Lack of Highly Pathogenic Avian Influenza H5N1 in the South Shetland Islands in Antarctica, Early 2023
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
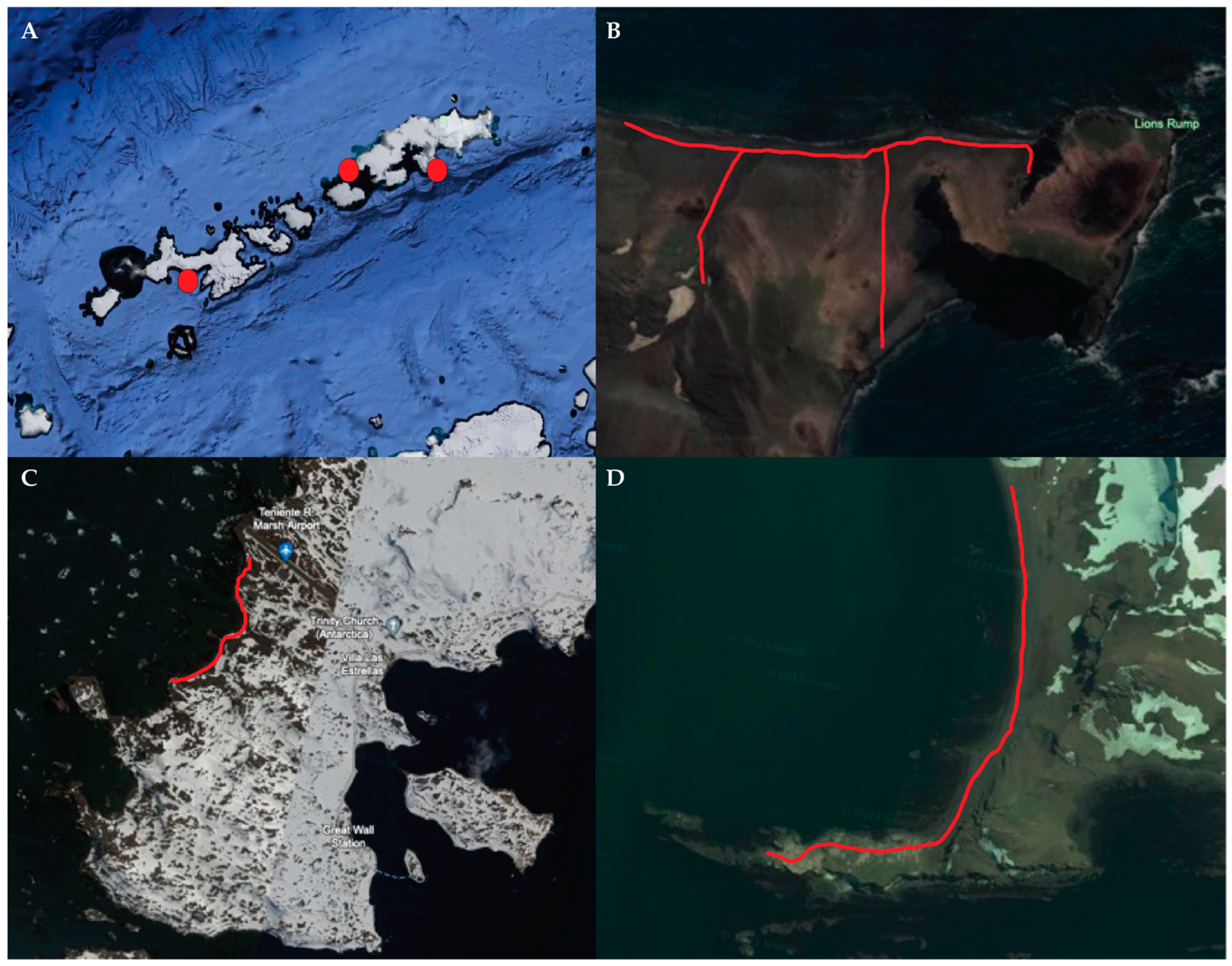
2.1. Locations
2.2. Sampling
2.3. Diagnostic Testing
3. Results
3.1. No Clinical Signs Related to HPAIV
3.2. Lack of Detection by Real-Time RT PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvey, J.A.; Mullinax, J.M.; Runge, M.C.; Prosser, D.J. The Changing Dynamics of Highly Pathogenic Avian Influenza H5N1: Next Steps for Management & Science in North America. Biol. Conserv. 2023, 282, 110041. [Google Scholar] [CrossRef]
- Ariyama, N.; Pardo-Roa, C.; Muñoz, G.; Aguayo, C.; Ávila, C.; Mathieu, C.; Brito, B.; Medina, R.; Johow, M.; Neira, V. Emergence and Rapid Dissemination of Highly Pathogenic Avian Influenza Virus H5N1 Clade 2.3.4.4b in Wild Birds, Chile. bioRxiv 2023. [Google Scholar] [CrossRef]
- Wong, J.B.; Lisovski, S.; Alisauskas, R.T.; English, W.; Giroux, M.-A.; Harrison, A.-L.; Kellett, D.; Lecomte, N.; Maftei, M.; Nagy-MacArthur, A.; et al. Arctic Terns from Circumpolar Breeding Colonies Share Common Migratory Routes. Mar. Ecol. Prog. Ser. 2021, 671, 191–206. [Google Scholar] [CrossRef]
- Egevang, C.; Stenhouse, I.J.; Phillips, R.A.; Petersen, A.; Fox, J.W.; Silk, J.R.D. Tracking of Arctic Terns Sterna Paradisaea Reveals Longest Animal Migration. Proc. Natl. Acad. Sci. USA 2010, 107, 2078–2081. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.; Peter, H.; Mustafa, O.; Lisovski, S.; Ritz, M.; Phillips, R.; Hahn, S. South Polar Skuas from a Single Breeding Population Overwinter in Different Oceans Though Show Similar Migration Patterns. Mar. Ecol. Prog. Ser. 2011, 435, 263–267. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Tarroux, A.; Chastel, O.; Delord, K.; Cherel, Y.; Descamps, S. Population-Specific Wintering Distributions of Adult South Polar Skuas over Three Oceans. Mar. Ecol. Prog. Ser. 2015, 538, 229–237. [Google Scholar] [CrossRef]
- Finger, J.V.G.; Krüger, L.; Corá, D.H.; Petry, M.V. Habitat Selection of Southern Giant Petrels: Potential Environmental Monitors of the Antarctic Peninsula. Antarct. Sci. 2023, 35, 256–269. [Google Scholar] [CrossRef]
- Delord, K.; Pinet, P.; Pinaud, D.; Barbraud, C.; De Grissac, S.; Lewden, A.; Cherel, Y.; Weimerskirch, H. Species-Specific Foraging Strategies and Segregation Mechanisms of Sympatric Antarctic Fulmarine Petrels throughout the Annual Cycle. IBIS 2016, 158, 569–586. [Google Scholar] [CrossRef]
