Different Evolutionary Trends of Galloanseres: Mitogenomics Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Assembly and Annotation of the Mitogenome
2.2. Phylogenetic Analysis and Divergence Time Inference
2.3. Selection Pressure Analysis of Mitochondrial Genes
3. Results
3.1. Mitogenome Structure and Annotation
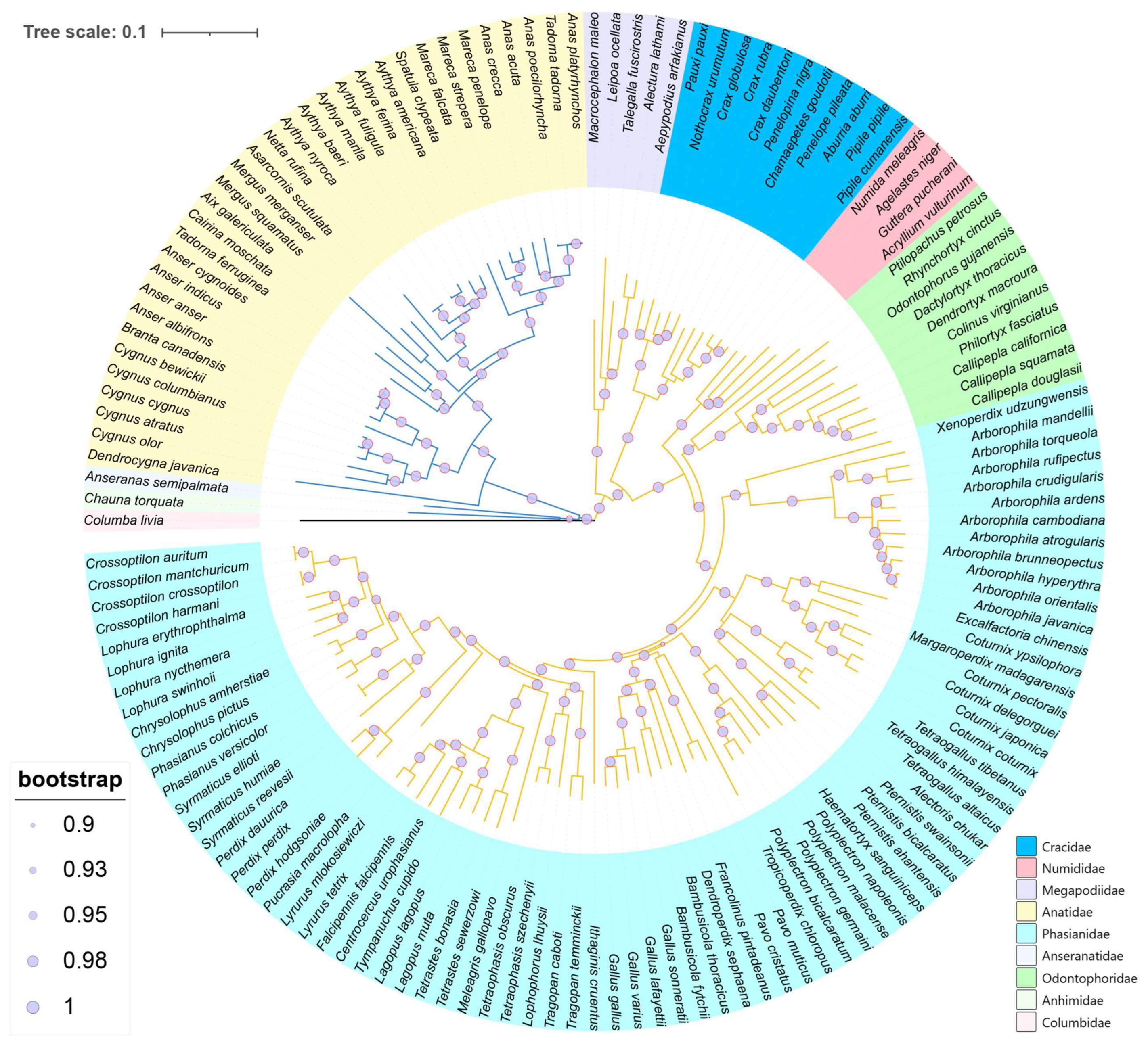
3.2. Phylogenetic and Divergence Time Analysis
3.3. Selection Pressure Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarvis, E.D.; Mirarab, S.; Aberer, A.J.; Li, B.; Houde, P.; Li, C.; Ho, S.Y.; Faircloth, B.C.; Nabholz, B.; Howard, J.T.; et al. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 2014, 346, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- De Mendoza, R.S.; Gomez, R.O. Ecomorphology of the tarsometatarsus of waterfowl (Anseriformes) based on geometric morphometrics and its application to fossils. Anat. Rec. 2022, 305, 3243–3253. [Google Scholar] [CrossRef] [PubMed]
- Hosner, P.A.; Tobias, J.A.; Braun, E.L.; Kimball, R.T. How do seemingly non-vagile clades accomplish trans-marine dispersal? Trait and dispersal evolution in the landfowl (Aves: Galliformes). Proc. Biol. Sci. 2017, 284. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, I.; Rathore, S.S.; Singh, R.P. An interplay between compositional constraint and natural selection dictates the codon usage pattern among select Galliformes. Bio Syst. 2021, 204, 104390. [Google Scholar] [CrossRef] [PubMed]
- Cordero, G.A.; Werneburg, I. Domestication and the comparative embryology of birds. J. Exp. Zool. B Mol. Dev. Evol. 2022, 338, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, I.; Sazanov, L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2022, 23, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.S.; D’Imprima, E.; Vonck, J. Mitochondrial Respiratory Chain Complexes. Subcell. Biochem. 2018, 87, 167–227. [Google Scholar]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Ng, M.Y.W.; Wai, T.; Simonsen, A. Quality control of the mitochondrion. Dev. Cell 2021, 56, 881–905. [Google Scholar] [CrossRef]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef]
- Collier, J.J.; Olahova, M.; McWilliams, T.G.; Taylor, R.W. Mitochondrial signalling and homeostasis: From cell biology to neurological disease. Trends Neurosci. 2023, 46, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef] [PubMed]
