Increased Erythrocyte Sedimentation Rate in Dogs: Frequency in Routine Clinical Practice and Association with Hematological Changes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Caseload and Sample Processing
2.2. Statistical Analysis
- Controls: samples from dogs that were clinically healthy at the time of collection and had no abnormalities in hematologic and biochemical analysis. These samples were collected as part of periodic health screenings, before elective surgeries (e.g., ovariectomy, orchiectomy) or diagnostic investigations (radiographs, ultrasound), or for monitoring patients in complete remission or those considered clinically cured. This group has been included in the study as a “negative” control, since changes in ESR values in this group were not expected.
- Mild chronic disorders: samples from dogs that, at the time of collection, had mild clinical signs or minimal laboratory changes associated with metabolic, degenerative, or chronic inflammatory conditions, mostly localized to a single organ or system. In this group, changes in the ESR were expected to be unlikely, either because some of the conditions included in this group do not have an inflammatory origin, or because the acute phase response associated with inflammation, if present, tends to become mild over time.
- Severe/acute diseases: samples from dogs that at the time of sampling had an acute clinical presentation or severe clinical conditions, likely associated with systemic involvement. This group may be of particular interest, because it included clinical events that may or may not have an inflammatory basis. For example, in some cases the acute clinical presentation may have led to sampling before the full development of inflammation (e.g., trauma or foreign bodies) or may have induced a stress response, with the production of cytokines that mimic an inflammatory pathogenesis. Additionally, in some of the acute diseases included in this group, the Ht is likely low, and this may influence the ESR, since a well-known inverse relationship between Ht and the ESR exists [23,28].
- Tumors: samples from dogs with different types of neoplasia, mainly lymphoproliferative disorders and mast cell tumors, collected either at first presentation or during chemotherapy. These samples, despite being collected from dogs affected by a chronic disorder, have been considered separately because they were particularly numerous and relatively homogeneous, and because it is known that the ESR may be increased in oncologic patients [29].
- Chronic Kidney Disease (CKD): samples from dogs that, at the time of collection, showed clinical and laboratory changes consistent with CKD in stage 2 of the International Renal Interest Society (IRIS) classification (http://www.iris-kidney.com, accessed on 1 March 2024) and/or a clinical presentation characterized by systemic symptoms referable to uremia. Dogs in the CKD group had a urine specific gravity (USG) < 1025, a documented history of azotemic CKD for more than 3 months, and no overt signs of uremia such as vomiting, anorexia, weakness, or uremic ulcers [30]. These samples, despite being affected by a chronic disorder, were considered as a separate group because they were relatively homogeneous in terms of pathogenesis and more easily clinically characterized.
