White Isthmus Transcriptome Analysis Reveals the Mechanism of Translucent Eggshell Formation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. RNA Extraction, Library Preparation, and Sequencing
2.3. RNA-Seq Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Selection of Hens and Collection of Oviduct Tissue
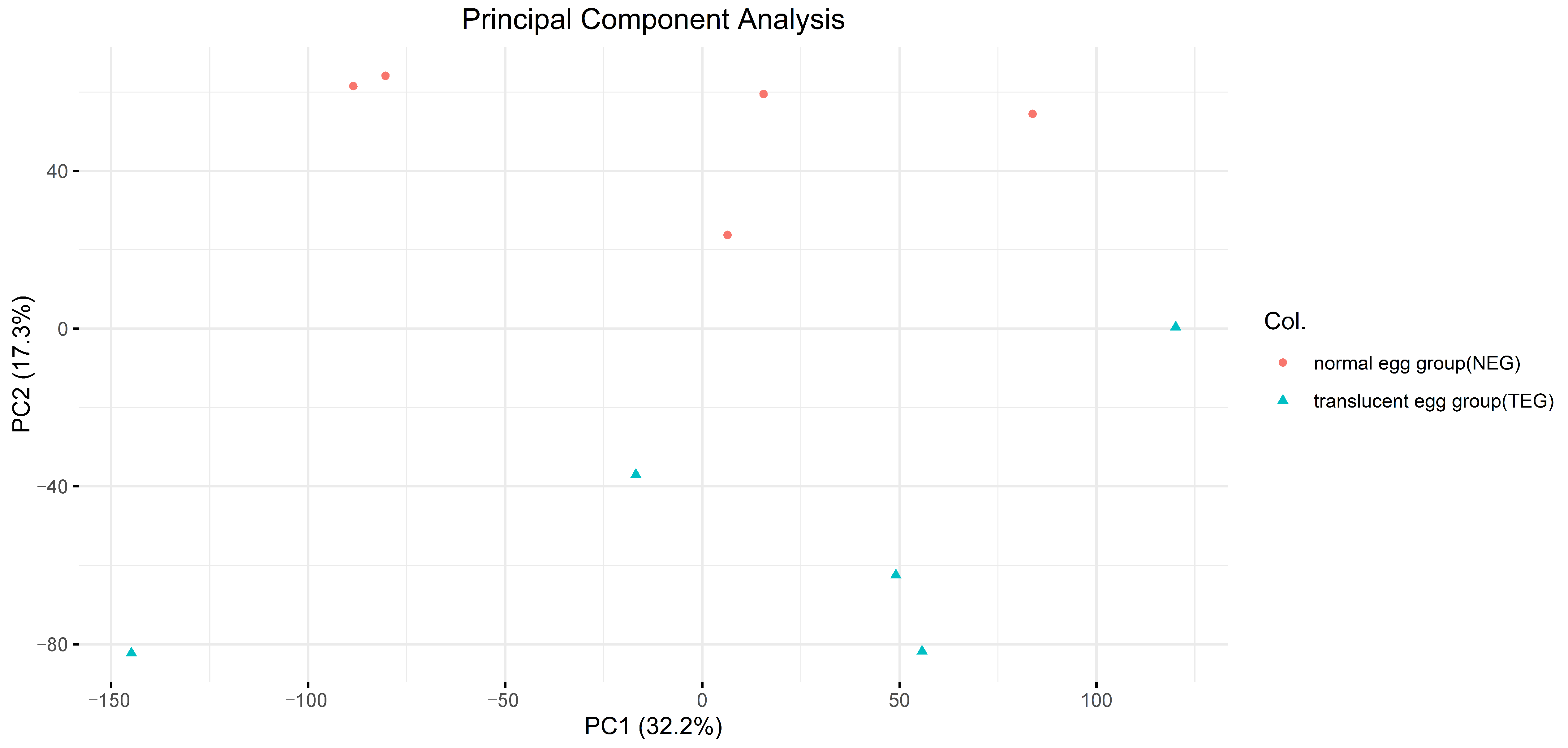
3.2. Construction of the Raw Reads, Mapping, and Batch Correction
3.3. Identification of DEGs between the Normal Egg Group (NEG) and Translucent Egg Group (TEG)
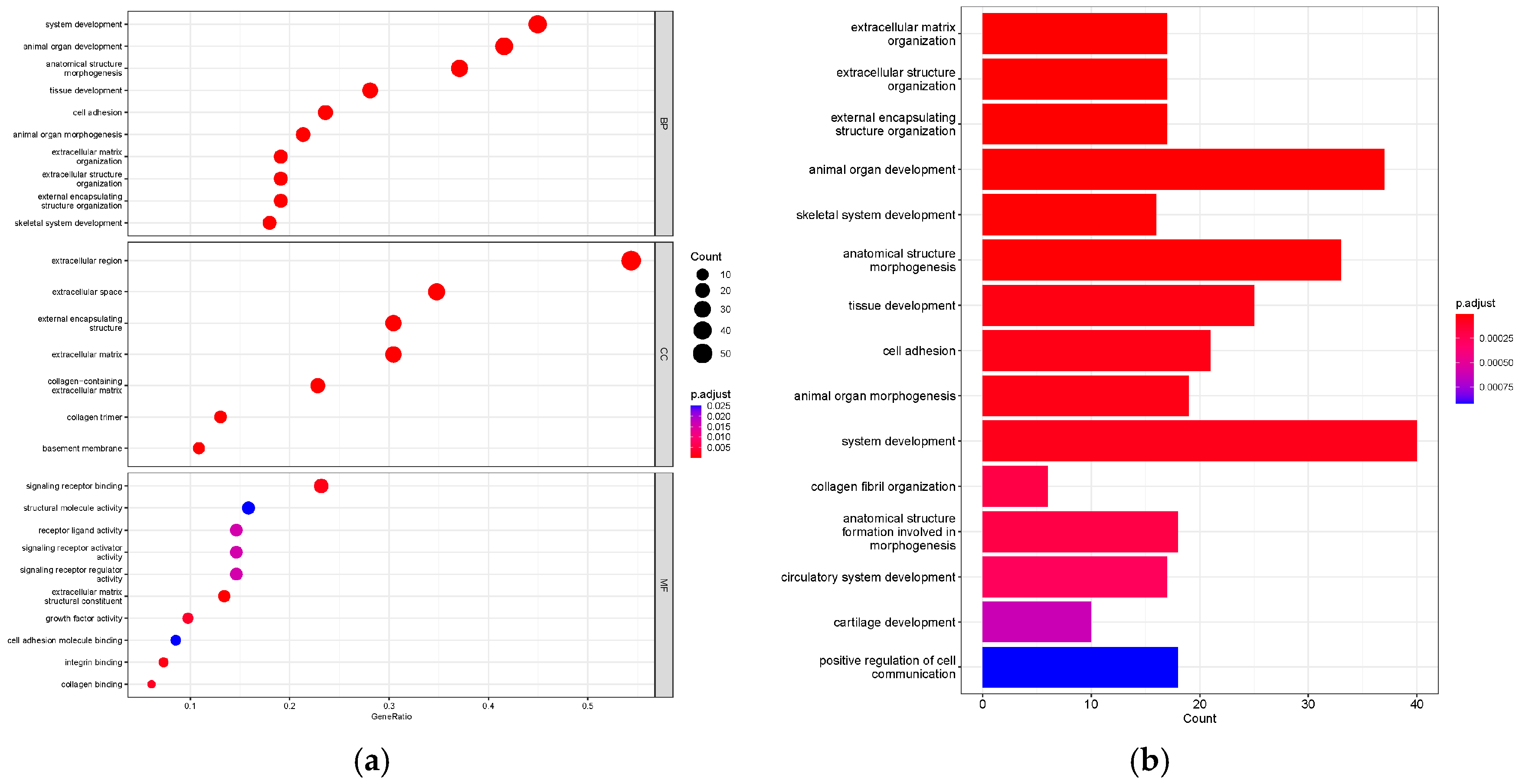
3.4. Function and Pathway Analysis of the DEGs
3.5. Integration of PPI Network and Module Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.-H.; Li, Y.-J.; Liu, L.; Liu, J.-S.; Bao, M.; Yang, N.; Zhuo-Cheng, H.; Ning, Z.-H. Traits of Eggshells and Shell Membranes of Translucent Eggs. Poult. Sci. 2017, 96, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ning, Z. Research Progress on Bird Eggshell Quality Defects: A Review. Poult. Sci. 2023, 102, 102283. [Google Scholar] [CrossRef] [PubMed]
- Holst, W.F.; Almquist, H.J.; Lorenz, F.W. A Study of Shell Texture of the Hen’s Egg. Poult. Sci. 1932, 11, 144–149. [Google Scholar] [CrossRef]
- Talbot, C.J.; Tyler, C. A Study of the Fundamental Cause of Natural Translucent Areas in Egg Shells. Br. Poult. Sci. 1974, 15, 197–204. [Google Scholar] [CrossRef]
- Solomon, S.E. Egg & Eggshell Quality; Iowa State University Press: Ames, IA, USA, 1997. [Google Scholar]
