Simulated Swine Digestion and Gut Microbiota Fermentation of Hydrolyzed Copra Meal
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrolysis Process of Copra Meal and Reducing Sugars and Total Carbohydrates Determination
2.3. Digestion Evaluation of CM and HCM by Swine Simulated Gastrointestinal (GI) Conditions
2.4. In Vitro Fermentation of Fecal Samples for Microbial Analysis and Volatile Fatty Acids Determination
2.4.1. DNA Extraction for Next-Generational Sequencing (NGS)
2.4.2. Microbial Analysis
2.4.3. Short-Chain Fatty Acid Analysis during Swine Microbiota Fermentation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Hydrolyzed Copra Meal
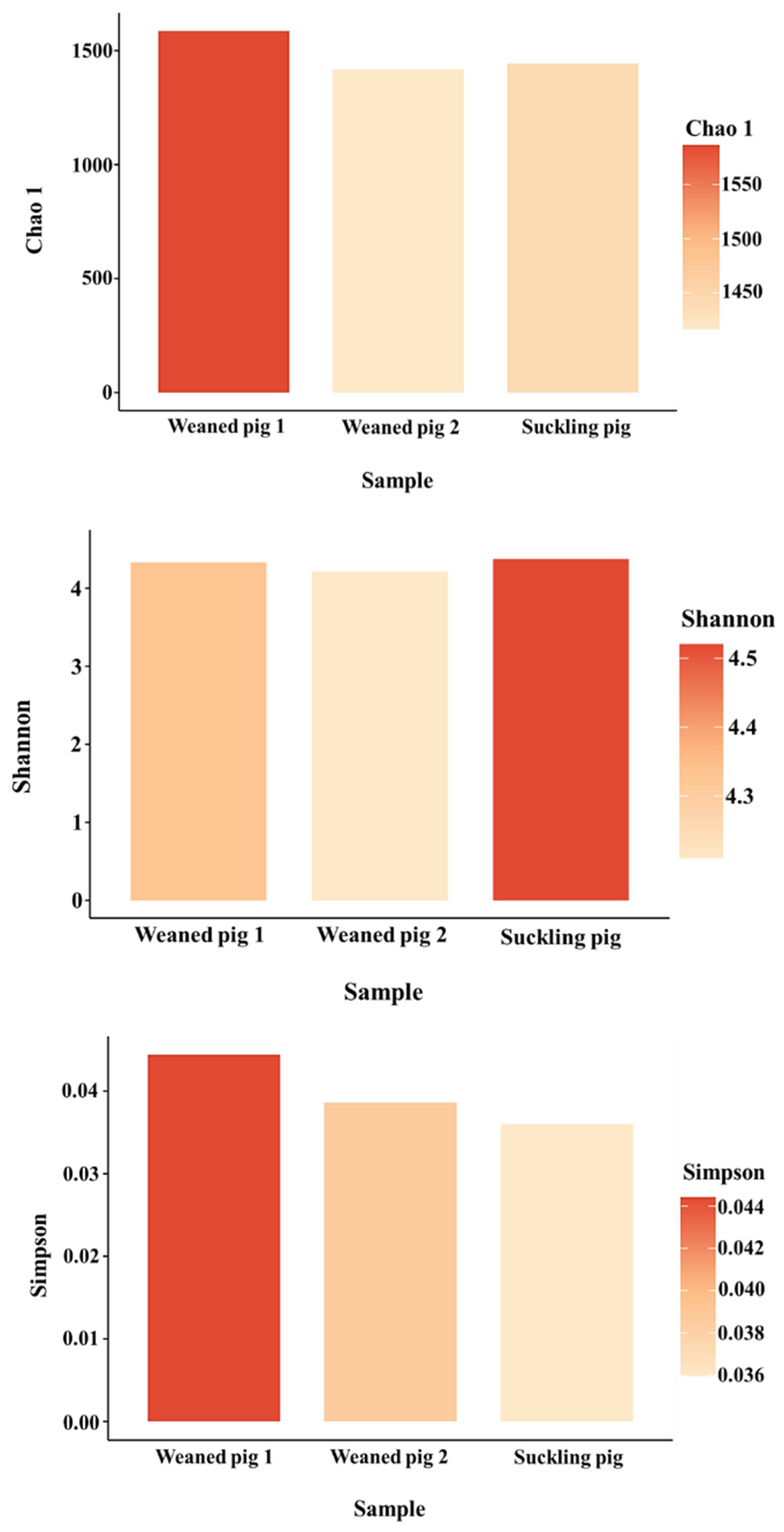
3.2. Understanding Microbiota of Swine Feces
3.3. Understanding the Modulation Effect of CM and HCM
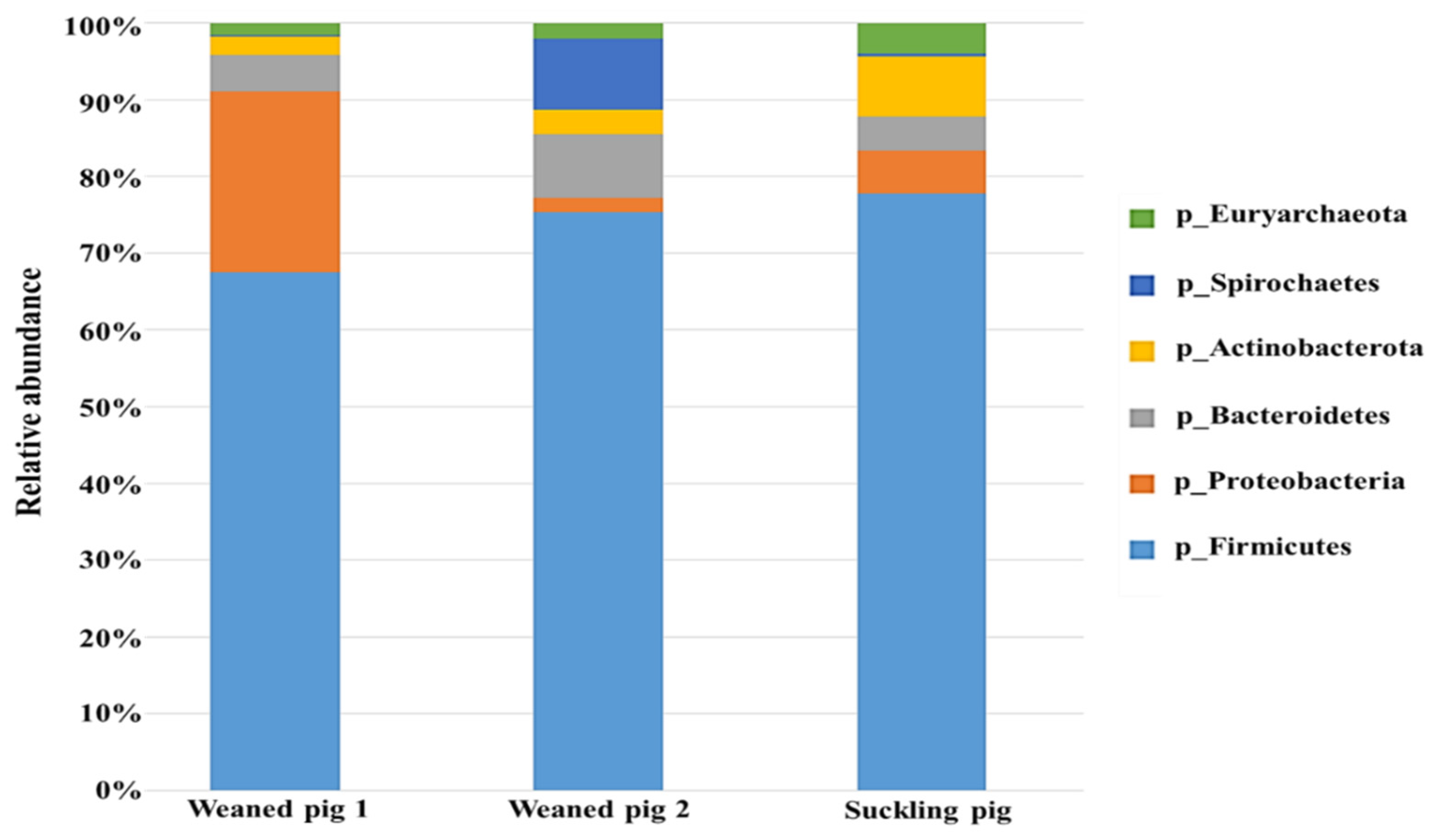
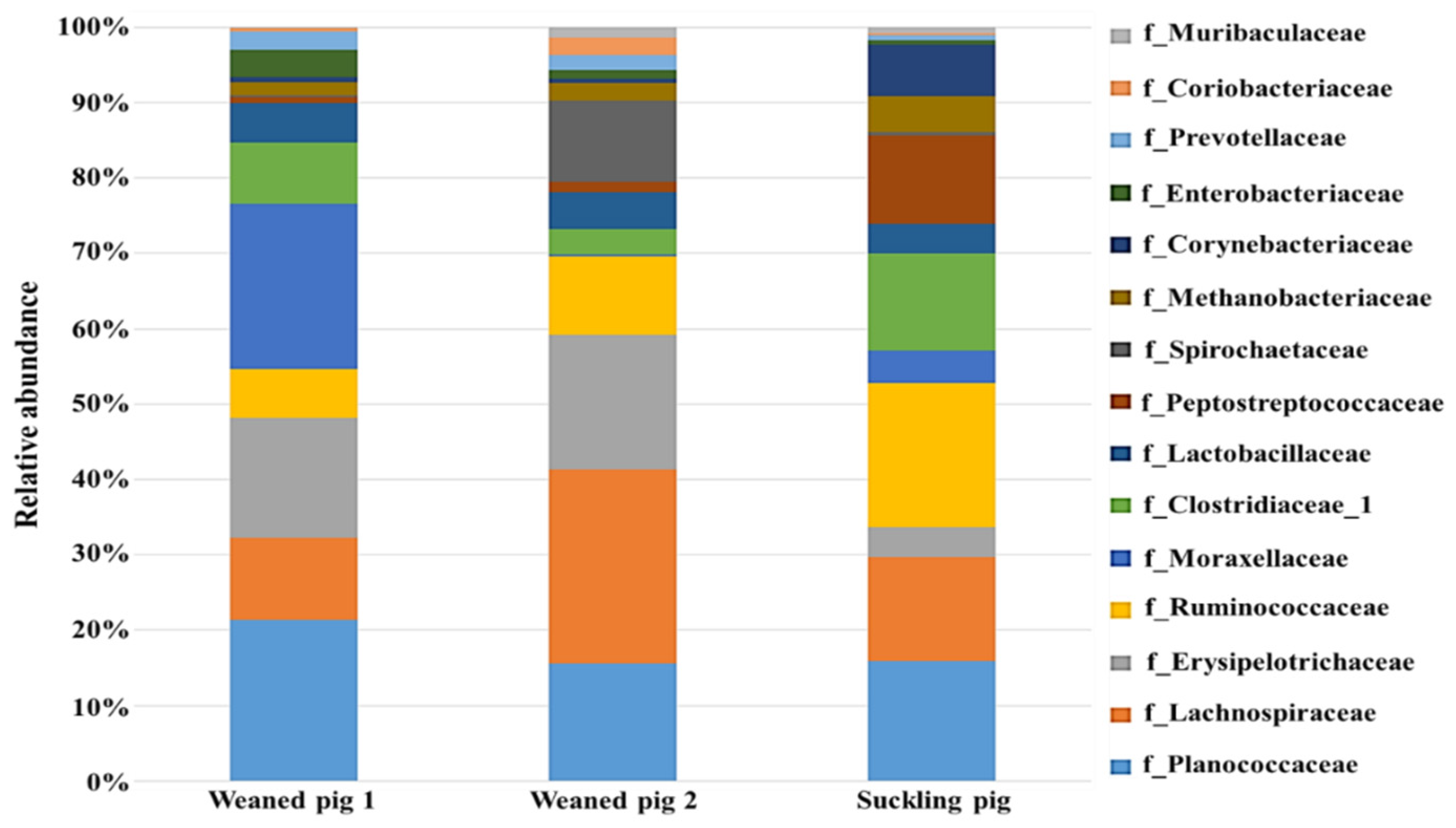
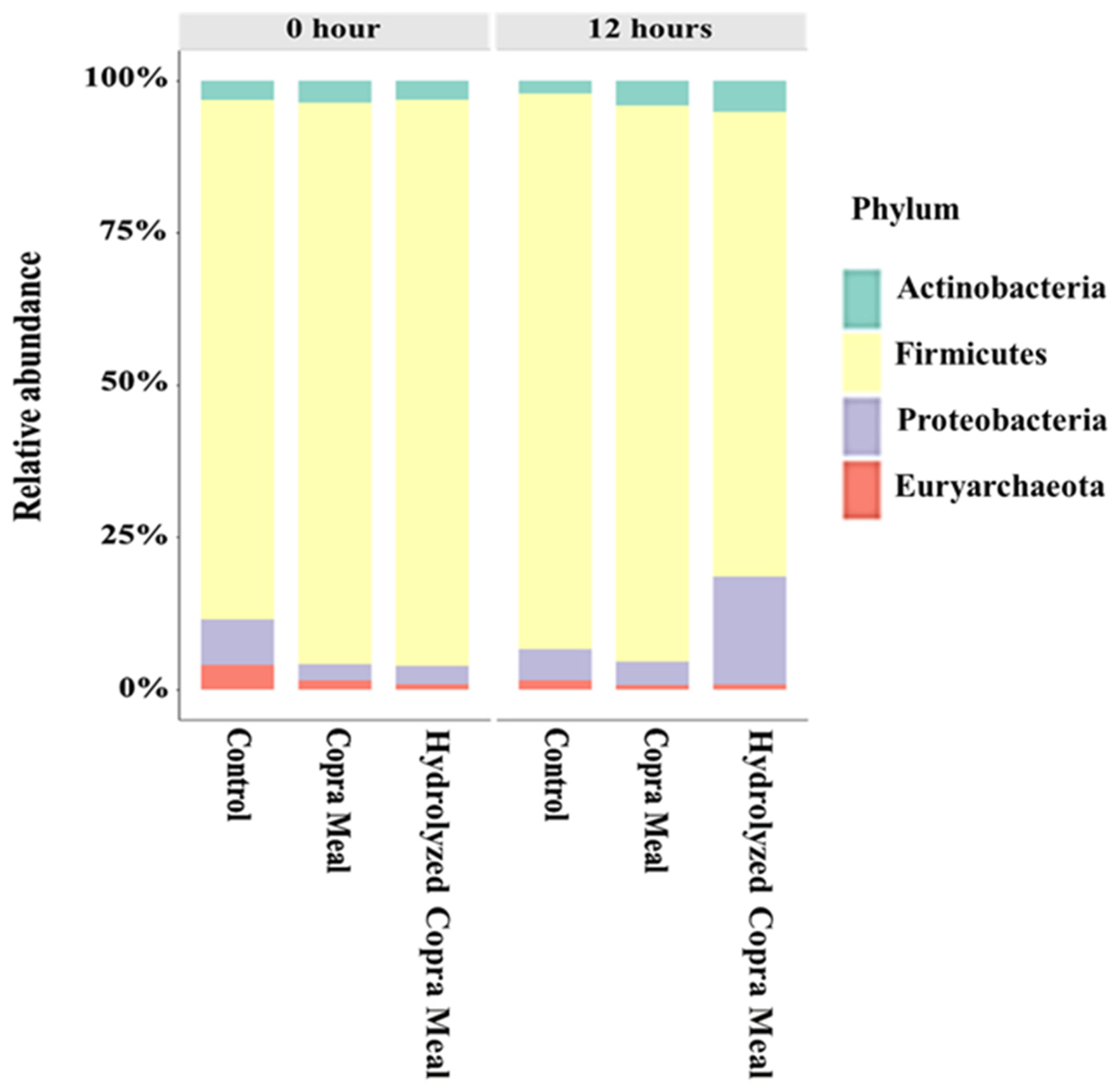
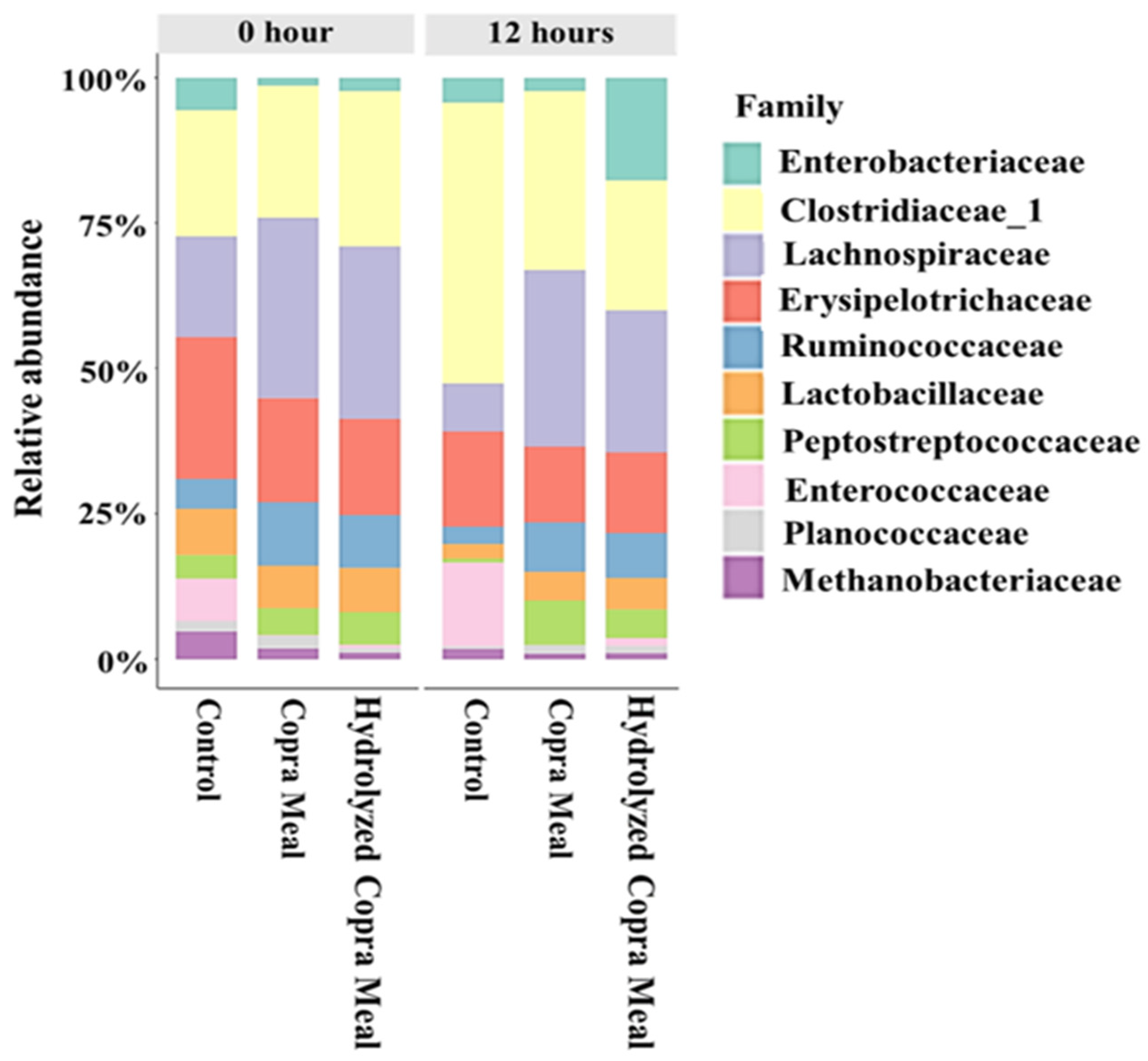
3.3.1. The Gut Microbiota Composition and Structure
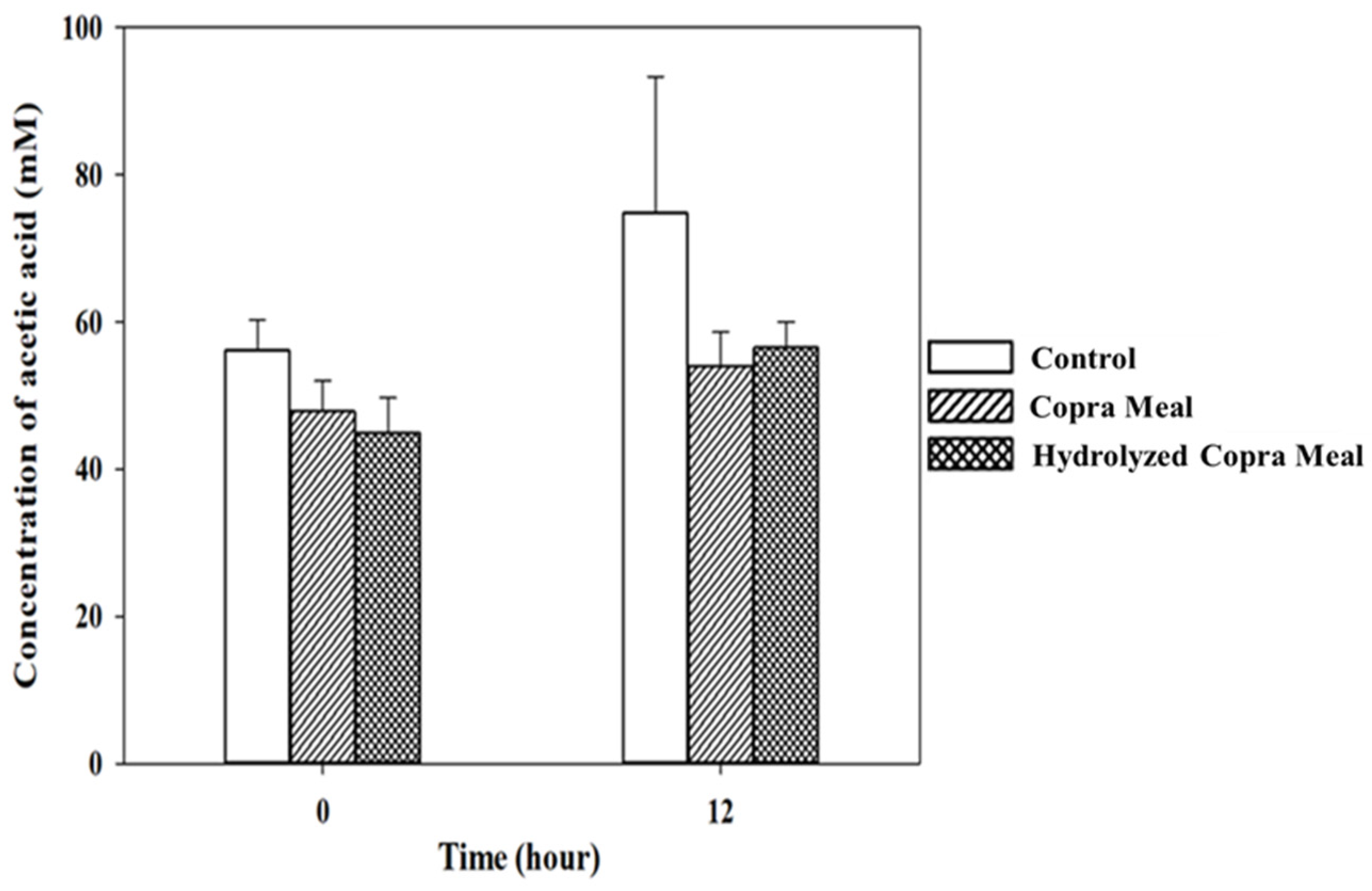
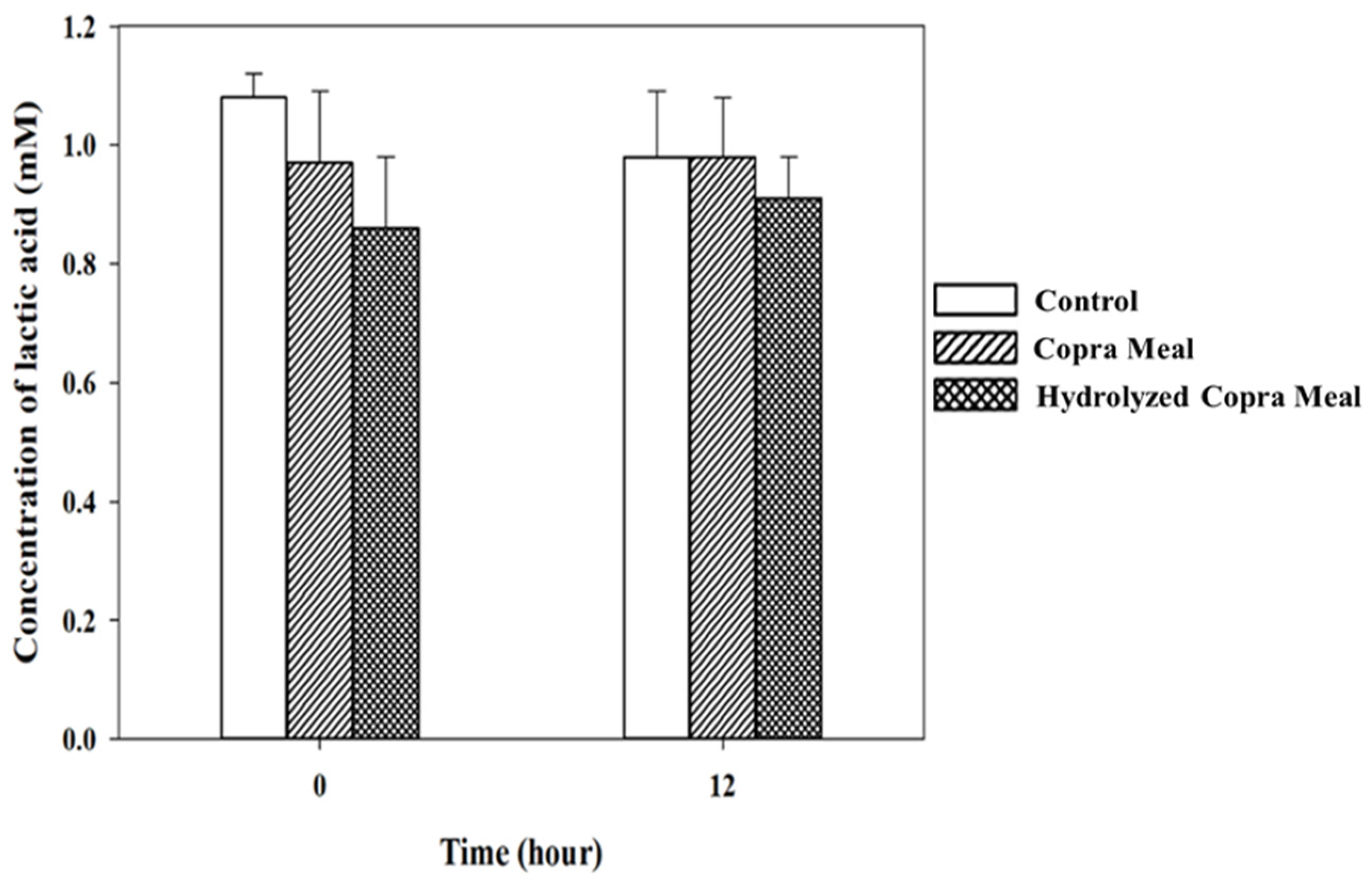
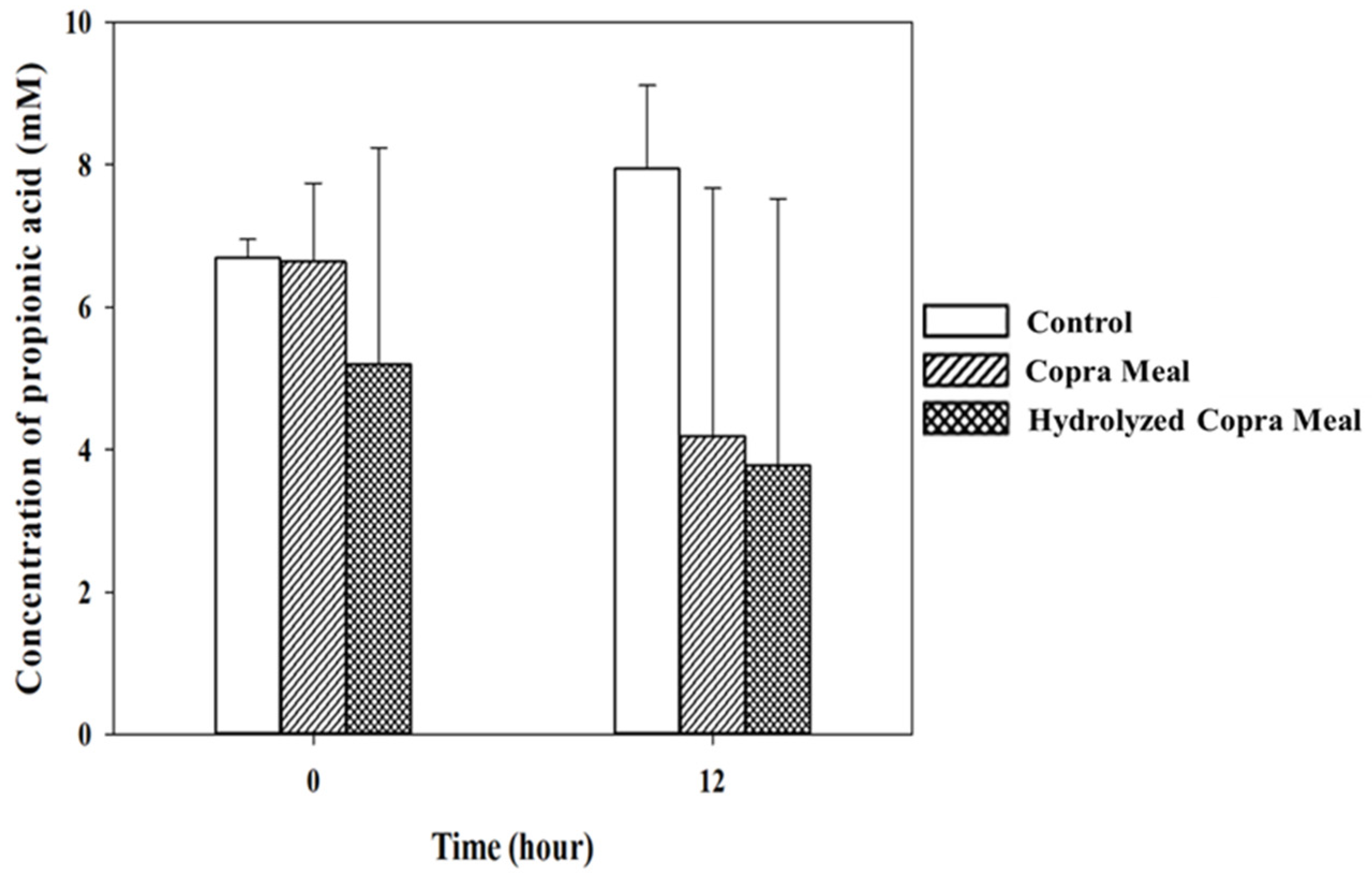
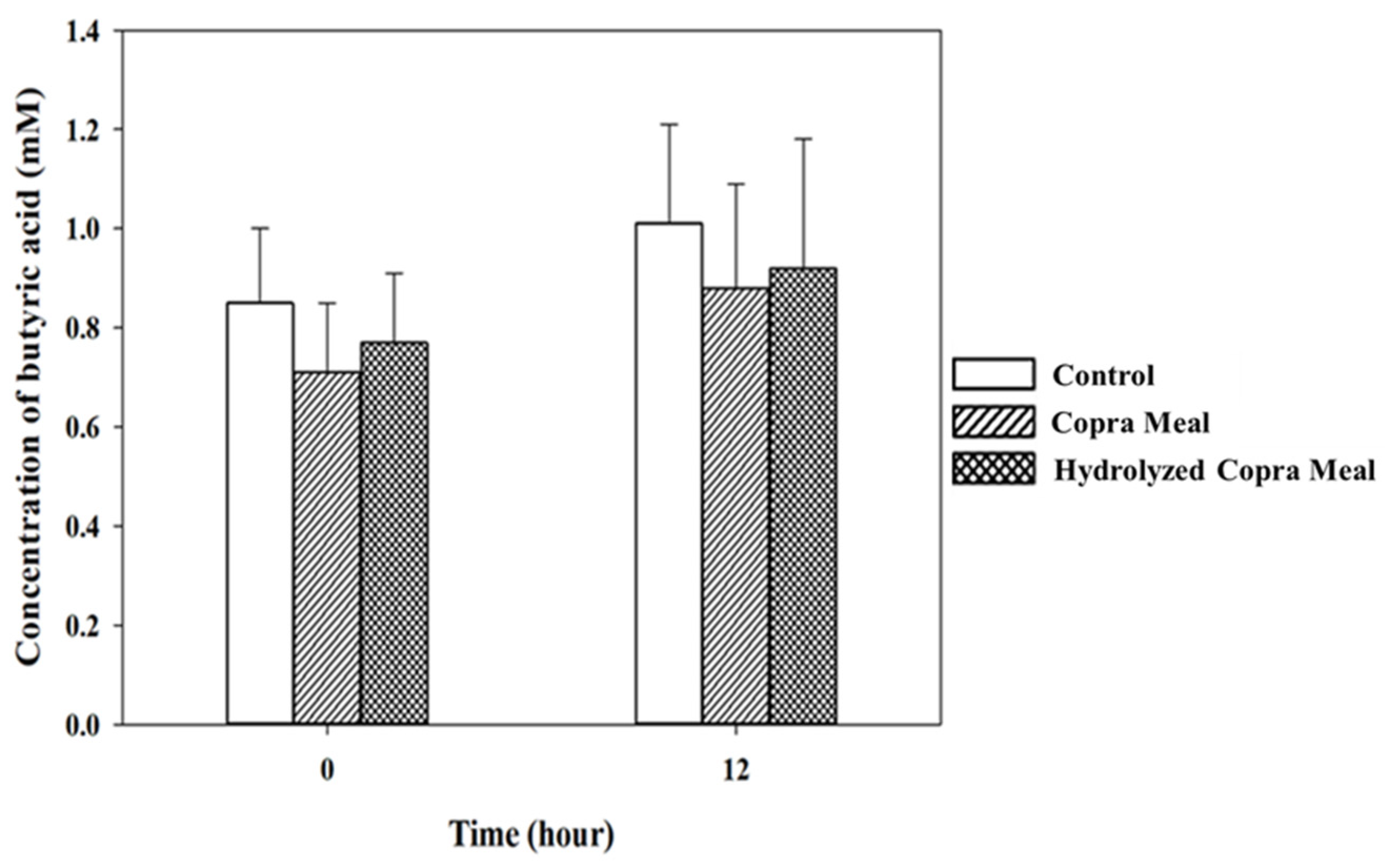
3.3.2. Short-Chain Fatty Acid during Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ayimbila, F.; Keawsompong, S. Characteristics and bioactive properties of agro-waste and yeast derived manno-oligosaccharides. Biocatal. Agric. Biotechnol. 2022, 119, 102522. [Google Scholar] [CrossRef]
- Tang, M.; Cheng, L.; Liu, Y.; Wu, Z.; Zhang, X.; Luo, S. Plant polysaccharides modulate immune function via the gut microbiome and may have potential in COVID-19 Therapy. Molecules 2022, 27, 2773. [Google Scholar] [CrossRef] [PubMed]
