Investigating the Influence of Varied Light-Emitting Diode (LED) Wavelengths on Phototactic Behavior and Opsin Genes in Vespinae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Maintenance and Experimental Conditions
2.2. Monitoring Phototactic Behavior
2.3. Behavioral Response of Vespula germanica and Vespa analis to Different Spectral Wavelength Light
2.4. Cloning of Opsin Genes
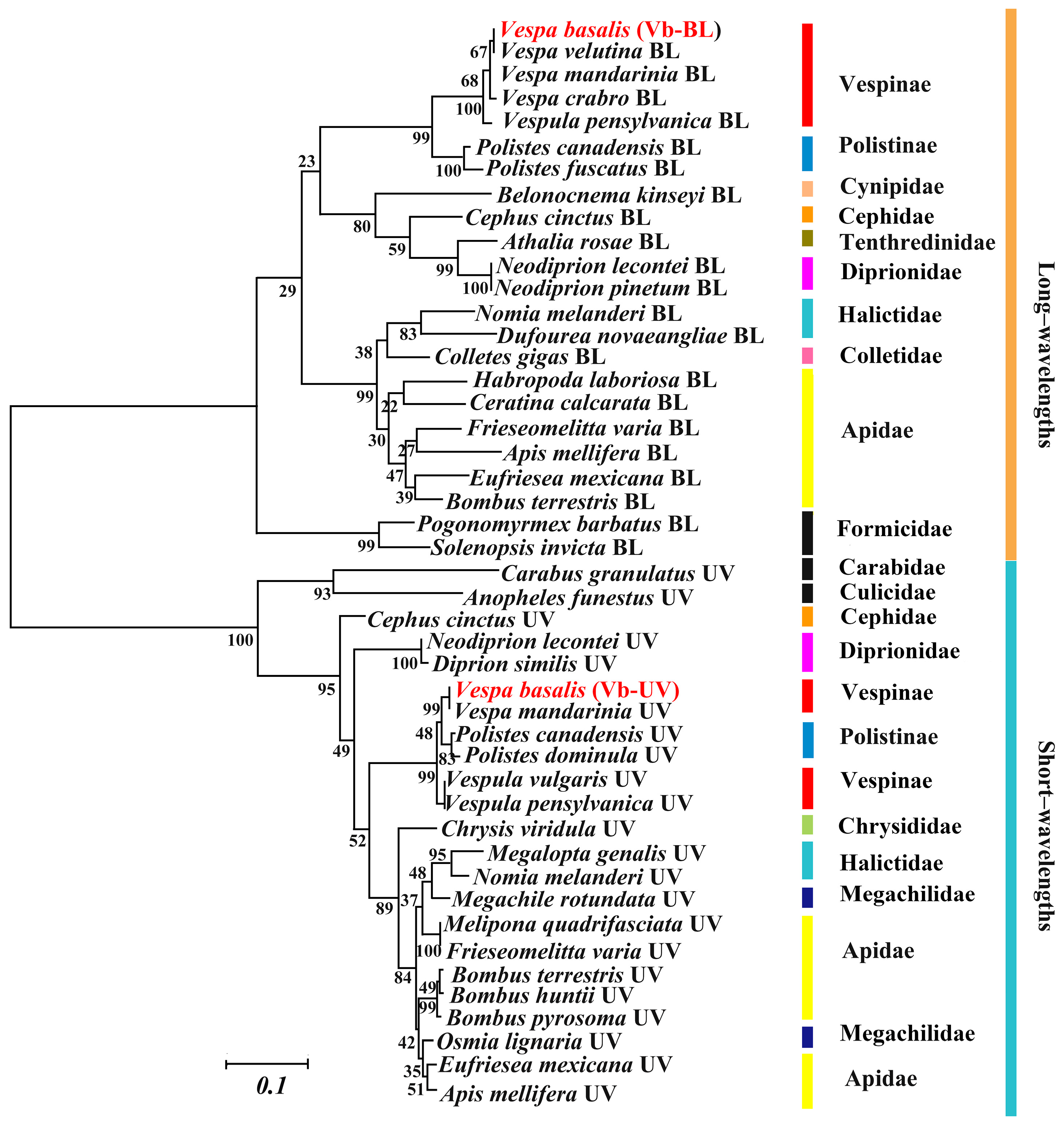
2.5. Statistical Analysis, Sequence Alignment, and Phylogenetic Tree
3. Results
3.1. Phototactic Rhythmicity of Vespula germanica at Different Times
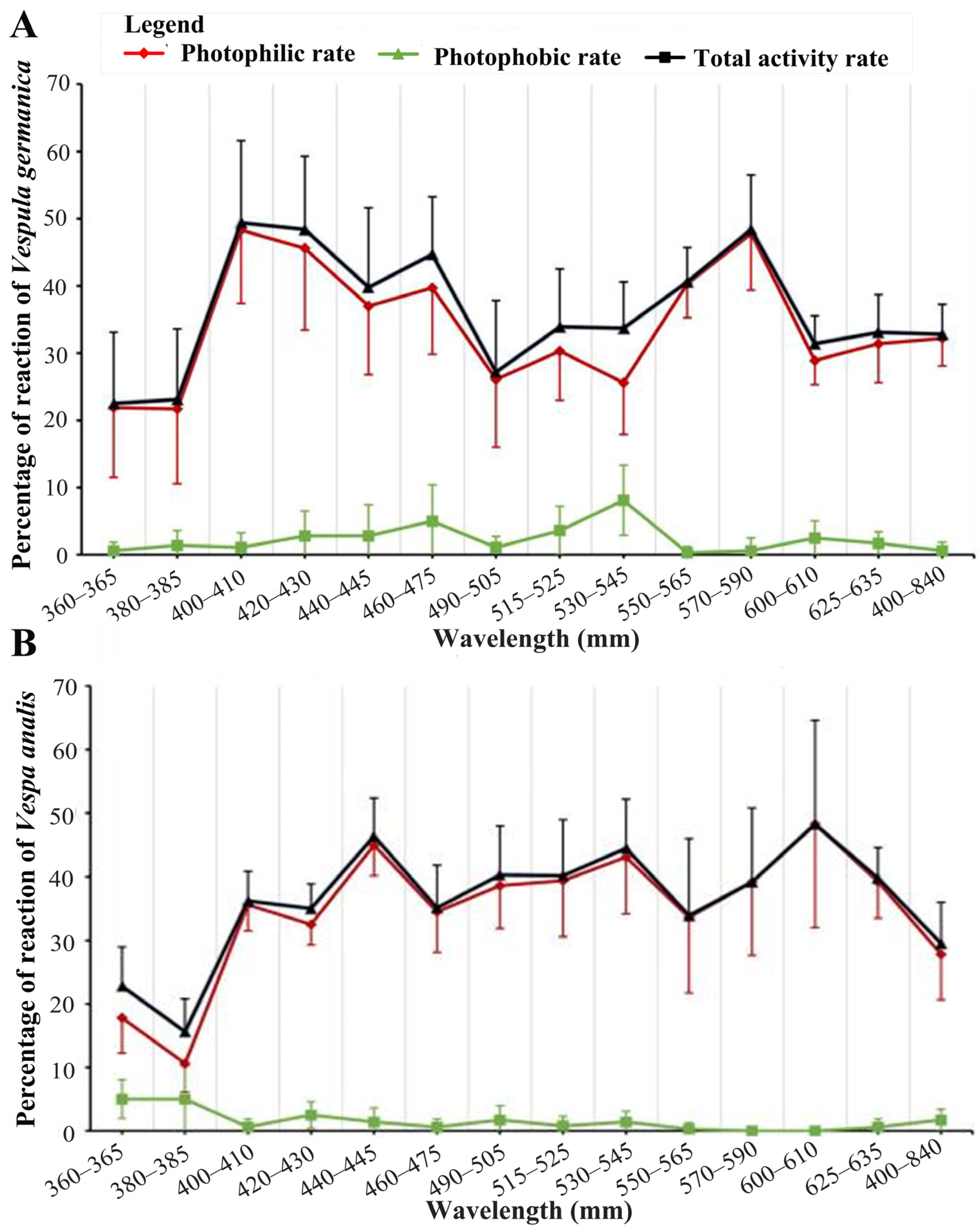
3.2. The Phototactic Behavior of Vespula germanica under Different Wavelengths of Light
3.3. Phototactic Rhythmicity of Vespa analis at Different Times
3.4. Phototactic Behavior of Vespa analis under Different Wavelengths of Light
3.5. Analyses of Optic Protein Genes in Vespa basalis
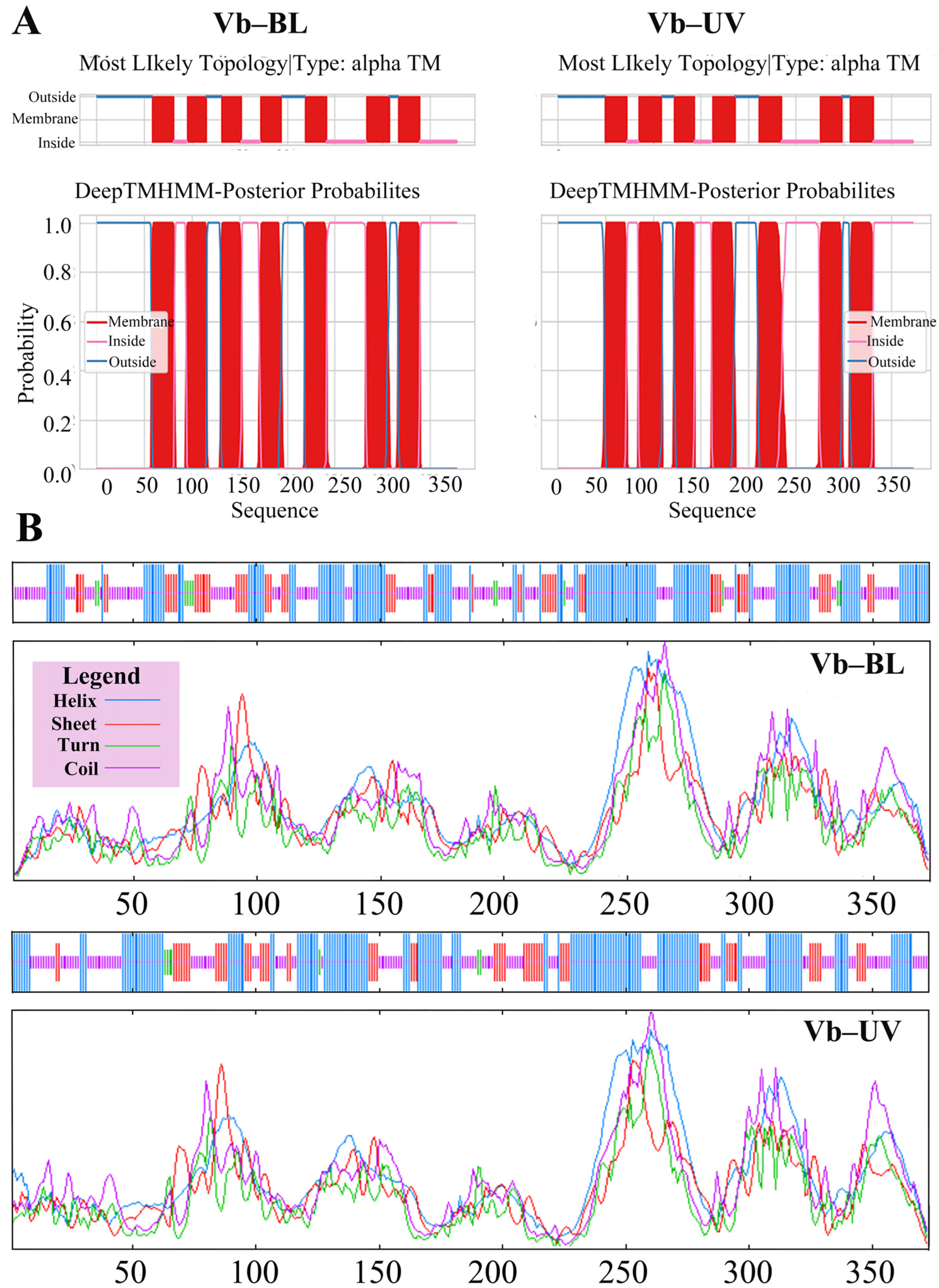
3.5.1. Physico-Chemical Characterization of Opsin Genes in Vespa basalis
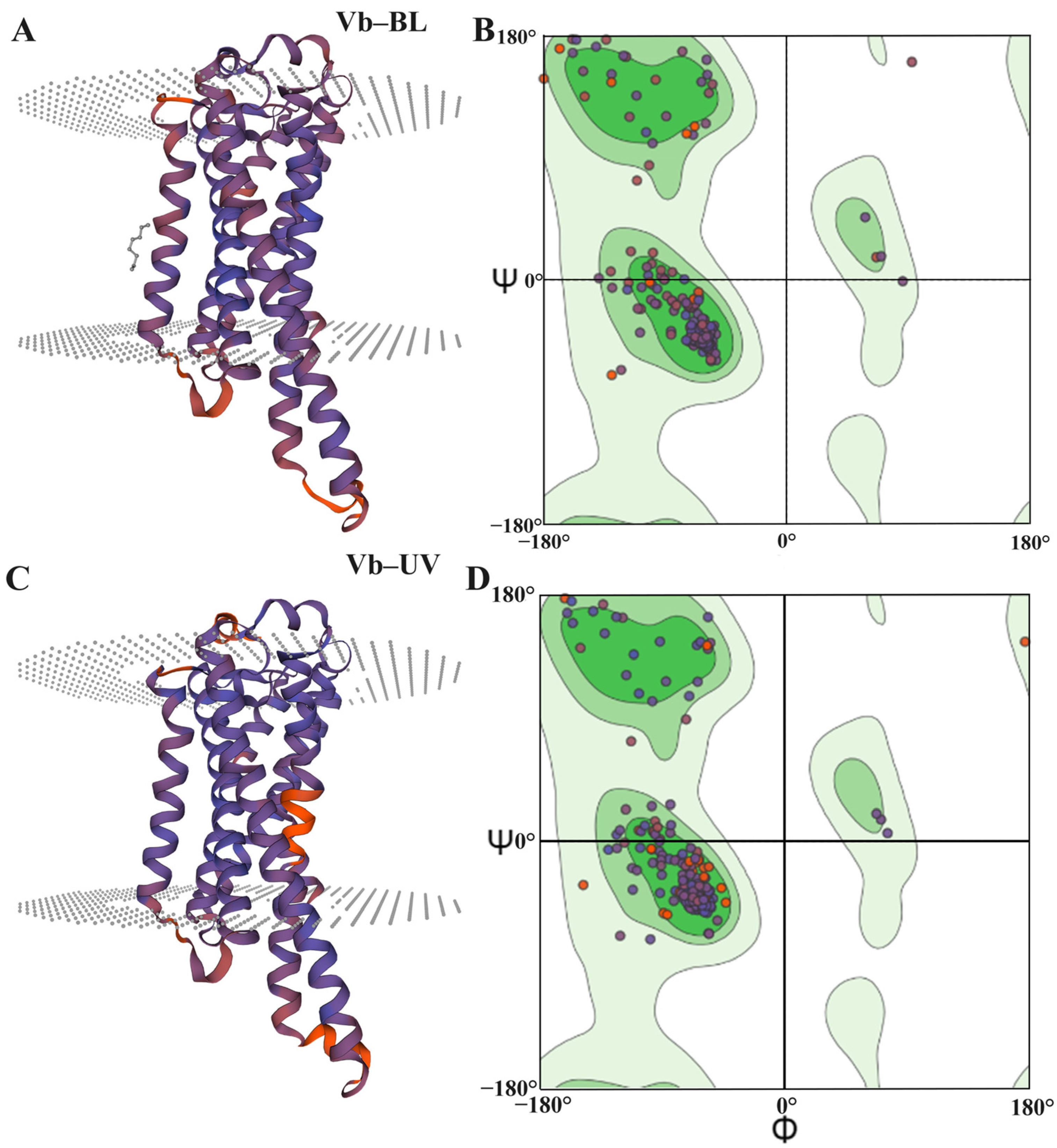
3.5.2. Predictive Analysis of the Structure of the Opsin Genes in Vespa basalis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.N.; Huang, Q.Y.; Lei, C.L. Advances in insect phototaxis and application to pest management: A review. Pest. Manag. Sci. 2019, 75, 3135–3143. [Google Scholar] [CrossRef]
- Neckameyer, W.S.; Bhatt, P. Protocols to Study Behavior in Drosophila. Methods Mol. Biol. 2016, 1478, 303–320. [Google Scholar]
- Pan, H.; Liang, G.; Lu, Y. Response of Different Insect Groups to Various Wavelengths of Light under Field Conditions. Insects 2021, 12, 427. [Google Scholar] [CrossRef]
- Hay, D.; Crossley, S. The design of mazes to study Drosophila behavior. Behav. Genet. 1977, 7, 389–402. [Google Scholar] [CrossRef]
- Paris, T.M.; Allan, S.A.; Udell, B.J.; Stansly, P.A. Wavelength and polarization affect phototaxis of the Asian Citrus Psyllid. Insects 2017, 8, 88. [Google Scholar] [CrossRef]
