.W.; Leue, C.; Masclee, A.A. Decreased levels of kynurenic acid in the intestinal mucosa of IBS patients: Relation to serotonin and psychological state. J. Psychosom. Res. 2013, 74, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Golubeva, A.V.; Joyce, S.A.; Moloney, G.; Burokas, A.; Sherwin, E.; Arboleya, S.; Flynn, I.; Khochanskiy, D.; Moya-Pérez, A.; Peterson, V.; et al. Microbiota-related Changes in Bile Acid & Tryptophan Metabolism are Associated with Gastrointestinal Dysfunction in a Mouse Model of Autism. eBioMedicine 2017, 24, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.K.; Essa, M.M.; de Paula Martins, R.; Lovejoy, D.B.; Bilgin, A.A.; Waly, M.I.; Al-Farsi, Y.M.; Al-Sharbati, M.; Al-Shaffae, M.A.; Guillemin, G.J. Altered kynurenine pathway metabolism in autism: Implication for immune-induced glutamatergic activity. Autism Res. 2016, 9, 621–631. [Google Scholar] [CrossRef]
- Rosado, B.; García-Belenguer, S.; León, M.; Chacón, G.; Villegas, A.; Palacio, J. Blood concentrations of serotonin, cortisol and dehydroepiandrosterone in aggressive dogs. Appl. Anim. Behav. Sci. 2010, 123, 124–130. [Google Scholar] [CrossRef]
- Amat, M.; Le Brech, S.; Camps, T.; Torrente, C.; Mariotti, V.M.; Ruiz, J.L.; Manteca, X. Differences in serotonin serum concentration between aggressive English cocker spaniels and aggressive dogs of other breeds. J. Vet. Behav. 2013, 8, 19–25. [Google Scholar] [CrossRef]
- León, M.; Rosado, B.; García-Belenguer, S.; Chacón, G.; Villegas, A.; Palacio, J. Assessment of serotonin in serum, plasma, and platelets of aggressive dogs. J. Vet. Behav. 2012, 7, 348–352. [Google Scholar] [CrossRef]
- Cannas, S.; Tonini, B.; Bela, B.; Di Prinzio, R.; Pignataro, G.; Di Simone, D.; Gramenzi, A. Effect of a novel nutraceutical supplement (Relaxigen Pet dog) on the fecal microbiome and stress-related behaviors in dogs: A pilot study. J. Vet. Behav. 2021, 42, 37–47. [Google Scholar] [CrossRef]
- Bardo, M.T. Neuropharmacological mechanisms of drug reward: Beyond dopamine in the nucleus accumbens. Crit. Rev. Neurobiol. 1998, 12, 37–67. [Google Scholar] [CrossRef]
- Baik, J.H. Dopamine signaling in reward-related behaviors. Front. Neural Circuits 2013, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Beninger, R.J.; Miller, R. Dopamine D1-like receptors and reward-related incentive learning. Neurosci. Biobehav. Rev. 1998, 22, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.G.; Florio, E.; Punzo, D.; Borrelli, E. The Brain’s Reward System in Health and Disease. Adv. Exp. Med. Biol. 2021, 1344, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Yadid, G.; Friedman, A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog. Brain Res. 2008, 172, 265–286. [Google Scholar] [CrossRef]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The function of gut microbiota in immune-related neurological disorders: A review. J. Neuroinflamm. 2022, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Aneman, A.; Friberg, P.; Hooper, D.; Fåndriks, L.; Lonroth, H.; Hunyady, B.; Mezey, E. Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab. 1997, 82, 3864–3871. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- Wright, H.F.; Mills, D.S.; Pollux, P.M. Behavioural and physiological correlates of impulsivity in the domestic dog (Canis familiaris). Physiol. Behav. 2012, 105, 676–682. [Google Scholar] [CrossRef]
- Riva, J.; Bondiolotti, G.; Michelazzi, M.; Verga, M.; Carenzi, C. Anxiety related behavioural disorders and neurotransmitters in dogs. Appl. Anim. Behav. Sci. 2008, 114, 168–181. [Google Scholar] [CrossRef]