- Chacher, M.F.A.; Kamran, Z.; Ahsan, U.; Ahmad, S.; Koutoulis, K.C.; Qutab Ud Din, H.G.; Cengiz, Ö. Use of mannan oligosaccharide in broiler diets: An overview of underlying mechanisms. World’s Poult. Sci. J. 2017, 73, 831–844. [Google Scholar] [CrossRef]
- Jana, U.K.; Kango, N. Characteristics and bioactive properties of mannooligosaccharides derived from agro-waste mannans. Int J. Biol. Macromol. 2020, 149, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Rungruangsaphakun, J.; Nakphaichit, M.; Keawsompong, S. Nutritional improvement of copra meal for swine feed. Biocatal. Agric. Biotechnol. 2022, 39, 102273. [Google Scholar] [CrossRef]
- Rungrassamee, W.; Kingcha, Y.; Srimarut, Y.; Maibunkaew, S.; Karoonuthaisiri, N.; Visessanguan, W. Mannooligosaccharides from copra meal improves survival of the Pacific white shrimp (Litopenaeus vannamei) after exposure to Vibrio harveyi. Aquaculture 2014, 434, 403–410. [Google Scholar] [CrossRef]
- Rungruangsaphakun, J.; Keawsompong, S. Optimization of hydrolysis conditions for the mannooligosaccharides copra meal hydrolysate production. 3 Biotech 2018, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Thrastardottir, T.O.; Copeland, V.J.; Constantinou, C. The Association Between the Gut Microbiome, Nutritional Habits, Antibiotics, and Gastric Cancer: A Scoping Review. Curr. Nutr. Rep. 2022, 11, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Prayoonthien, P.; Nitisinprasert, S.; Keawsompong, S. In vitro fermentation of copra meal hydrolysate by chicken microbiota. 