- Yao, M.C.; Lee, C.Y.; Chiu, H.W.; Feng, W.B.; Yang, E.C.; Lu, K.H. Efficiency of a Novel Light-Emitting Diode (LED) Trap for Trapping Rhyzopertha dominica (Coleoptera: Bostrichidae) in Paddy Rice Storehouses. J. Econ. Entomol. 2022, 115, 1294–1302. [Google Scholar] [CrossRef]
- Muri, R.B.; Jones, G.J. Microspectrophotometry of single rhabdoms in the retina of the honeybee drone (Apis mellifera male). J. Gen. Physiol. 1983, 82, 469–496. [Google Scholar] [CrossRef]
- Peitsch, D.; Fietz, A.; Hertel, H. The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. 1992, 170, 23–40. [Google Scholar] [CrossRef]
- Menzel, R.; Blakers, M. Colour receptors in the bee eye-morphology and spectral sensitivity. J. Comp. Physiol. 1976, 108, 11–33. [Google Scholar] [CrossRef]
- Hecht, S.; Wald, G. The visual acuity and intensity discrimination of Drosophila. J. Comp. Physiol. 1934, 17, 517–547. [Google Scholar] [CrossRef]
- Chen, Z.; Kuang, P.R.; Zhou, J.X. Phototactic behavior in Aphidius gifuensis (Hymenoptera: Braconidae). Biocontrol Sci. Technol. 2012, 22, 271–279. [Google Scholar] [CrossRef]
- Kim, J.G.; Lee, E.H.; Seo, Y.M.; Kim, N.Y. Cyclic behavior of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae) on host plants. J. Insect Behav. 2011, 24, 423–435. [Google Scholar] [CrossRef]
- Menzel, R.; Backhaus, W. Color Vision in Insects; MacMillan Press: London, UK, 1992; pp. 522–542. [Google Scholar]
- Nouvian, M.; Galizia, C.G. Complexity and plasticity in honey bee phototactic behavior. Sci. Rep. 2020, 10, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Campagna, C.; Fernandez, T.A. comparative analysis of the vision and mission statements of international environmental organisations. Environ. Values 2007, 16, 369–398. [Google Scholar] [CrossRef]