- Mallet, R.T.; Burtscher, J.; Pialoux, V.; Pasha, Q.; Ahmad, Y.; Millet, G.P.; Burtscher, M. Molecular Mechanisms of High-Altitude Acclimatization. Int. J. Mol. Sci. 2023, 24, 1698. [Google Scholar] [CrossRef] [PubMed]
- Hood, W.R.; Austad, S.N.; Bize, P.; Jimenez, A.G.; Montooth, K.L.; Schulte, P.M.; Scott, G.R.; Sokolova, I.; Treberg, J.R.; Salin, K. The Mitochondrial Contribution to Animal Performance, Adaptation, and Life-History Variation. Integr. Comp. Biol. 2018, 58, 480–485. [Google Scholar] [CrossRef]
- O’Brien, K.M. Mitochondrial biogenesis in cold-bodied fishes. J. Exp. Biol. 2011, 214 Pt 2, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Latorre-Muro, P.; Puigserver, P. Mechanisms of mitochondrial respiratory adaptation. Nat. Rev. Mol. Cell Biol. 2022, 23, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Francoso, E.; Zuntini, A.R.; Ricardo, P.C.; Santos, P.K.F.; de Souza Araujo, N.; Silva, J.P.N.; Goncalves, L.T.; Brito, R.; Gloag, R.; Taylor, B.A.; et al. Rapid evolution, rearrangements and whole mitogenome duplication in the Australian stingless bees Tetragonula (Hymenoptera: Apidae): A steppingstone towards understanding mitochondrial function and evolution. Int. J. Biol. Macromol. 2023, 242 Pt 1, 124568. [Google Scholar] [CrossRef]
- Sasaki, T.; Sato, M. Degradation of paternal mitochondria via mitophagy. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129886. [Google Scholar] [CrossRef]
- Lebedev, V.S.; Shenbrot, G.I.; Krystufek, B.; Mahmoudi, A.; Melnikova, M.N.; Solovyeva, E.N.; Lisenkova, A.A.; Undrakhbayar, E.; Rogovin, K.A.; Surov, A.V.; et al. Phylogenetic relations and range history of jerboas of the Allactaginae subfamily (Dipodidae, Rodentia). Sci. Rep. 2022, 12, 842. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, X.; Liu, G.; Wu, X.; Wei, Q.; Sun, G.; Mei, X.; Dong, Y.; Sha, W.; Zhang, H. Adaptability and Evolution of Gobiidae: A Genetic Exploration. Animals 2022, 12, 1741. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Wu, X.; Wei, Q.; Shang, Y.; Sun, G.; Mei, X.; Dong, Y.; Sha, W.; Zhang, H. High-altitude adaptation in vertebrates as revealed by mitochondrial genome analyses. Ecol. Evol. 2021, 11, 15077–15084. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Gao, S.; Zhao, M.; Lv, H.; Song, J.; Wang, H.; Zeng, Q.; Liu, J. Mitochondrial genomic analyses provide new insights into the “missing” atp8 and adaptive evolution of Mytilidae. BMC Genom. 2022, 23, 738. [Google Scholar] [CrossRef]