- Urinary disorders: samples from dogs with acute symptoms related to inflammatory or obstructive disorders of the urinary tract. These samples, despite the acute presentation and an inflammatory pathogenesis, have been considered separately due to their homogeneous clinical presentation (hematuria, crystalluria or urolithiasis).
- Acute/subacute inflammation: samples from dogs that at the time of sampling had an acute/subacute presentation, with clinical or laboratory signs associated with acute presentations with inflammation, on which an infectious cause was suspected based on the clinical signs (e.g., fever, leukocytosis, and/or purulent exudates) or confirmed through additional testing (e.g., cytological evidence of leishmania, bacteria or fungi). This group was included in the study as a positive control group, since, based on the current information on ESR pathophysiology, an increase in the ESR is expected in samples included in this group [19].
3. Results
3.1. Study Population
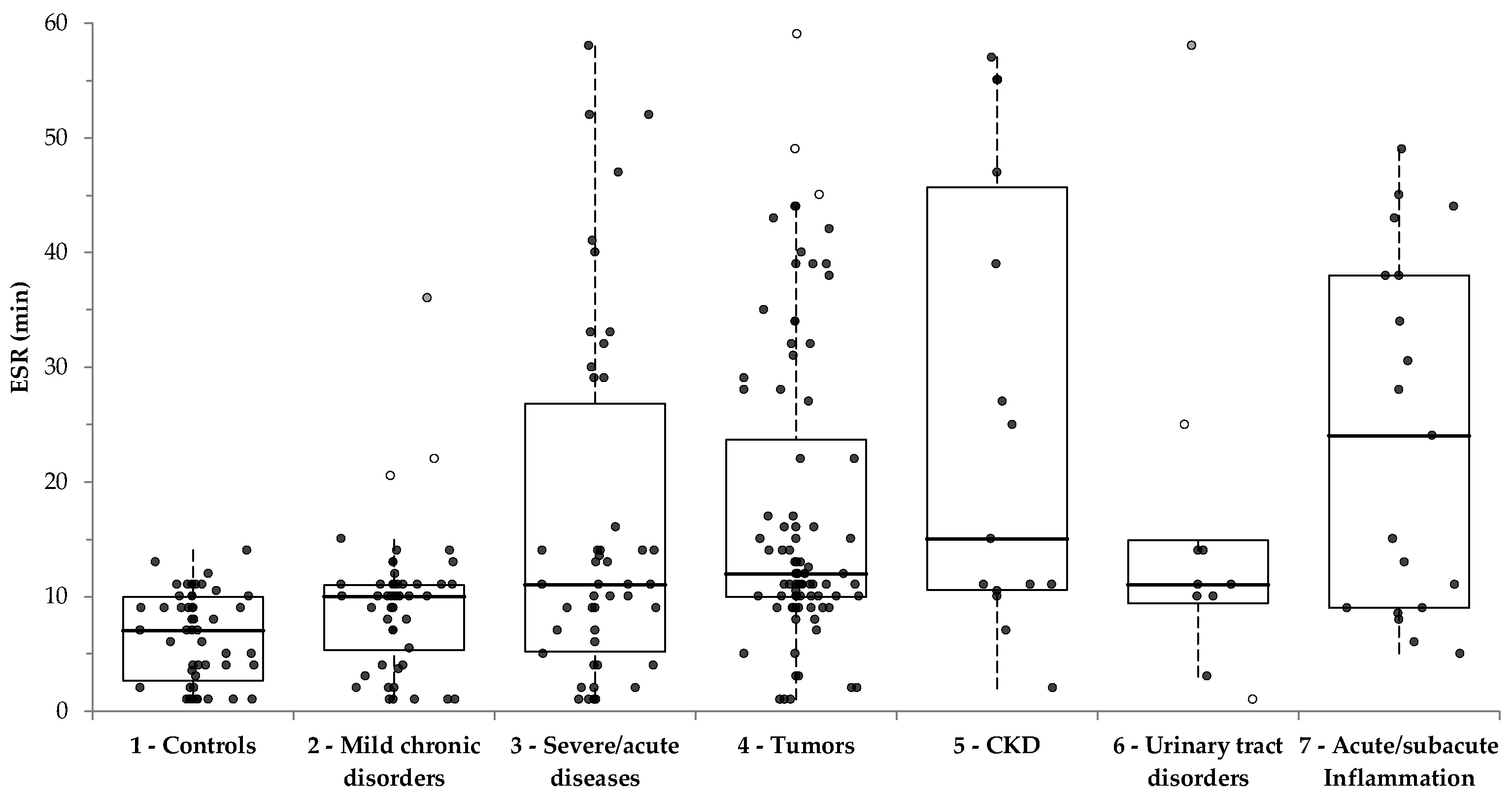
3.2. ESR Results
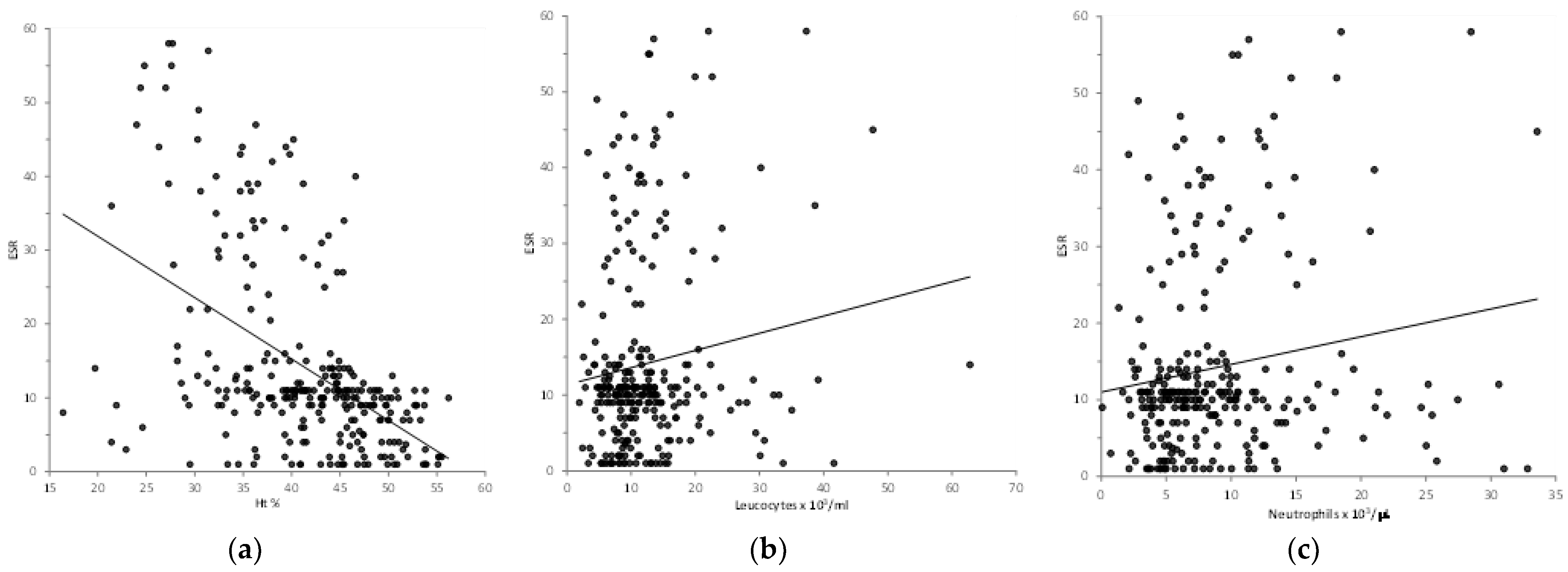
3.3. Hematological Parameters and ESR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baskurt, O.K.; Meiselman, H.J. Erythrocyte aggregation: Basic aspects and clinical importance. Clin. Hemorheol. Microcirc. 2013, 53, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Sak, J. A short history of the discovery of the erythrocyte sedimentation rate. Int. J. Lab. Hematol. 2012, 34, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Boroviczény, B.V.; Böttiger, L.E. Reference Method for the Erythrocyte Sedimentation Rate (ESR) Test on Human Blood. Br. J. Haematol. 1973, 24, 671–673. [Google Scholar] [CrossRef] [PubMed]
- ICSH recommendations for measurement of erythrocyte sedimentation rate. International Council for Standardization in Haematology (Expert Panel on Blood Rheology). J. Clin. Pathol. 1993, 46, 198–203. [CrossRef] [PubMed]
- Bain, B.J. Some influences on the ESR and the fibrinogen level in healthy subjects. Clin. Lab. Haematol. 1983, 5, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Brigden, M.L. Clinical utility of the erythrocyte sedimentation rate. Am. Fam. Physician 1999, 60, 1443–1450. [Google Scholar] [PubMed]
- Olshaker, J.S.; Jerrard, D.A. The erythrocyte sedimentation rate. J. Emerg. Med. 1997, 15, 869–874. [Google Scholar] [CrossRef]
- Alende-Castro, V.; Alonso-Sampedro, M. Factors influencing erythrocyte sedimentation rate in adults: New evidence for an old test. Medicine 2019, 98, 16816–16834. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Niedermüller, H. The Effect of Aging on Laboratory Values in Dogs. Zentralbl. Vet. A 1993, 40, 720–730. [Google Scholar] [CrossRef]