- González-Martínez, Á.; Muñiz de Miguel, S.; Graña, N.; Costas, X.; Diéguez, F.J. Serotonin and Dopamine Blood Levels in ADHD-like Dogs. Animals 2023, 13, 1037. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Heise, K.F.; Chalavi, S.; Puts, N.A.J.; Edden, R.A.E.; Swinnen, S.P. The role of MRS-assessed GABA in human behavioral performance. Prog. Neurobiol. 2022, 212, 102247. [Google Scholar] [CrossRef] [PubMed]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front. Neurosci. 2020, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Jie, F.; Yin, G.; Yang, W.; Yang, M.; Gao, S.; Lv, J.; Li, B. Stress in Regulation of GABA Amygdala System and Relevance to Neuropsychiatric Diseases. Front. Neurosci. 2018, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G.; van der Veen, J.W.; Tumonis, T.; Meyers, N.; Shen, J.; Drevets, W.C. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 2007, 64, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.W.; Narayan, M.; Woods, S.W.; Germine, M.; Kramer, G.L.; Davis, L.L.; Petty, F. Plasma levels of gamma-aminobutyric acid and panic disorder. Psychiatry Res. 1996, 63, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Dolfen, N.; Veldman, M.P.; Gann, M.A.; von Leupoldt, A.; Puts, N.A.J.; Edden, R.A.E.; Mikkelsen, M.; Swinnen, S.; Schwabe, L.; Albouy, G.; et al. A role for GABA in the modulation of striatal and hippocampal systems under stress. Commun. Biol. 2021, 4, 1033. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Xie, M.; Chen, H.H.; Nie, S.P.; Yin, J.Y.; Xie, M.Y. Gamma-Aminobutyric Acid Increases the Production of Short-Chain Fatty Acids and Decreases pH Values in Mouse Colon. Molecules 2017, 22, 653. [Google Scholar] [CrossRef]
- Park, K.B.; Oh, S.H. Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour. Technol. 2007, 98, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Inagawa, K.; Seki, S.; Bannai, M.; Takeuchi, Y.; Mori, Y.; Takahashi, M. Alleviative effects of gamma-aminobutyric acid (GABA) on behavioral abnormalities in aged dogs. J. Vet. Med. Sci. 2005, 67, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Uetake, K.; Okumoto, A.; Tani, N.; Goto, A.; Tanaka, T. Calming effect of orally administered γ-aminobutyric acid in Shih Tzu dogs. Anim. Sci. J. 2012, 83, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Unterer, S.; Suchodolski, J.S.; Honneffer, J.B.; Guard, B.C.; Lidbury, J.A.; Steiner, J.M.; Fritz, J.; Kölle, P. The fecal microbiome and metabolome differs between dogs fed Bones and Raw Food (BARF) diets and dogs fed commercial diets. PLoS ONE 2018, 13, e0201279. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- Kim, C.H. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell. Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Guard, B.C.; Barr, J.W.; Reddivari, L.; Klemashevich, C.; Jayaraman, A.; Steiner, J.M.; Vanamala, J.; Suchodolski, J.S. Characterization of microbial dysbiosis and metabolomic changes in dogs with acute diarrhea. PLoS ONE 2015, 10, e0127259. [Google Scholar] [CrossRef] [PubMed]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Fock, E.; Parnova, R. Mechanisms of Blood-Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Park. Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Chen, H.; Meng, L.; Shen, L. Multiple roles of short-chain fatty acids in Alzheimer disease. Nutrition 2022, 93, 111499. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Maltz, R.M.; Keirsey, J.; Kim, S.C.; Mackos, A.R.; Gharaibeh, R.Z.; Moore, C.C.; Xu, J.; Somogyi, A.; Bailey, M.T. Social Stress Affects Colonic Inflammation, the Gut Microbiome, and Short-chain Fatty Acid Levels and Receptors. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal Short Chain Fatty Acids Profile is Changed in Polish Depressive Women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.L.; Pan, J.X.; Zheng, P.; Xia, J.J.; Yin, B.M.; Liang, W.W.; Li, Y.F.; Wu, J.; Xu, F.; Wu, Q.Y.; et al. Metabonomics reveals peripheral and central short-chain fatty acid and amino acid dysfunction in a naturally occurring depressive model of macaques. Neuropsychiatr. Dis. Treat. 2019, 15, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Sun, C.L.; Lai, T.T.; Liou, C.W.; Lin, Y.Y.; Xue, J.Y.; Wang, H.W.; Chai, L.M.X.; Lee, Y.J.; Chen, S.L.; et al. Oral short-chain fatty acids administration regulates innate anxiety in adult microbiome-depleted mice. Neuropharmacology 2022, 214, 109140. [Google Scholar] [CrossRef] [PubMed]