- Wang, X.; Shang, Y.; Wu, X.; Wei, Q.; Zhou, S.; Sun, G.; Mei, X.; Dong, Y.; Sha, W.; Zhang, H. Divergent evolution of mitogenomics in Cetartiodactyla niche adaptation. Org. Divers. Evol. 2023, 23, 243–259. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017, 45, e18. [Google Scholar]
- Donath, A.; Juhling, F.; Al-Arab, M.; Bernhart, S.H.; Reinhardt, F.; Stadler, P.F.; Middendorf, M.; Bernt, M. Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Res. 2019, 47, 10543–10552. [Google Scholar] [CrossRef]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlic, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Kumar, S.; Suleski, M.; Craig, J.M.; Kasprowicz, A.E.; Sanderford, M.; Li, M.; Stecher, G.; Hedges, S.B. TimeTree 5: An Expanded Resource for Species Divergence Times. Mol. Biol. Evol. 2022, 39, msac174. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Carretero, S.; Kapli, P.; Yang, Z. Beginner’s Guide on the Use of PAML to Detect Positive Selection. Mol. Biol. Evol. 2023, 40, msad041. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Jiang, G.T.; Yun, L.; Li, G.J.; Dai, Q.Z.; Zhang, S.R.; Hou, D.X.; He, X. The complete mitochondrial genome of the Linwu duck. Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016, 27, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Pan, T.; Hu, C.; Sun, L.; Ding, H.; Wang, H.; Zhang, C.; Jin, H.; Chang, Q.; Kan, X.; et al. Rapid and recent diversification patterns in Anseriformes birds: Inferred from molecular phylogeny and diversification analyses. PLoS ONE 2017, 12, e0184529. [Google Scholar] [CrossRef] [PubMed]
- Kimball, R.T.; Hosner, P.A.; Braun, E.L. A phylogenomic supermatrix of Galliformes (Landfowl) reveals biased branch lengths. Mol. Phylogenet. Evol. 2021, 158, 107091. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, C.; Chang, Q.; Yan, L.; Zhang, B. The complete mitochondrial genome of Aythya fuligula (Anatidae: Aythya). Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016, 27, 1003–1004. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, C.; Chang, Q.; Yan, L.; Pan, T.; Zhang, B. The complete mitochondrial genome of Aythya ferina (Anatidae: Aythya). Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016, 27, 968–969. [Google Scholar] [CrossRef]