- Krietsch, J.; Hahn, S.; Kopp, M.; Phillips, R.; Peter, H.; Lisovski, S. Consistent Variation in Individual Migration Strategies of Brown Skuas. Mar. Ecol. Prog. Ser. 2017, 578, 213–225. [Google Scholar] [CrossRef]
- Carneiro, A.; Manica, A.; Clay, T.; Silk, J.; King, M.; Phillips, R. Consistency in Migration Strategies and Habitat Preferences of Brown Skuas over Two Winters, a Decade Apart. Mar. Ecol. Prog. Ser. 2016, 553, 267–281. [Google Scholar] [CrossRef]
- Ogrzewalska, M.; Couto Motta, F.; Resende, P.C.; Fumian, T.; Fonseca da Mendonça, A.C.; Appolinario Reis, L.; Lima Brandao, M.; Chame, M.; Arantes Gomes, I.L.; Mendonca Siqueira, M. Influenza A(H11N2) Virus Detection in Fecal Samples from Adélie (Pygoscelis adeliae) and Chinstrap (Pygoscelis antarcticus) Penguins, Penguin Island, Antarctica. Microbiol. Spectr. 2022, 10, e0142722. [Google Scholar] [CrossRef] [PubMed]
- Hurt, A.C.; Su, Y.C.F.; Aban, M.; Peck, H.; Lau, H.; Baas, C.; Deng, Y.-M.; Spirason, N.; Ellström, P.; Hernandez, J.; et al. Evidence for the Introduction, Reassortment, and Persistence of Diverse Influenza A Viruses in Antarctica. J. Virol. 2016, 90, 9674–9682. [Google Scholar] [CrossRef] [PubMed]
- Barriga, G.P.; Boric-Bargetto, D.; San Martin, M.C.; Neira, V.; van Bakel, H.; Thompsom, M.; Tapia, R.; Toro-Ascuy, D.; Moreno, L.; Vasquez, Y.; et al. Avian Influenza Virus H5 Strain with North American and Eurasian Lineage Genes in an Antarctic Penguin. Emerg. Infect. Dis. 2016, 22, 2221–2223. [Google Scholar] [CrossRef]
- Ulloa, M.; Fernández, A.; Ariyama, N.; Colom-Rivero, A.; Rivera, C.; Nuñez, P.; Sanhueza, P.; Johow, M.; Araya, H.; Torres, J.C.; et al. Mass Mortality Event in South American Sea Lions (Otaria flavescens) Correlated to Highly Pathogenic Avian Influenza (HPAI) H5N1 Outbreak in Chile. Vet. Q. 2023, 43, 1–10. [Google Scholar] [CrossRef]
- Molini, U.; Aikukutu, G.; Roux, J.-P.; Kemper, J.; Ntahonshikira, C.; Marruchella, G.; Khaiseb, S.; Cattoli, G.; Dundon, W.G. Avian Influenza H5N8 Outbreak in African Penguins (Spheniscus demersus), Namibia, 2019. J. Wildl. Dis. 2020, 56, 214–218. [Google Scholar] [CrossRef]
- Pardo-Roa, C.; Nelson, M.I.; Ariyama, N.; Aguayo, C.; Almonacid, L.I.; Munoz, G.; Navarro, C.; Avila, C.; Ulloa, M.; Reyes, R.; et al. Cross-Species Transmission and PB2 Mammalian Adaptations of Highly Pathogenic Avian Influenza A/H5N1 Viruses in Chile. bioRxiv 2023. [Google Scholar] [CrossRef]