- Chousalkar, K.K.; Flynn, P.; Sutherland, M.; Roberts, J.R.; Cheetham, B.F. Recovery of Salmonella and Escherichia Coli from Commercial Egg Shells and Effect of Translucency on Bacterial Penetration in Eggs. Int. J. Food Microbiol. 2010, 142, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Bain, M.M.; MacLeod, N.; Thomson, R.; Hancock, J.W. Microcracks in Eggs. Poult. Sci. 2006, 85, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, H.; Wang, L. The Causes of Thin Spots on Eggshells; China Poultry: Fountain Valley, CA, USA, 2007; pp. 49–51. [Google Scholar] [CrossRef]
- Nie, W. Effects of Dietary Phosphorus Level on Laying Performance, Eggshell Quality, and Calcium and Phosphorus Absorption in Bantam Hens. Ph.D. Dissertation, China Agricultural University, Beijing, China, 2013. [Google Scholar]
- Wang, D. Exploration of the Formation Mechanism of Dark Spots on Eggs. Ph.D. Dissertation, China Agricultural University, Beijing, China, 2017. [Google Scholar]
- Liu, G.-Y.; Shi, L.; Chen, Y.-F.; Chen, H.; Zhang, C.; Wang, Y.-T.; Ning, Z.-H.; Wang, D.-H. Estimation of Genetic Parameters of Eggshell Translucency and Production Traits in Different Genotypes of Laying Hens. Poult. Sci. 2023, 102, 102616. [Google Scholar] [CrossRef]
- Nys, Y.; Gautron, J.; Garcia-Ruiz, J.M.; Hincke, M.T. Avian Eggshell Mineralization: Biochemical and Functional Characterization of Matrix Proteins. Comptes Rendus Palevol 2004, 3, 549–562. [Google Scholar] [CrossRef]
- Nakano, T.; Ikawa, N.I.; Ozimek, L. Chemical Composition of Chicken Eggshell and Shell Membranes. Poult. Sci. 2003, 82, 510–514. [Google Scholar] [CrossRef]
- Chen, L.; Kang, J.; Sukigara, S. Preparation and Characterization of Polyurethane/Soluble Eggshell Membrane Nanofibers. BioMed. Mater. Eng. 2014, 24, 1979–1989. [Google Scholar] [CrossRef]
- Fernandez, M.S.; Araya, M.; Arias, J.L. Eggshells Are Shaped by a Precise Spatio-Temporal Arrangement of Sequentially Deposited Macromolecules. Matrix Biol. 1997, 16, 13–20. [Google Scholar] [CrossRef]