- Zhai, H.; Li, Z.; Mi, S.; Meng, D.; Yu, H.; Teng, L.; Liu, Z. The complete mitochondrial genome of the Ferruginous Duck (Aythya nyroca) from Ningxia, China. Mitochondrial DNA B Resour. 2021, 6, 546–547. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Meng, D.; Li, Z.; Si, Y.; Yu, H.; Teng, L.; Liu, Z. Complete mitochondrial genome of the common Pochard (Aythya ferina) from Ningxia Hui autonomous region, China. Mitochondrial DNA B Resour. 2022, 7, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, T.; Gao, X.; Yang, X.; Sun, G.; Zhao, C.; Liu, G.; Zhang, H. Characterization and Phylogenetic Analysis of the Complete Mitochondrial Genome of Aythya marila. Genes 2023, 14, 1205. [Google Scholar] [CrossRef] [PubMed]
- Lind, C.; Sund, J.; Åqvist, J. Codon-reading specificities of mitochondrial release factors and translation termination at non-standard stop codons. Nat. Commun. 2013, 4, 2940. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.L.; Enkler, L.; Megel, C.; Karim, L.; Laporte, D.; Becker, H.D.; Duchene, A.M.; Sissler, M.; Marechal-Drouard, L. Idiosyncrasies in decoding mitochondrial genomes. Biochimie 2014, 100, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Peng, X.; Huang, X.; Xiang, D.; Chen, X. Complete mitochodrial genome of Spinibarbus caldwelli (Cypriniformes, Cyprinidae). Mitochondrial DNA 2015, 26, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Xia, E.H.; Gao, C.W.; Gao, L.Z. The complete mitochondrial genome of western painted turtle, Chrysemys picta bellii (Chrysemys, Emydidae). Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016, 27, 787–788. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Chai, Y.L.; Ma, H.M. The complete sequence of the mitochondrial genome of Duroc pig (Sus Scrofa). Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016, 27, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Zhang, J.; Chen, L.; Sha, W.; Yang, X.; Liu, G. The complete mitochondrial genome sequence of the Tibetan wolf (Canis lupus laniger). Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016, 27, 7–8. [Google Scholar] [CrossRef]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Krüger, A.; Remes, C.; Shiriaev, D.I.; Liu, Y.; Spåhr, H.; Wibom, R.; Atanassov, I.; Nguyen, M.D.; Cooperman, B.S.; Rorbach, J. Human mitochondria require mtRF1 for translation termination at non-canonical stop codons. Nat. Commun. 2023, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Saurer, M.; Leibundgut, M.; Nadimpalli, H.P.; Scaiola, A.; Schönhut, T.; Lee, R.G.; Siira, S.J.; Rackham, O.; Dreos, R.; Lenarčič, T.; et al. Molecular basis of translation termination at noncanonical stop codons in human mitochondria. Science 2023, 380, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Claramunt, S.; Cracraft, J. A new time tree reveals Earth history’s imprint on the evolution of modern birds. Sci. Adv. 2015, 1, e1501005. [Google Scholar] [CrossRef] [PubMed]