- Park, Y.G.; Lee, Y.S.; Sarker, S.; Ham, E.H.; Lim, U.T. Attractiveness of four wavelengths of LED light: UV (385 nm), violet (405 nm), blue (450 nm), and red (660 nm) for seven species of natural enemies. Biol. Control 2023, 179, 105–166. [Google Scholar] [CrossRef]
- Cheng, W.J.; Zheng, X.L.; Wang, P.; Zhou, L.L.; Si, S.Y.; Wang, X.P. Male-Biased Capture in Light Traps in Spodoptera exigua (Lepidoptera: Noctuidae): Results from the Studies of Reproductive Activities. J. Insect Behav. 2016, 29, 368–378. [Google Scholar] [CrossRef]
- Yang, E.C.; Lee, D.W.; Wu, W.Y. Action spectra of phototactic responses of the flea beetle, Phyllotreta striolata. Physiol. Entomol. 2003, 28, 362–368. [Google Scholar] [CrossRef]
- Sun, G.; Liu, S.; Luo, H.; Feng, Z.; Yang, B.; Luo, J.; Tang, J.; Yao, Q.; Xu, J. Intelligent Monitoring System of Migratory Pests Based on Searchlight Trap and Machine Vision. Front. Plant Sci. 2022, 13, 739–897. [Google Scholar] [CrossRef]
- Robinson, H.S. On the behaviour of night-flying insects in the neighbourhood of a bright source of light. Entomol. Gen. 1952, 27, 13–21. [Google Scholar] [CrossRef]
- Callahan, P.S. Intermediate and far infrared sensing of nocturnal insects. Ann. Entomol. Soc. Am. 1965, 58, 727–745. [Google Scholar] [CrossRef]
- Michael, D.A. Introduction to Insect Behavior; Macmillan Publishing Company: New York, NY, USA, 1980; pp. 128–1134. [Google Scholar]
- Sang, W.; Huang, Q.; Wang, X.; Guo, S.H.; Lei, C.L. Development, achievement, and prospect of insect phototaxis and light trapping techniques in China. J. Appl. Entomol. 2019, 56, 907–916. [Google Scholar]
- Gebhardt, F.; Desplan, C. Retinal perception and ecological significance of color vision in insects. Curr. Opin. Insect Sci. 2017, 24, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, K.; Stavenga, D.G. Insect photopigments: Photoreceptor spectral sensitivities and visual adaptations. In Evolution of Visual and Non-Visual Pigments; Springer: Boston, MA, USA, 2014; pp. 137–162. [Google Scholar]
