Mycobacterium kansasii Infection in a Farmed White-Tailed Deer (Odocoileus virginianus) in Florida, USA
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Molecular Examination
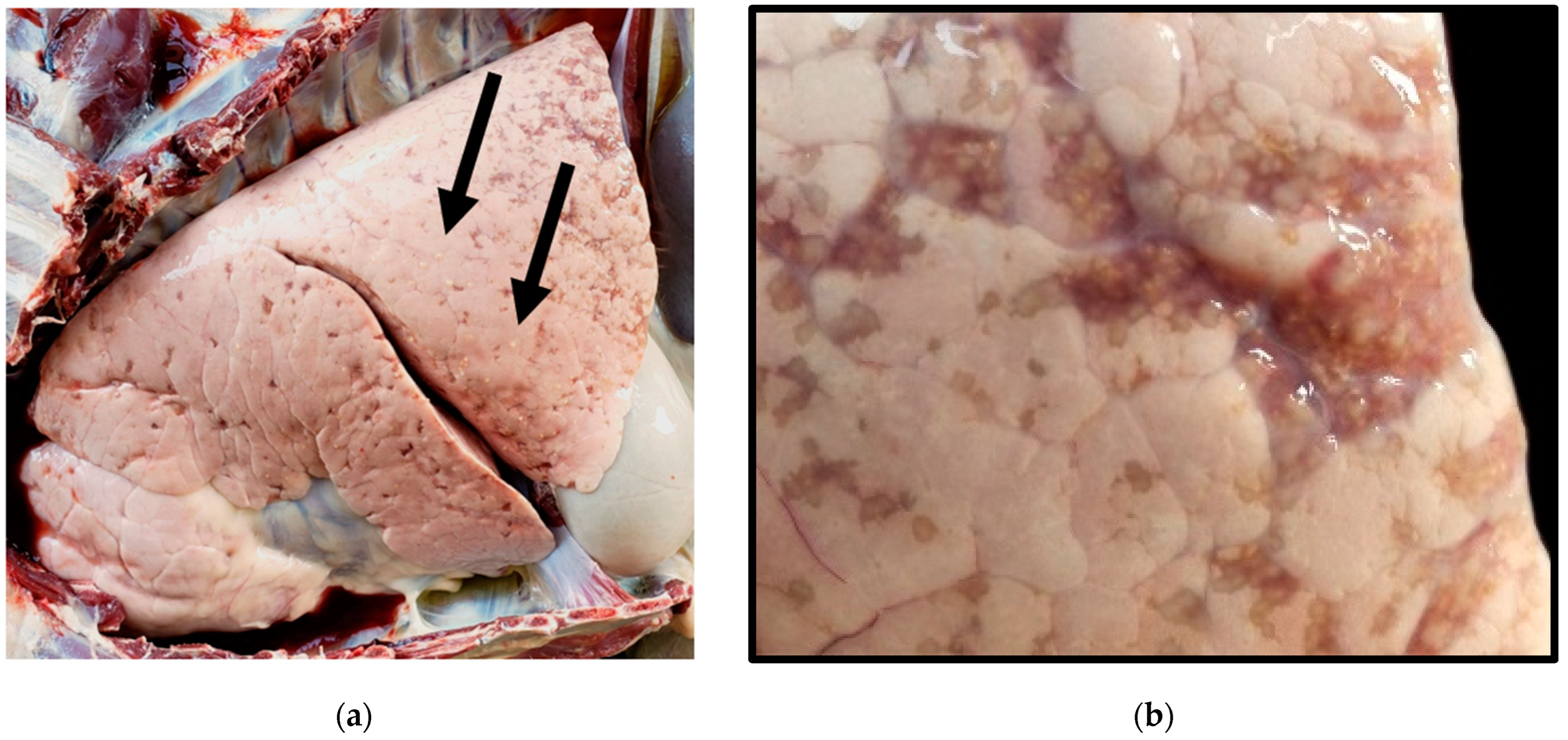
3. Results
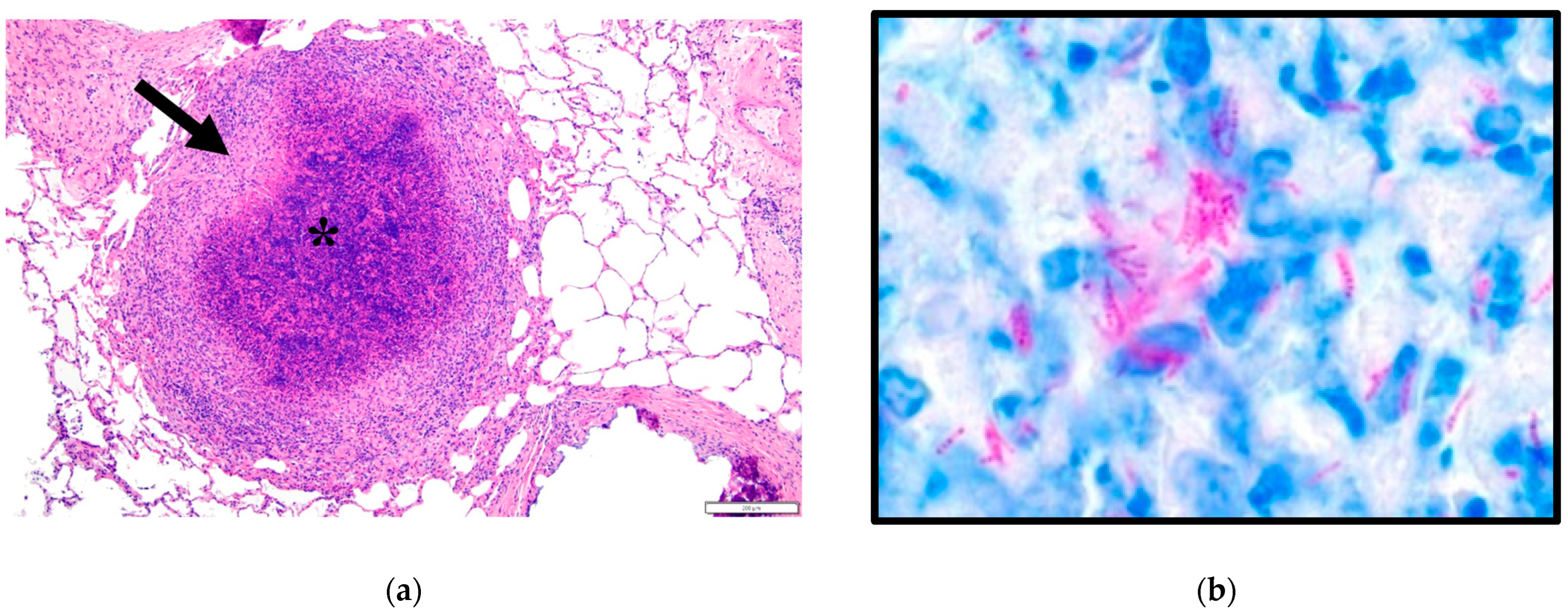
3.1. Microscopic Observations
3.2. Molecular Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; McIntosh, F.; Radomski, N.; Dewar, K.; Simeone, R.; Enninga, J.; Brosch, R.; Rocha, E.P.; Veyrier, F.J.; Behr, M.A. Insights on the Emergence of Mycobacterium tuberculosis from the Analysis of Mycobacterium kansasii. Genome Biol. Evol. 2015, 7, 856–870. [Google Scholar] [CrossRef] [PubMed]
- Machado, E.; Vasconcellos, S.E.G.; Cerdeira, C.; Gomes, L.L.; Junqueira, R.; Carvalho, L.D.; Ramos, J.P.; Redner, P.; Campos, C.E.D.; Caldas, P.C.S.; et al. Whole Genome Sequence of Mycobacterium kansasii Isolates of the Genotype 1 from Brazilian Patients with Pulmonary Disease Demonstrates Considerable Heterogeneity. Mem. Inst. Oswaldo Cruz 2018, 113, e180085. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Xu, P.; Zhang, Y.; Porter, J.L.; Ghanem, M.; Liu, Q.; Jiang, Y.; Li, J.; Miao, Q.; Hu, B.; et al. Population Genomics Provides Insights into the Evolution and Adaptation to Humans of the Waterborne Pathogen Mycobacterium Kansasii. Nat. Commun. 2021, 12, 2491. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Ramos, B.; Reis, A.C.; Cunha, M.V. Non-Tuberculous Mycobacteria: Molecular and Physiological Bases of Virulence and Adaptation to Ecological Niches. Microorganisms 2020, 8, 1380. [Google Scholar] [CrossRef] [PubMed]
- Vaerewijck, M.J.; Huys, G.; Palomino, J.C.; Swings, J.; Portaels, F. Mycobacteria in Drinking Water Distribution Systems: Ecology and Significance for Human Health. FEMS Microbiol. Rev. 2005, 29, 911–934. [Google Scholar] [CrossRef] [PubMed]
- Buhler, V.B.; Pollak, A. Human Infection with Atypical Acid-Fast Organisms; Report of Two Cases with Pathologic Findings. Am. J. Clin. Pathol. 