- Methner, K.; Campani, M.; Fiebig, J.; Loffler, N.; Kempf, O.; Mulch, A. Middle Miocene long-term continental temperature change in and out of pace with marine climate records. Sci. Rep. 2020, 10, 7989. [Google Scholar] [CrossRef] [PubMed]
- Vrba, E.S. Turnover-pulses, the Red Queen, and related topics. Am. J. Sci. 1993, 293, 418–452. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, X.; Dong, Y.; Shang, Y.; Sun, G.; Wu, X.; Zhao, C.; Sha, W.; Yang, G.; Zhang, H. Analysis of the Complete Mitochondrial Genome of Pteronura brasiliensis and Lontra canadensis. Animals 2023, 13, 3165. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, H.; Wu, X.; Sha, W. The selective constraints of ecological specialization in mustelidae on mitochondrial genomes. Mammal Res. 2020, 65, 85–92. [Google Scholar] [CrossRef]
- Marsh, J.A.; Teichmann, S.A. Predicting pathogenic protein variants. Science 2023, 381, 1284–1285. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Duanmu, X.; Zeng, L.; Liu, B.; Song, Z. Mitochondrial DNA: Distribution, Mutations, and Elimination. Cells 2019, 8, 379. [Google Scholar] [CrossRef]
- Kadenbach, B. Complex IV—The regulatory center of mitochondrial oxidative phosphorylation. Mitochondrion 2021, 58, 296–302. [Google Scholar] [CrossRef]
- Ohnishi, T.; Ohnishi, S.T.; Salerno, J.C. Five decades of research on mitochondrial NADH-quinone oxidoreductase (complex I). Biol. Chem. 2018, 399, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Y.; Shi, P.; Sun, Y.B.; Zhang, Y.P. Relaxation of selective constraints on avian mitochondrial DNA following the degeneration of flight ability. Genome Res. 2009, 19, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
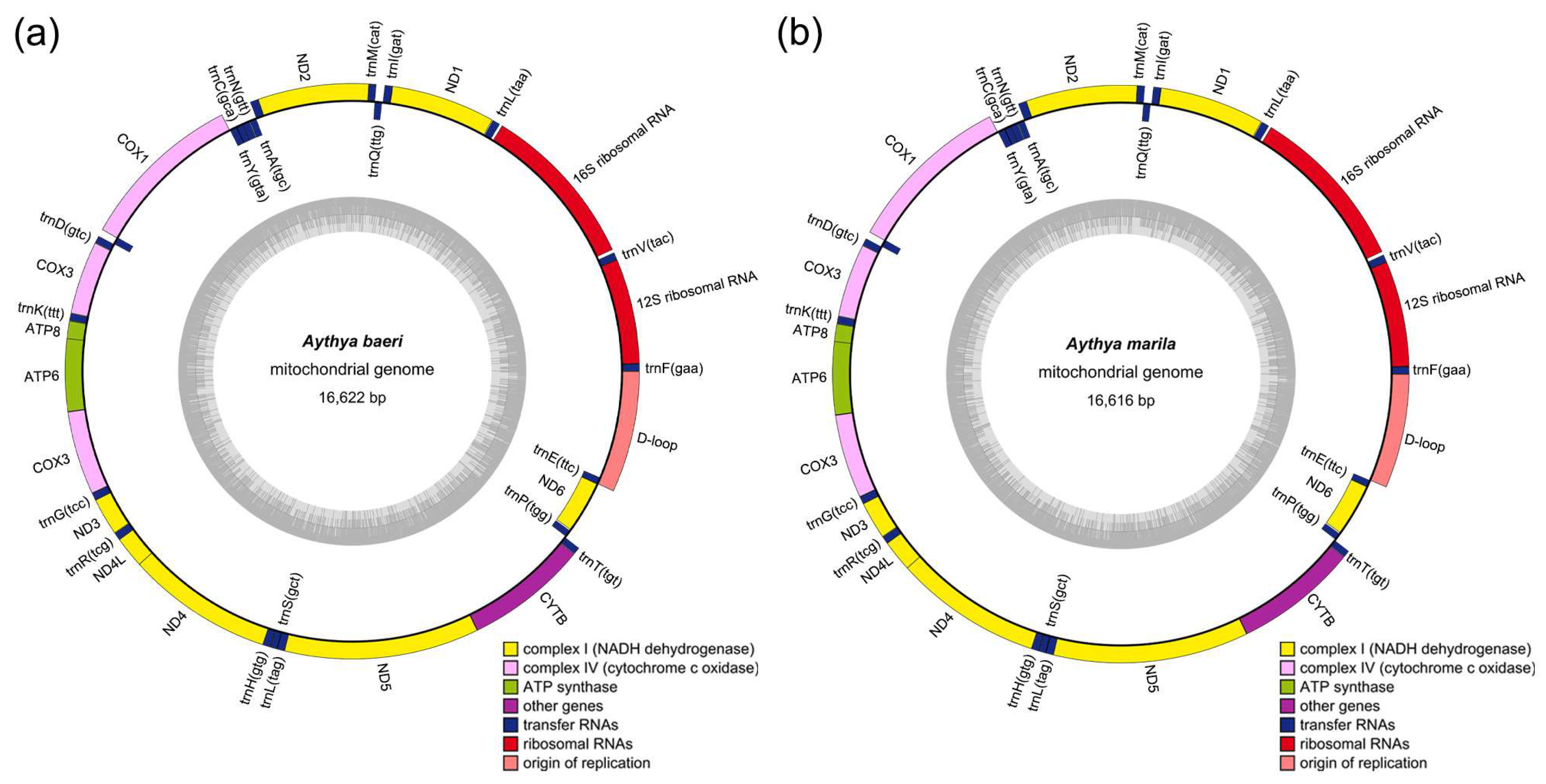



| Gene | Nucleotide Positions | Size (bp) | Stand | Intergenic Nucleotide | Start | Stop |
|---|---|---|---|---|---|---|
| trnF | 1–70 | 70 | + | |||
| 12s rRNA | 70–1053 | 984 | + | −1 | ||
| trnV | 1054–1124 | 71 | + | 0 | ||
| 16s rRNA | 1155–2704 | 1550 | + | 30 | ||
| trnL | 2729–2802 | 74 | + | 24 | ||
| ND1 | 2807–3784 | 978 | + | 4 | ATG | AGG |
| trnI | 3783–3854 | 72 | + | −2 | ||
| trnQ | 3863–3933 | 71 | − | 8 | ||