- Tizard, I.R.; Jones, S.W. The Microbiota Regulates Immunity and Immunologic Diseases in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Chen, W.D.; Wang, Y.D. Gut microbiota: An integral moderator in health and disease. Front. Microbiol. 2018, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, A.; Molin, G. Gut microbiota and inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Giaretta, P.R.; Rech, R.R.; Guard, B.C.; Blake, A.B.; Blick, A.K.; Steiner, J.M.; Lidbury, J.A.; Cook, A.K.; Hanifeh, M.; Spillmann, T.; et al. Comparison of intestinal expression of the apical sodium-dependent bile acid transporter between dogs with and without chronic inflammatory enteropathy. J. Vet. Intern. Med. 2018, 32, 1918–1926. [Google Scholar] [CrossRef]
- Guard, B.C.; Honneffer, J.B.; Jergens, A.E.; Jonika, M.M.; Toresson, L.; Lawrence, Y.A.; Webb, C.B.; Hill, S.; Lidbury, J.A.; Steiner, J.M.; et al. Longitudinal assessment of microbial dysbiosis, fecal unconjugated bile acid concentrations, and disease activity in dogs with steroid-responsive chronic inflammatory enteropathy. J. Vet. Intern. Med. 2019, 33, 1295–1305. [Google Scholar] [CrossRef]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 2015, 6, 33–47. [Google Scholar] [CrossRef]
- Blake, A.B.; Guard, B.C.; Honneffer, J.B.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Altered microbiota, fecal lactate, and fecal bile acids in dogs with gastrointestinal disease. PLoS ONE 2019, 14, e0224454. [Google Scholar] [CrossRef]
- Li, Q. Metabolic Reprogramming, Gut Dysbiosis, and Nutrition Intervention in Canine Heart Disease. Front. Vet. Sci. 2022, 9, 791754. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Matthewman, L.; Xia, D.; Wilshaw, J.; Chang, Y.M.; Connolly, D.J. The gut microbiome in dogs with congestive heart failure: A pilot study. Sci. Rep. 2020, 10, 13777. [Google Scholar] [CrossRef]
- Cintio, M.; Scarsella, E.; Sgorlon, S.; Sandri, M.; Stefanon, B. Gut Microbiome of Healthy and Arthritic Dogs. Vet. Sci. 2020, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Muscatello, M.R.; Bruno, A.; Scimeca, G.; Pandolfo, G.; Zoccali, R.A. Role of negative affects in pathophysiology and clinical expression of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 7570–7586. [Google Scholar] [CrossRef]
- Gądek-Michalska, A.; Tadeusz, J.; Rachwalska, P.; Bugajski, J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol. Rep. 2013, 65, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef]
- D’Mello, C.; Ronaghan, N.; Zaheer, R.; Dicay, M.; Le, T.; MacNaughton, W.K.; Surrette, M.G.; Swain, M.G. Probiotics Improve Inflammation-Associated Sickness Behavior by Altering Communication between the Peripheral Immune System and the Brain. J. Neurosci. 2015, 35, 10821–10830. [Google Scholar] [CrossRef]
- Han, S.K.; Kim, D.H. Lactobacillus mucosae and Bifidobacterium longum Synergistically Alleviate Immobilization Stress-Induced Anxiety/Depression in Mice by Suppressing Gut Dysbiosis. J. Microbiol. Biotechnol. 2019, 29, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Sechi, S.; Di Cerbo, A.; Canello, S.; Guidetti, G.; Chiavolelli, F.; Fiore, F.; Cocco, R. Effects in dogs with behavioural disorders of a commercial nutraceutical diet on stress and neuroendocrine parameters. Vet. Rec. 2017, 180, 18. [Google Scholar] [CrossRef] [PubMed]
- Di Cerbo, A.; Centenaro, S.; Beribè, F.; Laus, F.; Cerquetella, M.; Spaterna, A.; Guidetti, G.; Canello, S.; Terrazzano, G. Clinical evaluation of an antiinflammatory and antioxidant diet effect in 30 dogs affected by chronic otitis externa: Preliminary results. Vet. Res. Commun. 2016, 40, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Park, Y.M.; Hwang, H.M.; Shin, D.Y.; Jeong, H.N.; Kim, J.G.; Park, H.Y.; Kim, D.S.; Yoo, J.J.; Kim, M.S.; et al. The Effect of the Mixed Extract of Kalopanax pictus Nakai and Achyranthes japonica Nakai on the Improvement of Degenerative Osteoarthritis through Inflammation Inhibition in the Monosodium Iodoacetate-Induced Mouse Model. Curr. Issues Mol. Biol. 2023, 45, 6395–6414. [Google Scholar] [CrossRef] [PubMed]
- Re, S.; Zanoletti, M.; Emanuele, E. Association of inflammatory markers elevation with aggressive behavior in domestic dogs. J. Ethol. 2009, 27, 31–33. [Google Scholar] [CrossRef]
- Merchenthaler, I. Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides 1984, 5 (Suppl. S1), 53–69. [Google Scholar] [CrossRef] [PubMed]