1953, 23, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Matveychuk, A.; Fuks, L.; Priess, R.; Hahim, I.; Shitrit, D. Clinical and Radiological Features of Mycobacterium kansasii and Other Ntm Infections. Respir. Med. 2012, 106, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K.C.; Zwerling, L.; Pletcher, M.J.; Hahn, J.A.; Gerberding, J.L.; Ostroff, S.M.; Vugia, D.J.; Reingold, A.L. Incidence and Clinical Implications of Isolation of Mycobacterium kansasii: Results of a 5-Year, Population-Based Study. Ann. Intern. Med. 1998, 129, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.; Banavathi, K. Mycobacterium Kansasii Osteomyelitis—A Masquerading Disease. JMM Case Rep. 2018, 5, e005114. [Google Scholar] [CrossRef]
- Sheu, L.C.; Tran, T.M.; Jarlsberg, L.G.; Marras, T.K.; Daley, C.L.; Nahid, P. Non-Tuberculous Mycobacterial Infections at San Francisco General Hospital. Clin. Respir. J. 2015, 9, 436–442. [Google Scholar] [CrossRef]
- Taillard, C.; Greub, G.; Weber, R.; Pfyffer, G.E.; Bodmer, T.; Zimmerli, S.; Frei, R.; Bassetti, S.; Rohner, P.; Piffaretti, J.C.; et al. Clinical Implications of Mycobacterium Kansasii Species Heterogeneity: Swiss National Survey. J. Clin. Microbiol. 2003, 41, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.S.; Roberts, C.H.; Kaul, S.; Klein, J.L.; Milburn, H.J. Non-Tuberculous Slow-Growing Mycobacterial Pulmonary Infections in Non-Hiv-Infected Patients in South London. Scand. J. Infect. Dis. 2012, 44, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Arend, S.M.; de Palou, E.C.; de Haas, P.; Janssen, R.; Hoeve, M.A.; Verhard, E.M.; Ottenhoff, T.H.; van Soolingen, D.; van Dissel, J.T. Pneumonia Caused by Mycobacterium kansasii in a Series of Patients without Recognised Immune Defect. Clin. Microbiol. Infect. 2004, 10, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, W.M.; O’Shaughnessy, T.C.; van Ingen, J. Human-to-Human Transmission of Mycobacterium kansasii or Victims of a Shared Source? Eur. Respir. J. 2014, 44, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Acosta, B.; Real, F.; Ferrer, O.; Deniz, S.; Poveda, B. Isolation of Mycobacterium Kansasii from a Tuberculin-Positive Goat. Vet. Rec. 1998, 142, 195–196. [Google Scholar] [CrossRef]
- Sato, T.; Shibuya, H.; Ohba, S.; Nojiri, T.; Shirai, W. Mycobacteriosis in Two Captive Florida Manatees (Trichechus Manatus Latirostris). J. Zoo Wildl. Med. 2003, 34, 184–188. [Google Scholar] [PubMed]