| trnM | 3933–4001 | 69 | + | −1 | ||
| ND2 | 4002–5040 | 1039 | + | 0 | ATG | T |
| trnW | 5041–5116 | 76 | + | 0 | ||
| trnA | 5120–5188 | 69 | − | 3 | ||
| trnN | 5191–5263 | 73 | − | 2 | ||
| trnC | 5264–5329 | 66 | − | 0 | ||
| trnY | 5329–5399 | 71 | − | −1 | ||
| COX1 | 5401–6951 | 1551 | + | 1 | GTG | AGG |
| trnS | 6943–7015 | 73 | − | −9 | ||
| trnD | 7018–7086 | 69 | + | 2 | ||
| COX2 | 7088–7774 | 687 | + | 1 | GTG | TAA |
| trnK | 7776–7843 | 68 | + | 1 | ||
| ATP8 | 7845–8012 | 168 | + | 1 | ATG | TAA |
| ATP6 | 8003–8686 | 684 | + | −10 | ATG | TAA |
| COX3 | 8686–9469 | 784 | + | −1 | ATG | T |
| trnG | 9470–9538 | 69 | + | 0 | ||
| ND3 | 9539–9890 | 352 | + | 0 | ATG | TAA |
| trnR | 9892–9962 | 71 | + | 1 | ||
| ND4L | 9963–10,259 | 297 | + | 0 | ATG | TAA |
| ND4 | 10,253–11,630 | 1378 | + | −7 | ATG | T |
| trnH | 11,631–11,699 | 69 | + | 0 | ||
| trnS | 11,700–11,765 | 66 | + | 0 | ||
| trnL | 11,765–11,835 | 71 | + | −1 | ||
| ND5 | 11,836–13,659 | 1824 | + | 0 | GTG | TAA |
| CYTB | 13,659–14,801 | 1143 | + | −1 | ATG | TAA |
| trnT | 14,804–14,872 | 69 | + | 2 | ||
| trnP | 14,883–14,952 | 70 | − | 10 | ||
| ND6 | 14,963–15,484 | 522 | − | 10 | ATG | TAG |
| trnE | 15,485–15,552 | 68 | − | 0 |
| Gene | Nucleotide Positions | Size (bp) | Stand | Intergenic Nucleotide | Start | Stop |
|---|---|---|---|---|---|---|
| trnF | 1–70 | 70 | + | |||
| 12s rRNA | 70–1054 | 985 | + | −1 | ||
| trnV | 1055–1125 | 71 | + | 0 | ||
| 16s rRNA | 1156–2705 | 1550 | + | 30 | ||
| trnL | 2729–2802 | 74 | + | 23 | ||
| ND1 | 2807–3784 | 978 | + | 4 | ATG | AGG |
| trnI | 3783–3854 | 72 | + | −2 | ||
| trnQ | 3863–3933 | 71 | − | 8 | ||
| trnM | 3933–4001 | 69 | + | −1 | ||
| ND2 | 4002–5040 | 1039 | + | 0 | ATG | T |
| trnW | 5041–5116 | 76 | + | 0 | ||
| trnA | 5120–5188 | 69 | − | 3 | ||
| trnN | 5191–5263 | 73 | − | 2 | ||
| trnC | 5264–5329 | 66 | − | 0 | ||
| trnY | 5329–5399 | 71 | − | −1 | ||
| COX1 | 5401–6951 | 1551 | + | 1 | GTG | AGG |
| trnS | 6943–7015 | 73 | − | −9 | ||
| trnD | 7018–7086 | 69 | + | 2 | ||
| COX2 | 7088–7774 | 687 | + | 1 | GTG | TAA |
| trnK | 7776–7843 | 68 | + | 1 | ||
| ATP8 | 7845–8012 | 168 | + | 1 | ATG | TAA |
| ATP6 | 8003–8686 | 684 | + | −10 | ATG | TAA |
| COX3 | 8686–9469 | 784 | + | −1 | ATG | T |
| trnG | 9470–9538 | 69 | + | 0 | ||
| ND3 | 9539–9890 | 352 | + | 0 | ATG | TAA |
| trnR | 9892–9961 | 70 | + | 1 | ||
| ND4L | 9962–10,258 | 297 | + | 0 | ATG | TAA |