- Terakita, A. The opsins. Genome Biol. 2005, 6, 213. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Bernard, G.D.; Le, J. Contrasting modes of evolution of the visual pigments in Heliconius butterflies. Mol. Biol. Evol. 2010, 27, 2392–2405. [Google Scholar] [CrossRef] [PubMed]
- Henze, M.J.; Oakley, T.H. The dynamic evolutionary history of pancrustacean eyes and opsins. Integr. Comp. Biol. 2015, 55, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Oba, Y.; Kainuma, T. Diel changes in the expression of long wavelength-sensitive and ultraviolet-sensitive opsin genes in the Japanese firefly, Luciola cruciata. Gene 2009, 43, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, A.D.; Bybee, S.M.; Bernard, G.D. Positive selection of a duplicated UV-sensitive visual pigment coincides with wing pigment evolution in Heliconius butterflies. Proc. Natl. Acad. Sci. USA 2010, 107, 3628–3633. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.G.; Chittka, L. Bumblebee search time without ultraviolet light. J. Exp. Biol. 2004, 207, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Froy, O.; Gotter, A.L.; Casselman, A.L. Illuminating the circadian clock in monarch butterfly migration. Science 2003, 300, 1303–1305. [Google Scholar] [CrossRef]
- Li, C.F.; Tian, F.J.; Lin, T.; Wang, Z.B.; Liu, J.L.; Zeng, X.N. The expression and function of opsin genes related to the phototactic behavior of Asian citrus psyllid. Pest Manag. Sci. 2000, 76, 1578–1587. [Google Scholar] [CrossRef]
- Tan, J.L.; van Achterberg, C.; Chen, X.X. Potentially Lethal Social Wasps, Fauna of the Chinese Vespinae (Hymenoptera: Vespidae); Beijing Science Press: Beijing, China, 2015; pp. 352–369. [Google Scholar]