- Packard, A.E.; Egan, A.E.; Ulrich-Lai, Y.M. HPA Axis Interactions with Behavioral Systems. Compr. Physiol. 2016, 6, 1897–1934. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.E.; Papilloud, A.; Huzard, D.; Sandi, C. The link between aberrant hypothalamic-pituitary-adrenal axis activity during development and the emergence of aggression-Animal studies. Neurosci. Biobehav. Rev. 2018, 91, 138–152. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Cubała, W.J.; Landowski, J. Serotoninergic system and limbic-hypothalamic-pituitary-adrenal axis (LHPA axis) in depression. Psychiatr. Pol. 2006, 40, 415–430. [Google Scholar] [PubMed]
- Du, X.; Pang, T.Y. Is Dysregulation of the HPA-Axis a Core Pathophysiology Mediating Co-Morbid Depression in Neurodegenerative Diseases? Front. Psychiatry 2015, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. Blood-brain barrier transport of cytokines: A mechanism for neuropathology. Curr. Pharm. Des. 2005, 11, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zeng, B.; Zeng, L.; Du, X.; Li, B.; Huo, R.; Liu, L.; Wang, H.; Dong, M.; Pan, J.; et al. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl. Psychiatry 2018, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Markovszky, A.K.; Weber, C.; Biksi, O.; Danes, M.; Dumitrescu, E.; Muselin, F.; Tufarelli, V.; Puvača, N.; Cristina, R.T. Is ECLIA Serum Cortisol Concentration Measurement, an Accurate Indicator of Pain Severity in Dogs with Locomotor Pain? Animals 2020, 10, 2036. [Google Scholar] [CrossRef]
- d’Angelo, D.; d’Ingeo, S.; Ciani, F.; Visone, M.; Sacchettino, L.; Avallone, L.; Quaranta, A. Cortisol Levels of Shelter Dogs in Animal Assisted Interventions in a Prison: An Exploratory Study. Animals 2021, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Lensen, R.C.M.M.; Moons, C.P.H.; Diederich, C. Physiological stress reactivity and recovery related to behavioral traits in dogs (Canis familiaris). PLoS ONE 2019, 14, e0222581. [Google Scholar] [CrossRef]
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, prebiotics and postbiotics on mitigation of depression symptoms: Modulation of the brain-gut-microbiome axis. Biomolecules 2021, 11, 1000. [Google Scholar] [CrossRef]
- Nigam, M.; Panwar, A.S.; Singh, R.K. Orchestrating the fecal microbiota transplantation: Current technological advancements and potential biomedical application. Front. Med. Technol. 2022, 4, 961569. [Google Scholar] [CrossRef] [PubMed]
- Settanni, C.R.; Ianiro, G.; Bibbò, S.; Cammarota, G.; Gasbarrini, A. Gut microbiota alteration and modulation in psychiatric disorders: Current evidence on fecal microbiota transplantation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110258. [Google Scholar] [CrossRef]
- Borody, T.J.; Paramsothy, S.; Agrawal, G. Fecal microbiota transplantation: Indications, methods, evidence, and future directions. Curr. Gastroenterol. Rep. 2013, 15, 337. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.K.; Michaelsen, T.Y.; Bundgaard-Nielsen, C.; Nielsen, R.E.; Hjerrild, S.; Leutscher, P.; Wegener, G.; Sørensen, S. Faecal microbiota transplantation from patients with depression or healthy individuals into rats modulates mood-related behaviour. Sci. Rep. 2021, 11, 21869. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Xie, R.; Lin, L.; Jiang, J.; Du, L.; Zeng, X.; Li, G.; Wang, C.; Qiao, Y. Fecal microbiota transplantation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depressive-like behavior. Eur. J. Neurosci. 2021, 53, 3598–3611. [Google Scholar] [CrossRef] [PubMed]
- Doll, J.P.K.; Vázquez-Castellanos, J.F.; Schaub, A.C.; Schweinfurth, N.; Kettelhack, C.; Schneider, E.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Fecal Microbiota Transplantation (FMT) as an Adjunctive Therapy for Depression-Case Report. Front. Psychiatry 2022, 13, 815422. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Guo, Q.; Wen, Z.; Tan, S.; Chen, J.; Lin, L.; Chen, P.; He, J.; Wen, J.; Chen, Y. The multiple effects of fecal microbiota transplantation on diarrhea-predominant irritable bowel syndrome (IBS-D) patients with anxiety and depression behaviors. Microb. Cell Factories 2021, 20, 233. [Google Scholar] [CrossRef]