| ND4 | 10,252–11,629 | 1378 | + | −7 | ATG | T |
| trnH | 11,630–11,698 | 69 | + | 0 | ||
| trnS | 11,699–11,764 | 66 | + | 0 | ||
| trnL | 11,764–11,834 | 71 | + | −1 | ||
| ND5 | 11,835–13,658 | 1824 | + | 0 | GTG | TAA |
| CYTB | 13,658–14,800 | 1143 | + | −1 | ATG | TAA |
| trnT | 14,803–14,871 | 69 | + | 2 | ||
| trnP | 14,882–14,951 | 70 | − | 10 | ||
| ND6 | 14,962–15,483 | 522 | − | 10 | ATG | TAG |
| trnE | 15,484–15,551 | 68 | − | 0 |
| Gene | Model Compared | |2ΔlnL| | p-Value | M2a_rel | CmC | |
|---|---|---|---|---|---|---|
| ω | ωANS | ωGAL | ||||
| ATP6 | CmC vs. M2a_rel | 15.9197 | 3.492 × 10−4 ** | 0.1565 | 0.1679 | 0.1582 |
| ATP8 | 4.2318 | 0.1205 | 0.2575 | 0.1702 | 0.2763 | |
| COX1 | 13.7733 | 0.0010 ** | 0.1073 | 0.1034 | 0.1099 | |
| COX2 | 1.5259 | 0.4663 | 0.1133 | 0.1290 | 0.1109 | |
| COX3 | 8.0060 | 0.0183 * | 0.0633 | 0.0613 | 0.0660 | |
| CYTB | 12.8937 | 0.0016 ** | 0.0886 | 0.0832 | 0.0918 | |
| ND1 | 13.7817 | 0.0010 ** | 0.1661 | 0.1557 | 0.1697 | |
| ND2 | 60.4582 | 7.438 × 10−14 ** | 0.1537 | 0.1038 | 0.1696 | |
| ND3 | 12.5340 | 0.0019 ** | 0.1720 | 0.1232 | 0.1907 | |
| ND4 | 26.8034 | 1.513 × 10−6 ** | 0.1313 | 0.1074 | 0.1402 | |
| ND4L | 3.3608 | 0.1863 | 0.1003 | 0.1267 | 0.0936 | |
| ND5 | 16.7277 | 2.33 × 10−4 ** | 0.1346 | 0.1075 | 0.1443 | |
| ND6 | 8.9346 | 0.0115 * | 0.1288 | 0.1004 | 0.1388 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Wang, X.; Wang, L.; Gao, X.; Lyu, T.; Xia, T.; Shi, L.; Dong, Y.; Mei, X.; Zhang, Z.; et al. Different Evolutionary Trends of Galloanseres: Mitogenomics Analysis. Animals 2024, 14, 1437. https://doi.org/10.3390/ani14101437
Zhou S, Wang X, Wang L, Gao X, Lyu T, Xia T, Shi L, Dong Y, Mei X, Zhang Z, et al. Different Evolutionary Trends of Galloanseres: Mitogenomics Analysis. Animals. 2024; 14(10):1437. https://doi.org/10.3390/ani14101437
Chicago/Turabian StyleZhou, Shengyang, Xibao Wang, Lidong Wang, Xiaodong Gao, Tianshu Lyu, Tian Xia, Lupeng Shi, Yuehuan Dong, Xuesong Mei, Zhihao Zhang, and et al. 2024. "Different Evolutionary Trends of Galloanseres: Mitogenomics Analysis" Animals 14, no. 10: 1437. https://doi.org/10.3390/ani14101437
APA StyleZhou, S., Wang, X., Wang, L., Gao, X., Lyu, T., Xia, T., Shi, L., Dong, Y., Mei, X., Zhang, Z., & Zhang, H. (2024). Different Evolutionary Trends of Galloanseres: Mitogenomics Analysis. Animals, 14(10), 1437. https://doi.org/10.3390/ani14101437