3 Biotech 2018, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Pongsapipatana, N.; Damrongteerapap, P.; Chantorn, S.; Sintuprapa, W.; Keawsompong, S.; Nitisinprasert, S. Molecular cloning of kman coding for mannanase from Klebsiella oxytoca KUB-CW2-3 and its hybrid mannanase characters. Enzyme Microb. Technol. 2016, 89, 39–51. [Google Scholar] [CrossRef]
- Beaumont, M.; Cauquil, L.; Bertide, A.; Ahn, I.; Barilly, C.; Gil, L.; Canlet, C.; Zemb, O.; Pascal, G.; Samson, A. Gut microbiota-derived metabolite signature in suckling and weaned piglets. J. Proteome Res. 2020, 20, 982–994. [Google Scholar] [CrossRef]
- Phothichitto, K.; Nitisinprasert, S.; Keawsompong, S. Isolation, screening and identification of mannanase producing microorganisms. Agric. Nat. Resour. 2006, 40 (Suppl. S6), 26–38. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Son, A.R.; Park, C.S.; Kim, B.G. Determination and prediction of digestible and metabolizable energy concentrations in byproduct feed ingredients fed to growing pigs. Asian Australas. J. Anim. Sci. 2017, 30, 546. [Google Scholar] [CrossRef]
- Plupjeen, S.-N.; Chawjiraphan, W.; Charoensiddhi, S.; Nitisinprasert, S.; Nakphaichit, M. Lactococcus lactis KA-FF 1-4 reduces vancomycin-resistant enterococci and impacts the human gut microbiome. 3 Biotech 2020, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Onumpai, C.; Kolida, S.; Bonnin, E.; Rastall, R.A. Microbial utilization and selectivity of pectin fractions with various structures. Appl. Environ. Microbiol. 2011, 77, 5747–5754. [Google Scholar] [CrossRef] [PubMed]