- Hall, P.B.; Bender, L.C.; Garner, M.M. Mycobacteriosis in a Black-Tailed Deer (Odocoileus hemionus columbianus) Caused by Mycobacterium kansasii. J. Zoo Wildl. Med. 2005, 36, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Previtali, M.; Gautschi, A.; Forster, E.; Steininger, K.; Irmer, M.; Reichle, S.; Sydler, T.; Wiederkehr, D.; Ruetten, M.; et al. Sonographic Findings in an Alpaca with Mycobacterium kansasii Infection. Schweiz. Arch. Tierheilkd. 2009, 151, 287–290. [Google Scholar] [CrossRef]
- Houlihan, M. Isolation of Mycobacterium kansasii from Calf Reactors in a Tb-Restricted Dairy Herd. Vet. Rec. 2010, 166, 272–273. [Google Scholar] [CrossRef]
- Miller, M.; Terrell, S.; Lyashchenko, K.; Greenwald, R.; Harris, B.; Thomsen, B.V.; Fontenot, D.; Stetter, M.; Neiffer, D.; Fleming, G. Mycobacterium Kansasii Infection in a Bontebok (Damaliscus pygaragus dorcas) Herd: Diagnostic Challenges in Differentiating from the Mycobacterium Tuberculosis Complex. J. Zoo Wildl. Med. 2011, 42, 468–472. [Google Scholar] [CrossRef]
- Thompson, K.A.; Campbell, M.; Levens, G.; Lim, A.; Bolin, S. Mycobacterium kansasii Infection in a Captive Sichuan Takin (Budorcas taxicolortibetana) and a Siamang (Hylobates syndactylus) at a Zoological Facility. Vet. Rec. Case Rep. 2016, 4, e000280. [Google Scholar] [CrossRef]
- Shipley, S.T.; Johnson, D.K.; Roodgar, M.; Smith, D.G.; Montgomery, C.A.; Lloyd, S.M.; Higgins, J.A.; Kriel, E.H.; Klein, H.J.; Porter, W.P.; et al. Isolated from Tuberculin-Positive Rhesus Macaques (Macaca mulatta) in the Absence of Disease. Comp. Med. 2017, 67, 368–375. [Google Scholar] [PubMed]
- Ghielmetti, G.; Friedel, U.; Scherrer, S.; Sarno, E.; Landolt, P.; Dietz, O.; Hilbe, M.; Zweifel, C.; Stephan, R. Non-Tuberculous Mycobacteria Isolated from Lymph Nodes and Faecal Samples of Healthy Slaughtered Cattle and the Abattoir Environment. Transbound. Emerg. Dis. 2018, 65, 711–718. [Google Scholar] [CrossRef]
- Cerna, P.; Mitchell, J.L.; Lodzinska, J.; Cazzini, P.; Varjonen, K.; Gunn-Moore, D.A. Systemic Mycobacterium Kansasii Infection in Two Related Cats. Pathogens 2020, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.K.; Niedringhaus, K.D.; Anderson, A.N.; LaCour, J.M.; Nemeth, N.M. Disseminated Mycobacterium Kansasii Infection in a White-Tailed Deer and Implications for Public and Livestock Health. J. Vet. Diagn. Investig. 