- Kurokawa, S.; Kishimoto, T.; Mizuno, S.; Masaoka, T.; Naganuma, M.; Liang, K.C.; Kitazawa, M.; Nakashima, M.; Shindo, C.; Suda, W.; et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: An open-label observational study. J. Affect. Disord. 2018, 235, 506–512. [Google Scholar] [CrossRef]
- Gal, A.; Barko, P.C.; Biggs, P.J.; Gedye, K.R.; Midwinter, A.C.; Williams, D.A.; Burchell, R.K.; Pazzi, P. One dog’s waste is another dog’s wealth: A pilot study of fecal microbiota transplantation in dogs with acute hemorrhagic diarrhea syndrome. PLoS ONE 2021, 16, e0250344. [Google Scholar] [CrossRef] [PubMed]
- Chaitman, J.; Ziese, A.L.; Pilla, R.; Minamoto, Y.; Blake, A.B.; Guard, B.C.; Isaiah, A.; Lidbury, J.A.; Steiner, J.M.; Unterer, S.; et al. Fecal Microbial and Metabolic Profiles in Dogs with Acute Diarrhea Receiving Either Fecal Microbiota Transplantation or Oral Metronidazole. Front. Vet. Sci. 2020, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Bottero, E.; Benvenuti, E.; Ruggiero, P. Faecal Microbiota Transplantation in 16 Dogs with Idiopathic Inflammatory Bowel Disease. Veterinaria 2017, 31, 31–45. [Google Scholar]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Fecal microbiota transplantation as a new treatment for canine inflammatory bowel disease. Biosci. Microbiota Food Health 2021, 40, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Toresson, L.; Spillmann, T.; Pilla, R.; Ludvigsson, U.; Hellgren, J.; Olmedal, G.; Suchodolski, J.S. Clinical Effects of Faecal Microbiota Transplantation as Adjunctive Therapy in Dogs with Chronic Enteropathies—A Retrospective Case Series of 41 Dogs. Vet. Sci. 2023, 10, 271. [Google Scholar] [CrossRef]
- Sugita, K.; Shima, A.; Takahashi, K.; Ishihara, G.; Kawano, K.; Ohmori, K. Pilot evaluation of a single oral fecal microbiota transplantation for canine atopic dermatitis. Sci. Rep. 2023, 13, 8824. [Google Scholar] [CrossRef]
| Fecal Microbiome Transplantation (FMT) Procedure in Dogs | |||
|---|---|---|---|
| Recipient | No of Recipients | FMT Method | Effects |
| Dogs with acute hemorrhagic diarrhea syndrome [181] | 8 | Endoscopic | No clinical benefit; however, increased abundance of SCFA-producing bacteria (beneficial for the organism) was observed |
| Dogs with acute diarrhea [182] | 11 | Rectal enema | Fecal consistency significantly improved in all dogs, with proper microbial (based on dysbiosis index) and metabolic profiles (in contrast to dogs treated with metronidazole) |
| Dogs with inflammatory bowel diseases [183,184] | 16 [183]; 9 [184] | Oral/endoscopic [183]; rectal enema [184] | Clinical improvement in most of dogs [183]; improvement in canine inflammatory bowel disease activity index in all dogs [184] |
| Dogs with chronic enteropathies (FMT used as add-on therapy) [185] | 41 | Rectal enema | Thirty-one dogs responded to treatment, resulting in improved fecal quality and/or activity level |
| Dogs with atopic dermatitis [186] | 12 | Oral | Eleven dogs presented significantly decreased skin lesions and pruritus scores and beneficially changed gut microbiota |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiełbik, P.; Witkowska-Piłaszewicz, O. The Relationship between Canine Behavioral Disorders and Gut Microbiome and Future Therapeutic Perspectives. Animals 2024, 14, 2048. https://doi.org/10.3390/ani14142048
Kiełbik P, Witkowska-Piłaszewicz O. The Relationship between Canine Behavioral Disorders and Gut Microbiome and Future Therapeutic Perspectives. Animals. 2024; 14(14):2048. https://doi.org/10.3390/ani14142048
Chicago/Turabian StyleKiełbik, Paula, and Olga Witkowska-Piłaszewicz. 2024. "The Relationship between Canine Behavioral Disorders and Gut Microbiome and Future Therapeutic Perspectives" Animals 14, no. 14: 2048. https://doi.org/10.3390/ani14142048
APA StyleKiełbik, P., & Witkowska-Piłaszewicz, O. (2024). The Relationship between Canine Behavioral Disorders and Gut Microbiome and Future Therapeutic Perspectives. Animals, 14(14), 2048. https://doi.org/10.3390/ani14142048