- Kogan, M. Integrated pest management: Historical perspectives and contemporary developments. Annu. Rev. Entomol. 1998, 43, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Wakefield, A.; Roberts, N.; Jones, G. Artificial light and biting flies: The parallel development of attractive light traps and unattractive domestic lights. Parasites Vectors 2021, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Meng, J.Y.; Zhou, L.C.; Zhang, Y.; Yu, C. Expression and function of opsin genes associated with phototaxis in Zeugodacus cucurbitae Coquillett (Diptera: Tephritidae). Pest Manag. Sci. 2023, 79, 4490–4500. [Google Scholar] [CrossRef]
- Jiang, X.; Hai, X.; Bi, Y.; Zhao, F.; Wang, Z.; Lyu, F. Research on Photoinduction-Based Technology for Trapping Asian Longhorned Beetle (Anoplophora glabripennis) (Motschulsky, 1853) (Coleoptera: Cerambycidae). Insects 2023, 14, 465. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Jiang, Y.L.; Zhou, G.T.; Zhang, G.P.; Miao, J.; Gong, Z.J.; Duan, Y.; Li, T. A review of studies of insect phototaxis. J. Environ. Entomol. 2023, 18, 1137–1149. [Google Scholar]
- Rode, J.B.; Ringel, M.M. Statistical Software Output in the Classroom: A Comparison of R and SPSS. Teach. Psychol. 2019, 46, 319–327. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Barioch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2005, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022, 8, 487–609. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S. SWISS-MODEL: Homology modeling of protein structures and complexes. Nucleic Acids Res. 2018, 46, 296–303. [Google Scholar] [CrossRef]
- Notredame, C.; Higgins, D.G.; Heringa, J. T–Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Lester, P.J.; Beggs, J.R. Invasion Success and Management Strategies for Social Vespula Wasps. Annu. Rev. Entomol. 2019, 64, 51–71. [Google Scholar] [CrossRef]
- Dyson, C.J.; Crossley, H.G.; Ray, C.H.; Goodisman, M.A.D. Social structure of perennial Vespula squamosa wasp colonies. Ecol. Evol. 2022, 12, e8569. [Google Scholar] [CrossRef]
- Ishay, J. Contributions to the bionomics of the Oriental hornet Vespa orientalis F. Isr. J. Entomol. 1967, 2, 45–106. [Google Scholar]