2020, 32, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Fukano, H.; Terazono, T.; Hirabayashi, A.; Yoshida, M.; Suzuki, M.; Wada, S.; Ishii, N.; Hoshino, Y. Human Pathogenic Mycobacterium Kansasii (Former Subtype I) with Zoonotic Potential Isolated from a Diseased Indoor Pet Cat, Japan. Emerg. Microbes Infect. 2021, 10, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Radulski, L.; Krajewska-Wedzina, M.; Lipiec, M.; Szulowski, K. Infection of a Free-Living Wild Boar (Sus scrofa) with a Bacterium from the Mycobacterium kansasii Complex. Animals 2022, 12, 964. [Google Scholar] [CrossRef]
- Gomez-Buendia, A.; Alvarez, J.; Bezos, J.; Mourelo, J.; Amado, J.; Saez, J.L.; de Juan, L.; Romero, B. Non-Tuberculous Mycobacteria: Occurrence in Skin Test Cattle Reactors from Official Tuberculosis-Free Herds. Front. Vet. Sci. 2024, 11, 1361788. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cottingham, S.L.; Cheng, A.-C.; de Oliveira Viadanna, P.H.; Subramaniam, K.; Craft, W.F.; Iredale, M.E.; Wisely, S.M.; Campos Krauer, J.M. Mycobacterium kansasii Infection in a Farmed White-Tailed Deer (Odocoileus virginianus) in Florida, USA. Animals 2024, 14, 1511. https://doi.org/10.3390/ani14101511
Cottingham SL, Cheng A-C, de Oliveira Viadanna PH, Subramaniam K, Craft WF, Iredale ME, Wisely SM, Campos Krauer JM. Mycobacterium kansasii Infection in a Farmed White-Tailed Deer (Odocoileus virginianus) in Florida, USA. Animals. 2024; 14(10):1511. https://doi.org/10.3390/ani14101511
Chicago/Turabian StyleCottingham, Sydney L., An-Chi Cheng, Pedro H. de Oliveira Viadanna, Kuttichantran Subramaniam, William F. Craft, Marley E. Iredale, Samantha M. Wisely, and Juan M. Campos Krauer. 2024. "Mycobacterium kansasii Infection in a Farmed White-Tailed Deer (Odocoileus virginianus) in Florida, USA" Animals 14, no. 10: 1511. https://doi.org/10.3390/ani14101511
APA StyleCottingham, S. L., Cheng, A.-C., de Oliveira Viadanna, P. H., Subramaniam, K., Craft, W. F., Iredale, M. E., Wisely, S. M., & Campos Krauer, J. M. (2024). Mycobacterium kansasii Infection in a Farmed White-Tailed Deer (Odocoileus virginianus) in Florida, USA. Animals, 14(10), 1511. https://doi.org/10.3390/ani14101511