- Sathitkowitchai, W.; Suratannon, N.; Keawsompong, S.; Weerapakorn, W.; Patumcharoenpol, P.; Nitisinprasert, S.; Nakphaichit, M. A randomized trial to evaluate the impact of copra meal hydrolysate on gastrointestinal symptoms and gut microbiome. PeerJ 2021, 9, e12158. [Google Scholar] [CrossRef]
- Nakphaichit, M.; Thanomwongwattana, S.; Phraephaisarn, C.; Sakamoto, N.; Keawsompong, S.; Nakayama, J.; Nitisinprasert, S. The effect of including Lactobacillus reuteri KUB-AC5 during post-hatch feeding on the growth and ileum microbiota of broiler chickens. Poult. Sci. 2011, 90, 2753–2765. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Flyvbjerg, H. Octave plots for visualizing diversity of microbial OTUs. Biorxiv 2018, 389833. [Google Scholar] [CrossRef]
- Ayimbila, F.; Siriwong, S.; Nakphaichit, M.; Keawsompong, S. In vitro gastrointestinal digestion of Lentinus squarrosulus powder and impact on human fecal microbiota. Sci. Rep. 2022, 12, 2655. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shen, H.; Zhang, F.; Liu, X.; Xiao, Q.; Tan, B.; Ma, X. Applications and prospects of functional oligosaccharides in pig nutrition: A review. Anim. Nutr. 2023, 13, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Y.; Wen, Z.; Jiang, X.; Ma, X.; Han, X. Weaning stress perturbs gut microbiome and its metabolic profile in piglets. Sci. Rep. 2018, 8, 18068. [Google Scholar] [CrossRef] [PubMed]
- Saladrigas-García, M.; Ko, H.-L.; Nolis, P.; Ramayo-Caldas, Y.; Folch, J.M.; Llonch, P.; Solà-Oriol, D.; Pérez, J.F.; Martín-Orúe, S.M. Understanding host-microbiota interactions in the commercial piglet around weaning. Sci. Rep. 2021, 11, 23488. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Zhong, R.; Liu, L.; Lin, C.; Xiao, L.; Chen, L.; Zhang, H.; Beckers, Y.; Everaert, N. Effects of xylo-oligosaccharides on growth and gut microbiota as potential replacements for antibiotic in weaning piglets. Front. Microbiol. 2021, 12, 641172. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pan, L.; Shang, Q.H.; Ma, X.K.; Long, S.F.; Xu, Y.T.; Piao, X.S. Effects of isomalto-oligosaccharides as potential prebiotics on performance, immune function and gut microbiota in weaned pigs. Anim. Feed Sci. Technol. 2017, 230, 126–135. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras, D.F.; Dunière, L.; Blanquet-Diot, S.; Forano, E. Microbiota composition and functional profiling throughout the gastrointestinal tract of commercial weaning piglets. Microorganisms 2019, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Balagurunathan, R.; Radhakrishnan, M.; Shanmugasundaram, T.; Gopikrishnan, V.; Jerrine, J. Protocols in Actinobacterial Research; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Moon, C.D.; Young, W.; Maclean, P.H.; Cookson, A.L.; Bermingham, E.N. Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats. Microbiologyopen 2018, 7, e00677. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huang, X.; Zhao, S.; Sun, W.; Yan, Z.; Wang, P.; Li, S.; Huang, W.; Zhang, S.; Liu, L. Structure and function of the fecal microbiota in diarrheic neonatal piglets. Front. Microbiol. 2017, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Petry, A.L.; Patience, J.F.; Koester, L.R.; Huntley, N.F.; Bedford, M.R.; Schmitz-Esser, S. Xylanase modulates the microbiota of ileal mucosa and digesta of pigs fed corn-based arabinoxylans likely through both a stimbiotic and prebiotic mechanism. PLoS ONE 2021, 16, e0246144. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Qiu, X.; Zhu, S.; Liu, J.; Wang, J.; Wang, Q.; Liu, Z.; Yang, F.; Yun, T.; Qi, R. Soybean oligosaccharides combined with probiotics reduce faecal odour compound content by improving intestinal microbiota in pigs. J. Anim. Physiol. Anim. 2023, 107, 839–849. [Google Scholar] [CrossRef]
- Yamashita, H. Biological function of acetic acid–improvement in obesity and glucose tolerance by acetic acid in type 2 diabetic rats. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S171–S175. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.-G.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health benefits and side effects of short-chain fatty acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Trif, M.; Schwarze, A.-K.; Bethke, M.; Penedo, B.A.; Rusu, A. Individualized dietary supplements enriched with microbial propionic acid for athletes and the elderly with benefits on gut microbiota. Proceedings 2021, 66, 23. [Google Scholar] [CrossRef]
- Yin, C.; Li, Y.; Li, J.; Fan, X.; Yao, F.; Shi, D.; Cheng, Y.; Liu, M.; Lu, Q.; Gao, H. Gastrointestinal digestion, probiotic fermentation behaviors and immunomodulatory effects of polysaccharides from Sanghuangporus vaninii. Int. J. Biol. Macromol. 2022, 223, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Littmann, E.R.; Fontana, E.; Moody, T.U.; Kohout, C.E.; Gjonbalaj, M.; Eaton, V.; Seok, R.; Leiner, I.M.; Pamer, E.G. Functional and genomic variation between human-derived isolates of Lachnospiraceae reveals inter-and intra-species diversity. Cell Host Microbe 2020, 28, 134–146.e4. [Google Scholar] [CrossRef]