- Wolf, E.; Zerrahn-Wolf, G. The effect of light intensity, area, and flicker frequency on the visual reactions of the honey bee. J. Gen. Physiol. 1935, 18, 853–863. [Google Scholar] [CrossRef]
- Makarova, A.; Polilov, A.; Fischer, S. Comparative morphological analysis of compound eye miniaturization in minute hymenoptera. Arthropod Struct. Dev. 2015, 44, 21–32. [Google Scholar] [CrossRef]
- Dai, B.; Zhang, L.; Zhao, C.; Bachman, H.; Becker, R.; Mai, J.; Jiao, Z.; Li, W.; Zheng, L.; Wan, X.; et al. Biomimetic apposition compound eye fabricated using microfluidic-assisted 3D printing. Nat. Commun. 2021, 12, 6458. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, G.; Xu, P.; Gao, B.; Liu, X.; Qi, X.; Zhang, G.; Cao, S.; Li, Z.; Ren, X.; et al. Behavioral and genomic divergence between a generalist and a specialist fly. Cell Rep. 2022, 41, 1152–1158. [Google Scholar] [CrossRef]
- Mishra, M.; Knust, E. Analysis of the Drosophila compound eye with light and electron microscopy. Methods Mol. Biol. 2013, 935, 161–182. [Google Scholar]
- Song, Y.; Liu, C.; Cai, P.; Chen, W.; Guo, Y.; Lin, J.; Zhang, S. Host-Seeking Behavior of Aphidius gifuensis (Hymenoptera: Braconidae) Modulated by Chemical Cues Within a Tritrophic Context. J. Insect Sci. 2021, 21, 9. [Google Scholar] [CrossRef]
- Menzel, R.; Backhaus, W. Colour vision in nocturnal insects. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 1862. [Google Scholar]
- Briscoe, A.D.; Chittka, L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001, 46, 471–510. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, A.D. Six opsins from the butterfly Papilio glaucus: Molecular phylogenetic evidence for paralogous origins of red-sensitive visual pigments in insects. J. Mol. Evol. 2000, 51, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kinoshita, M.; Stavenga, D.G. Sex-specific retinal pigmentation results in sexually dimorphic long-wavelength-sensitive photoreceptors in the