- Arias, J.L.; Carrino, D.A.; Fernández, M.S.; Rodríguez, J.P.; Dennis, J.E.; Caplan, A.I. Partial Biochemical and Immunochemical Characterization of Avian Eggshell Extracellular Matrices. Arch. Biochem. Biophys. 1992, 298, 293–302. [Google Scholar] [CrossRef]
- Anwar, K. A Study on the Primary Characterisation of Avian Egg-Shell Membranes. Master’s Thesis, University of St Andrews, St Andrews, UK, 1980. [Google Scholar]
- Wang, D.-H.; Chen, H.; Zhou, R.-Y.; Huang, C.-X.; Gao, H.-X.; Fan, B.-L.; Liu, G.-J.; Ning, Z.-H. Study of Measurement Methods on Phenotype of Translucent Eggs. Poult. Sci. 2019, 98, 6677–6683. [Google Scholar] [CrossRef] [PubMed]
- The Avian Egg: Chemistry and Biology by Burley, R.W.; Vadehra, D.V.: Fine Hardcover (1989)|CorgiPack. Available online: https://www.abebooks.com/Avian-Egg-Chemistry-Biology-Burley-Vadehra/17743611499/bd (accessed on 12 September 2023).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium Gene Ontology Consortium: Going Forward. Nucleic Acids Res. 2015, 43, D1049–D1056. [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; Von Mering, C.; et al. STRING v9.1: Protein-Protein Interaction Networks, with Increased Coverage and Integration. Nucleic Acids Res. 2012, 41, D808–D815. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Effect of Dietary Nonphytate Phosphorus on Laying Performance and Small Intestinal Epithelial Phosphate Transporter Expression in Dwarf Pink-Shell Laying Hens|Journal of Animal Science and Biotechnology|Full Text. Available online: https://jasbsci.biomedcentral.com/articles/10.1186/2049-1891-4-34 (accessed on 12 September 2023).