- Leguia, M.; Garcia-Glaessner, A.; Muñoz-Saavedra, B.; Juarez, D.; Barrera, P.; Calvo-Mac, C.; Jara, J.; Silva, W.; Ploog, K.; Amaro, L.; et al. Highly Pathogenic Avian Influenza A (H5N1) in Marine Mammals and Seabirds in Peru. Nat. Commun. 2023, 14, 5489. [Google Scholar] [CrossRef] [PubMed]
- Campagna, C.; Uhart, M.; Falabella, V.; Campagna, J.; Zavattieri, V.; Vanstreels, R.E.T.; Lewis, M.N. Catastrophic Mortality of Southern Elephant Seals Caused by H5N1 Avian Influenza. Mar. Mammal Sci. 2024, 40, 322–325. [Google Scholar] [CrossRef]
- Mena, J.; Brito, B.; Moreira, R.; Tadich, T.; González, I.; Cruces, J.; Ortega, R.; van Bakel, H.; Rathnasinghe, R.; Pizarro-Lucero, J.; et al. Reemergence of H3N8 Equine Influenza A Virus in Chile, 2018. Transbound. Emerg. Dis. 2018, 65, 1408–1415. [Google Scholar] [CrossRef]
- Tapia, R.; García, V.; Mena, J.; Bucarey, S.; Medina, R.A.; Neira, V. Infection of Novel Reassortant H1N2 and H3N2 Swine Influenza A Viruses in the Guinea Pig Model. Vet. Res. 2018, 49, 73. [Google Scholar] [CrossRef]
- Lisovski, S.; Günther, A.; Dewar, M.; Ainley, D.; Arce, R.; Ballard, G.; Belliure, J.; Boulinier, T.; Bennison, A.; Cary, C.; et al. No Evidence for Highly Pathogenic Avian Influenza Virus H5N1 (Clade 2.3.4.4b) in the Antarctic Region during the Austral Summer 2022/23. bioRxiv 2023. [Google Scholar] [CrossRef]
- Boulinier, T. Avian Influenza Spread and Seabird Movements between Colonies. Trends Ecol. Evol. 2023, 38, 391–395. [Google Scholar] [CrossRef]
- European Food Safety Authority, European Centre for Disease Prevention and Control, European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; Terregino, C.; Aznar, I.; et al. Avian Influenza Overview September–December 2022. EFSA J. 2023, 21, e07786. [Google Scholar] [CrossRef]
- Giacinti, J.A.; Signore, A.V.; Jones, M.E.B.; Bourque, L.; Lair, S.; Jardine, C.; Stevens, B.; Bollinger, T.; Goldsmith, D.; British Columbia Wildlife AIV Surveillance Program (BC WASPs); et al. Avian Influenza Viruses in Wild Birds in Canada Following Incursions of Highly Pathogenic H5N1 Virus from Eurasia in 2021/2022. bioRxiv 2023. [Google Scholar] [CrossRef]
- Bird Flu Hits Northumberland Arctic Terns Colony. BBC News, 10 July 2023.
- IA|SAG. Available online: https://websag.azurewebsites.net/ia (accessed on 18 December 2023).