eastern pale clouded yellow butterfly, Colias erate. J. Exp. Biol. 2013, 216, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Yang, C.L.; Meng, J.Y.; Zhou, L.; Zhang, C.Y. Comparative transcriptome and metabolome analysis of Ostrinia furnacalis female adults under UV-A exposure. Sci. Rep. 2021, 11, 6797. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.Y.; Zhang, C.Y.; Zhu, F.; Wang, X.P.; Lei, C.L. Ultraviolet light induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 2009, 55, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Sauman, I.; Briscoe, A.D.; Zhu, H.; Shi, D.; Froy, O.; Stalleicken, J.; Yuan, Q.; Casselman, A.; Reppert, S.M. Connecting the navigational clock to sun compass input in monarch butterfly brain. Neuron 2005, 46, 457–467. [Google Scholar] [CrossRef]
- Koyanagi, M.; Terakita, A. Diversity of animal opsin-based pigments and their optogenetic potential. BBA Bioenerg. 2014, 26, 710–716. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |


| Primer Name | Primer Sequence 5′-3′ |
|---|---|
| Vb-BL-F1 | CATTCGATCTGTTTGATATAAAAGAGC |
| Vb-BL-R1 | CTTTAGCGATTCTAACTTCGACACT |
| Vb-BL-F2 | GGGATACCTAACTACGTGCAGTTTC |
| Vb-BL-R2 | TCCATTTGATGCTTGCTTTATTC |
| Vb-UV-F1 | ATCTTTTAAAAAAGAACCGATTTGTTG |
| Vb-UV-R1 | GGTATCGGTTAAATAATCAAAAGAACAG |
| Vb-UV-F2 | ATGGAAAATTATCACGTGGTCAAGT |
| Vb-UV-R2 | TTATATGCCAGGAATTTACTTTATTGG |
| Species | V. germanica and V. analis | V. germanica | V. analis |
|---|---|---|---|
| Type | p-Value | F (DFn, DFd) = F(13, 154) | F (DFn, DFd) = F(13, 154) |
| Photophilic rate | <0.0001 | 13.12 | 18.11 |
| Photophobic rate | <0.0001 | 5.746 | 6.891 |
| Total activity rate | <0.0001 | 12.41 | 13.46 |
| Type | Vespula germanica | Vespa analis | ||||
|---|---|---|---|---|---|---|
| Photophilic /Photophobic Rate | Photophobic /Total Activity Rate | Photophobic /Total Activity Rate | Photophilic /Photophobic Rate | Photophobic /Total Activity Rate | Photophobic /Total Activity Rate | |