- Goryszewska-Szczurek, E.; Baryla, M.; Kaczynski, P.; Waclawik, A. Prokineticin 1–Prokineticin Receptor 1 Signaling in Trophoblast Promotes Embryo Implantation and Placenta Development. Sci. Rep. 2021, 11, 13715. [Google Scholar] [CrossRef]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ Is a Novel Adipose-Specific Gene Dysregulated in Obesity (*). J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Bonni, A.; Ginty, D.D.; Dudek, H.; Greenberg, M.E. Serine 133-Phosphorylated CREB Induces Transcription via a Cooperative Mechanism That May Confer Specificity to Neurotrophin Signals. Mol. Cell. Neurosci. 1995, 6, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Alder, J.; Thakker-Varia, S.; Bangasser, D.A.; Kuroiwa, M.; Plummer, M.R.; Shors, T.J.; Black, I.B. Brain-Derived Neurotrophic Factor-Induced Gene Expression Reveals Novel Actions of VGF in Hippocampal Synaptic Plasticity. J. Neurosci. 2003, 23, 10800–10808. [Google Scholar] [CrossRef]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-Based Propagation of Functional Annotations within the Gene Ontology Consortium. Brief. Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef]
- Laheri, S.; Ashary, N.; Bhatt, P.; Modi, D. Oviductal Glycoprotein 1 (OVGP1) Is Expressed by Endometrial Epithelium That Regulates Receptivity and Trophoblast Adhesion. J. Assist. Reprod. Genet. 2018, 35, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.C.; Casadio, A.; Zhu, H.; Yaping, E.; Rose, J.C.; Chen, M.; Bailey, C.H.; Kandel, E.R. Synapse-Specific, Long-Term Facilitation of Aplysia Sensory to Motor Synapses: A Function for Local Protein Synthesis in Memory Storage. Cell 1997, 91, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Suk, C.; Shin, S.-I.; Hong, J.-Y. Salivary Cortisol, Dehydroepiandrosterone, and Chromogranin A Levels in Patients with Gingivitis and Periodontitis and a Novel Biomarker for Psychological Stress. Front. Endocrinol. 2023, 14, 1147739. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E.; Haigh, M. ’Small’-Proteoglycan:Collagen Interactions: Keratan Sulphate Proteoglycan Associates with Rabbit Corneal Collagen Fibrils at the “a” and “c” Bands. Biosci. Rep. 1985, 5, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Conte, F.S.; McInnis, E.; Fong, T.H.; Cherr, G.N. Identification of the Origin and Localization of Chorion (Egg Envelope) Proteins in an Ancient Fish, the White Sturgeon, Acipenser Transmontanus. Biol. Reprod. 2014, 90, 132. [Google Scholar] [CrossRef] [PubMed]
- Kretova, M.; Selicky, T.; Cipakova, I.; Cipak, L. Regulation of Pre-mRNA Splicing: Indispensable Role of Post-Translational Modifications of Splicing Factors. Life 2023, 13, 604. [Google Scholar] [CrossRef] [PubMed]
- Gulati, P.; Thomas, G. Chapter 6—Amino Acid Regulation of hVps34 and mTORC1 Signaling. In The Enzymes; The Enzymes; Academic Press: Cambridge, MA, USA, 2010; Volume 27, pp. 89–100. [Google Scholar]
- Braakman, I.; Hebert, D.N. Protein Folding in the Endoplasmic Reticulum. Cold Spring Harb. Perspect. Biol. 2013, 5, a013201. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Baisakhiya, S.; Aggarwal, A.; Singh, A.; Abdelgawad, M.A.; Deepak, A.; Ansari, M.J.; Pramanik, S. Chondroitin Sulfate-Based Composites: A Tour d’horizon of Their Biomedical Applications. J. Mater. Chem. B 2022, 10, 9125–9178. [Google Scholar] [CrossRef] [PubMed]
- Hummel, S.; Christian, S.; Osanger, A.; Heid, H.; Nimpf, J.; Schneider, W.J. Identification of a Novel Chondroitin-Sulfated Collagen in the Membrane Separating Theca and Granulosa Cells in Chicken Ovarian Follicles: The Granulosa-Theca Cell Interface Is Not a Bona Fide Basement Membrane. J. Biol. Chem. 2007, 282, 8011–8018. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Li, L.; Li, G.; He, W.; Linhardt, R.J. Compositional Analysis and Structural Elucidation of Glycosaminoglycans in Chicken Eggs. Glycoconj. J. 2014, 31, 593–602. [Google Scholar] [CrossRef]