- Killian, M.L. Avian Influenza Virus Sample Types, Collection, and Handling. Anim. Influenza Virus Methods Protoc. 2020, 2123, 113–121. [Google Scholar] [CrossRef]
- Bennison, A.; Byrne, A.M.P.; Reid, S.M.; Lynton-Jenkins, J.G.; Mollett, B.; Sliva, D.D.; Peers-Dent, J.; Finlayson, K.; Hall, R.; Blockley, F.; et al. Detection and Spread of High Pathogenicity Avian Influenza Virus H5N1 in the Antarctic Region. bioRxiv 2023. [Google Scholar] [CrossRef]
- Corá, D.H.; Finger, J.V.G.; Krüger, L. Coprophagic Behaviour of Southern Giant Petrels (Macronectes giganteus) during Breeding Period. Polar Biol. 2020, 43, 2111–2116. [Google Scholar] [CrossRef]
- MAPPPD|Institute for Advanced Computational Science. Available online: https://www.stonybrook.edu/commcms/iacs/research/products/software/mapppd.php (accessed on 18 December 2023).
- Tomás, G.; Marandino, A.; Panzera, Y.; Rodríguez, S.; Wallau, G.D.L.; Dezordi, F.; Pérez, R.; Bassetti, L.; Negro, R.; Williman, J.; et al. Highly Pathogenic Avian Influenza H5N1 Virus Infections in Pinnipeds and Seabirds in Uruguay: A Paradigm Shift to Virus Transmission in South America. bioRxiv 2023. [Google Scholar] [CrossRef]
- de Seixas, M.M.M.; de Araújo, J.; Krauss, S.; Fabrizio, T.; Walker, D.; Ometto, T.; Matsumiya Thomazelli, L.; Vanstreels, R.E.T.; Hurtado, R.F.; Krüger, L.; et al. H6N8 Avian Influenza Virus in Antarctic Seabirds Demonstrates Connectivity between South America and Antarctica. Transbound. Emerg. Dis. 2022, 69, e3436–e3446. [Google Scholar] [CrossRef]
- Simeone, A.; Hiriart-Bertrand, L.; Reyes-Arriagada, R.; Halpern, M.; Dubach, J.; Wallace, R.; Pütz, K.; Lüthi, B. Heterospecific Pairing and Hybridization between Wild Humboldt and Magellanic Penguins in Southern Chile. Condor 2009, 111, 544–550. [Google Scholar] [CrossRef]
| Locations | Especies | Environmental | Direct | Total |
|---|---|---|---|---|
| Hanna Point, Livingstone Island | Southern elephant seal | 53 | 0 | 53 |
| Kelp gull | 13 | 0 | 13 | |
| Snowy sheathbill | 0 | 3 | 3 | |
| Chinstrap penguin | 0 | 3 | 3 | |
| Gentoo penguin | 0 | 8 | 8 | |
| Lions Rump, King George Island | Southern elephant seal | 68 | 0 | 68 |
| Gentoo penguin | 0 | 33 | 33 | |
| Adelie penguin | 0 | 1 | 1 | |
| Chinstrap penguin | 0 | 2 | 2 | |
| Fildes Peninsula western coast, King George Island | Southern elephant seal | 16 | 0 | 16 |
| Unidentified mammal | 6 | 0 | 6 | |
| Brown skua | 0 | 1 | 1 | |
| Total | 156 | 51 | 207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, G.; Mendieta, V.; Ulloa, M.; Agüero, B.; Torres, C.G.; Kruger, L.; Neira, V. Lack of Highly Pathogenic Avian Influenza H5N1 in the South Shetland Islands in Antarctica, Early 2023. Animals 2024, 14, 1008. https://doi.org/10.3390/ani14071008
Muñoz G, Mendieta V, Ulloa M, Agüero B, Torres CG, Kruger L, Neira V. Lack of Highly Pathogenic Avian Influenza H5N1 in the South Shetland Islands in Antarctica, Early 2023. Animals. 2024; 14(7):1008. https://doi.org/10.3390/ani14071008
Chicago/Turabian StyleMuñoz, Gabriela, Vanessa Mendieta, Mauricio Ulloa, Belén Agüero, Cristian G. Torres, Lucas Kruger, and Victor Neira. 2024. "Lack of Highly Pathogenic Avian Influenza H5N1 in the South Shetland Islands in Antarctica, Early 2023" Animals 14, no. 7: 1008. https://doi.org/10.3390/ani14071008
APA StyleMuñoz, G., Mendieta, V., Ulloa, M., Agüero, B., Torres, C. G., Kruger, L., & Neira, V. (2024). Lack of Highly Pathogenic Avian Influenza H5N1 in the South Shetland Islands in Antarctica, Early 2023. Animals, 14(7), 1008. https://doi.org/10.3390/ani14071008