| 360–365 nm | **** | ns | **** | **** | ns | **** |
| 380–385 nm | **** | ns | **** | * | * | **** |
| 400–410 nm | **** | ns | **** | **** | ns | **** |
| 420–430 nm | **** | ns | **** | **** | ns | **** |
| 440–445 nm | **** | ns | **** | **** | ns | **** |
| 460–475 nm | **** | ns | **** | **** | ns | **** |
| 490–505 nm | **** | ns | **** | **** | ns | **** |
| 515–525 nm | **** | ns | **** | **** | ns | **** |
| 530–545 nm | **** | * | **** | **** | ns | **** |
| 570–590 nm | **** | ns | **** | **** | ns | **** |
| 600–610 nm | **** | ns | **** | **** | ns | **** |
| 625–635 nm | **** | ns | **** | **** | ns | **** |
| 625–635 nm | **** | ns | **** | **** | ns | **** |
| 400–840 nm | **** | ns | **** | **** | ns | **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zhou, T.; Ullah, H.; Zhu, D.; Tang, Y.; Xu, H.; Wang, H.; Tan, J. Investigating the Influence of Varied Light-Emitting Diode (LED) Wavelengths on Phototactic Behavior and Opsin Genes in Vespinae. Animals 2024, 14, 1543. https://doi.org/10.3390/ani14111543
Huang X, Zhou T, Ullah H, Zhu D, Tang Y, Xu H, Wang H, Tan J. Investigating the Influence of Varied Light-Emitting Diode (LED) Wavelengths on Phototactic Behavior and Opsin Genes in Vespinae. Animals. 2024; 14(11):1543. https://doi.org/10.3390/ani14111543
Chicago/Turabian StyleHuang, Xiaojuan, Tong Zhou, Hasin Ullah, Danyang Zhu, Yan Tang, Hongli Xu, Hang Wang, and Jiangli Tan. 2024. "Investigating the Influence of Varied Light-Emitting Diode (LED) Wavelengths on Phototactic Behavior and Opsin Genes in Vespinae" Animals 14, no. 11: 1543. https://doi.org/10.3390/ani14111543
APA StyleHuang, X., Zhou, T., Ullah, H., Zhu, D., Tang, Y., Xu, H., Wang, H., & Tan, J. (2024). Investigating the Influence of Varied Light-Emitting Diode (LED) Wavelengths on Phototactic Behavior and Opsin Genes in Vespinae. Animals, 14(11), 1543. https://doi.org/10.3390/ani14111543