- Gianakas, C.A.; Keeley, D.P.; Ramos-Lewis, W.; Park, K.; Jayadev, R.; Kenny, I.W.; Chi, Q.; Sherwood, D.R. Hemicentin-Mediated Type IV Collagen Assembly Strengthens Juxtaposed Basement Membrane Linkage. J. Cell Biol. 2022, 222, e202112096. [Google Scholar] [CrossRef]
- Burch, G.H.; Gong, Y.; Liu, W.; Dettman, R.W.; Curry, C.J.; Smith, L.; Miller, W.L.; Bristow, J. Tenascin-X Deficiency Is Associated with Ehlers-Danlos Syndrome. Nat. Genet. 1997, 17, 104–108. [Google Scholar] [CrossRef]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted Disruption of Decorin Leads to Abnormal Collagen Fibril Morphology and Skin Fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef]
- Trowbridge, J.M.; Gallo, R.L. Dermatan Sulfate: New Functions from an Old Glycosaminoglycan. Glycobiology 2002, 12, 117R–125R. [Google Scholar] [CrossRef] [PubMed]
- Praissman, J.L.; Wells, L. Mammalian O-Mannosylation Pathway: Glycan Structures, Enzymes, and Protein Substrates. Biochemistry 2014, 53, 3066–3078. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.O.; Halmo, S.M.; Wells, L. Recent Advancements in Understanding Mammalian O-Mannosylation. Glycobiology 2017, 27, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Nucleocytoplasmic Transport: Mechanisms and Involvement in Neurodegenerative Disease. Neurology 2019, 92, 757–764. [Google Scholar] [CrossRef]
- KEGG PATHWAY: Protein Export. Available online: https://www.genome.jp/kegg-bin/show_pathway?ko03060+K03076 (accessed on 13 October 2023).
- Xiang, G.; Huang, L.; Zhang, X.; Wang, N.; Wang, H.; Mu, Y.; Li, K.; Liu, Z. Molecular Characteristics and Promoter Analysis of Porcine COL1A1. Genes 2022, 13, 1971. [Google Scholar] [CrossRef]
- Hudson, D.M.; Weis, M.; Eyre, D.R. Insights on the Evolution of Prolyl 3-Hydroxylation Sites from Comparative Analysis of Chicken and Xenopus Fibrillar Collagens. PLoS ONE 2011, 6, e19336. [Google Scholar] [CrossRef]
- Sakata-Takatani, K.; Matsuo, N.; Sumiyoshi, H.; Tsuda, T.; Yoshioka, H. Identification of a Functional CBF-Binding CCAAT-like Motif in the Core Promoter of the Mouse pro-Alpha 1(V) Collagen Gene (Col5a1). Matrix Biol. 2004, 23, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Mitulović, G.; Panahipour, L.; Gruber, R. Proteomic Analysis of Porcine-Derived Collagen Membrane and Matrix. Materials 2020, 13, 5187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wu, M.; Qin, X.; Wen, P.; Wu, Y.; Zhuang, J. Asporin Is a Potential Promising Biomarker for Common Heart Failure. DNA Cell Biol. 2021, 40, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Haq, F.; Ahmed, N.; Qasim, M. Comparative Genomic Analysis of Collagen Gene Diversity. 3 Biotech 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Troncoso, O.P.; Piaggio, F.; Hijar, A. Structure-Property Relationships of a Biopolymer Network: The Eggshell Membrane. Acta Biomater. 2010, 6, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Bellairs, R.; Boyde, A. Scanning Electron Microscopy of the Shell Membranes of the Hen’s Egg. Z. Zellforsch. Mikrosk. Anat. 1969, 96, 237–249. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Luo, Y.; Li, W.; Wang, D.; Ning, Z. White Isthmus Transcriptome Analysis Reveals the Mechanism of Translucent Eggshell Formation. Animals 2024, 14, 1477. https://doi.org/10.3390/ani14101477
Ma Y, Luo Y, Li W, Wang D, Ning Z. White Isthmus Transcriptome Analysis Reveals the Mechanism of Translucent Eggshell Formation. Animals. 2024; 14(10):1477. https://doi.org/10.3390/ani14101477
Chicago/Turabian StyleMa, Ying, Yuxing Luo, Wen Li, Dehe Wang, and Zhonghua Ning. 2024. "White Isthmus Transcriptome Analysis Reveals the Mechanism of Translucent Eggshell Formation" Animals 14, no. 10: 1477. https://doi.org/10.3390/ani14101477
APA StyleMa, Y., Luo, Y., Li, W., Wang, D., & Ning, Z. (2024). White Isthmus Transcriptome Analysis Reveals the Mechanism of Translucent Eggshell Formation. Animals, 14(10), 1477. https://doi.org/10.3390/ani14101477