- Darnaud, M.; Dos Santos, A.; Gonzalez, P.; Augui, S.; Lacoste, C.; Desterke, C.; De Hertogh, G.; Valentino, E.; Braun, E.; Zheng, J. Enteric delivery of regenerating family member 3 alpha alters the intestinal microbiota and controls inflammation in mice with colitis. Gastroenterology 2018, 154, 1009–1023.e14. [Google Scholar] [CrossRef] [PubMed]
- Göhl, B. Les Aliments du Bétail Sous les Tropiques. Division de Production et Santé Animale 1982; FAO: Rome, Italy, 1982. [Google Scholar]
- Lin, T.-C.; Chen, C. Enhanced mannanase production by submerged culture of Aspergillus niger NCH-189 using defatted copra based media. Process Biochem. 2004, 39, 1103–1109. [Google Scholar] [CrossRef]
- Sulabo, R.; Mathai, J.K.; Usry, J.L.; Ratliff, B.W.; McKilligan, D.M.; Moline, J.D.; Xu, G.; Stein, H.H. Nutritional value of dried fermentation biomass, hydrolyzed porcine intestinal mucosa products, and fish meal fed to weanling pigs. J. Anim. Sci. 2013, 91, 2802–2811. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.H.; Casas, G.A.; Abelilla, J.J.; Liu, Y.; Sulabo, R.C. Nutritional value of high fiber co-products from the copra, palm kernel, and rice industries in diets fed to pigs. J. Anim. Sci. Biotechnol. 2015, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, N.; Serão, N.V.L.; Kerr, B.J.; Zijlstra, R.T.; Patience, J.F. Relationships among dietary fiber components and the digestibility of energy, dietary fiber, and amino acids and energy content of nine corn coproducts fed to growing pigs. J. Anim. Sci. 2014, 92, 4505–4517. [Google Scholar] [CrossRef] [PubMed]
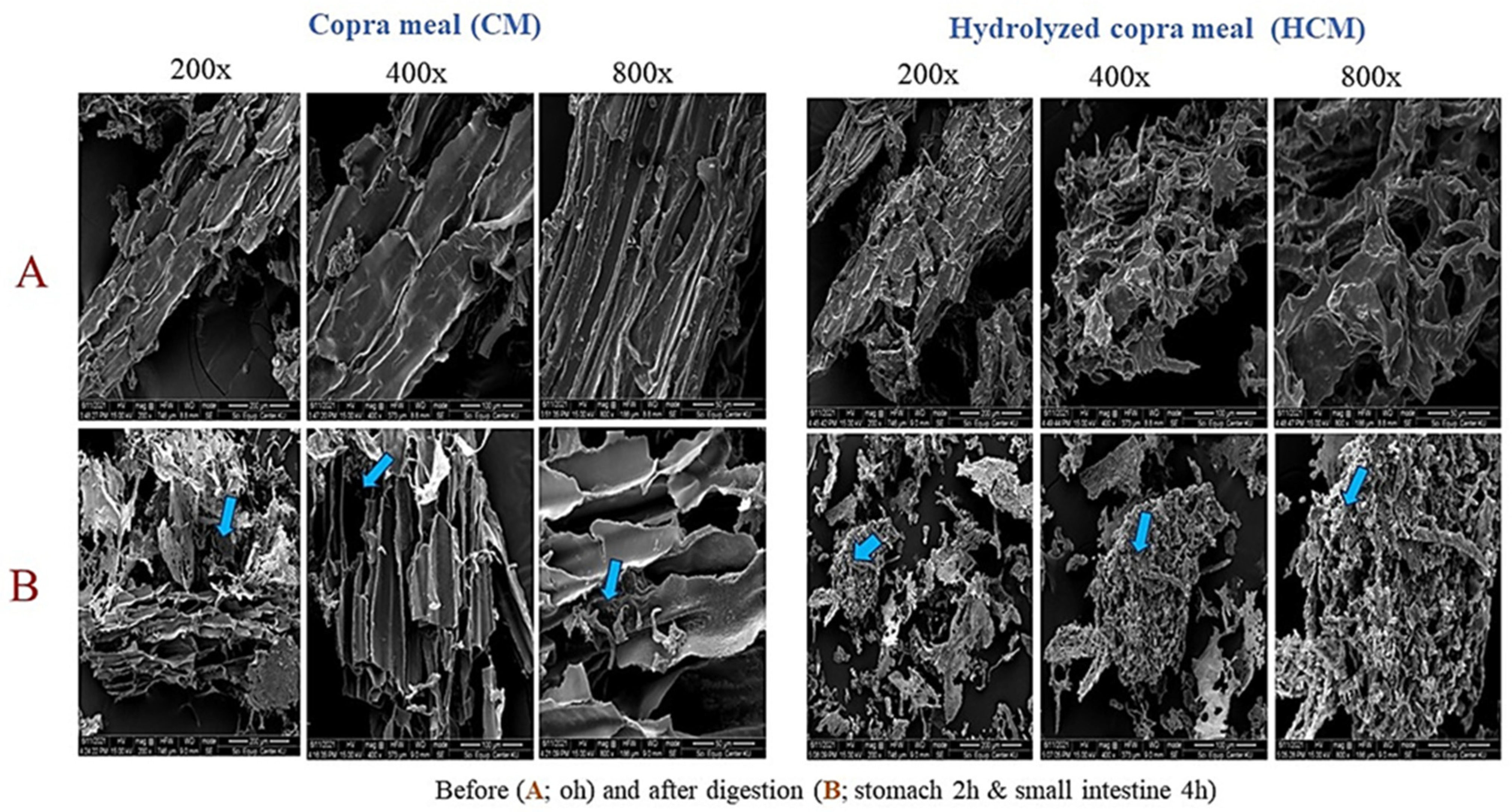

| Simulated Gastrointestinal (GI) Conditions | Copra Meal (CM) | Hydrolyzed Copra Meal (HCM) |
|---|---|---|
| Before digestion (0 h) | 1.0 | 1.0 |
| Stomach (2 h) | 0.94 | 0.70 |
| Small intestine (4 h) | 0.82 | 0.68 |
| Copra Meal (CM) | Hydrolyzed Copra Meal (HCM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M6 | M5 | M4 | M3 | M2 | M6 | M5 | M4 | M3 | M2 | |
| Stomach | 275.44 | 278.92 | 3.64 | 11.00 | 16.44 | 6.88 | ||||
| Small intestine | 405.72 | 0.54 | 9.30 | 2.40 | 3.36 | 0.060 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rungruangsaphakun, J.; Ayimbila, F.; Nakphaichit, M.; Keawsompong, S. Simulated Swine Digestion and Gut Microbiota Fermentation of Hydrolyzed Copra Meal. Animals 2024, 14, 1677. https://doi.org/10.3390/ani14111677
Rungruangsaphakun J, Ayimbila F, Nakphaichit M, Keawsompong S. Simulated Swine Digestion and Gut Microbiota Fermentation of Hydrolyzed Copra Meal. Animals. 2024; 14(11):1677. https://doi.org/10.3390/ani14111677
Chicago/Turabian StyleRungruangsaphakun, Jurairat, Francis Ayimbila, Massalin Nakphaichit, and Suttipun Keawsompong. 2024. "Simulated Swine Digestion and Gut Microbiota Fermentation of Hydrolyzed Copra Meal" Animals 14, no. 11: 1677. https://doi.org/10.3390/ani14111677
APA StyleRungruangsaphakun, J., Ayimbila, F., Nakphaichit, M., & Keawsompong, S. (2024). Simulated Swine Digestion and Gut Microbiota Fermentation of Hydrolyzed Copra Meal. Animals, 14(11), 1677. https://doi.org/10.3390/ani14111677