- Hiraga, A.; Sugano, S. Studies on exercise physiology of the racehorse performed in Japan during the period from the 1930s to the 1970s: Respiration and heart rate during exercise and the effect of exercise on blood characteristics. J. Equine Sci. 2016, 27, 37–48. [Google Scholar] [CrossRef]
- Bray, C.; Bell, L.N. Erythrocyte Sedimentation Rate and C-reactive Protein Measurements and Their Relevance in Clinical Medicine. WMJ 2016, 115, 317–321. [Google Scholar] [PubMed]
- Tishkowski, K.; Gupta, V. Erythrocyte Sedimentation Rate. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Aytekin, M. The Current Use and the Evolution of Erythrocyte Sedimentation Rate Measurement. Middle Black Sea J. Health Sci. 2018, 4, 17–23. [Google Scholar] [CrossRef]
- Fabry, T.L. Mechanism of Erythrocyte Aggregation and Sedimentation. Blood 1987, 70, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Cavalera, M.A.; Gernone, F. Erythrocyte sedimentation rate in canine leishmaniosis diagnosis: A new resource. Front. Vet. Sci. 2022, 9, 949372. [Google Scholar] [CrossRef] [PubMed]
- Asawapattanakul, T.; Pintapagung, T. Erythrocyte sedimentation rate, C-reactive protein, and interleukin-6 as inflammatory biomarkers in dogs naturally infected with Ehrlichia canis. Vet. World 2021, 14, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Matijatko, V.; Mrljak, V. Evidence of an acute phase response in dogs naturally infected with Babesia canis. Vet. Parasitol. 2007, 144, 242–250. [Google Scholar] [CrossRef] [PubMed]
- De Melo Benvenutti, M.E.; de Miranda, J.A.L. Diagnostic efficacy of erythrocyte sedimentation rate in horses: An integrative systematic review. Res. Soc. Dev. 2022, 11, e26911326416. [Google Scholar] [CrossRef]
- Gori, E.; Pasquini, A. Effect of time and storage temperature on canine and feline erythrocyte sedimentation rate. MethodsX 2022, 9, 101934. [Google Scholar] [CrossRef] [PubMed]
- Bland, S.D. Canine osteoarthritis and treatments: A review. Vet. Sci. Dev. 2015, 5, 5931. [Google Scholar] [CrossRef]
- Ghosh, C.K. Ameliorative effect of hemodialysis in a dog having chronic kidney disease. Pharma Innov. J. 2022, 11, 904–907. [Google Scholar]
- Osbaldiston, G.W. Erythrocyte sedimentation rate studies in sheep, dog, and horse. Cornell Vet. 1971, 61, 386–399. [Google Scholar] [PubMed]
- Jain, N.C.; Kono, C.S. Erythrocyte sedimentation rate in the dog and cat: Comparison of two methods and influence of packed cell volume, temperature and storage of blood. J. Small Anim. Prac. 1975, 16, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Cerón, J.J.; Eckersall, D.P. Acute phase proteins in dogs and cats: Current knowledge and future perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Takahashi, M. C-Reactive Protein Concentration in Dogs with Various Diseases. J. Vet. Med. Sci. 2008, 70, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Militello, C.; Pasquini, A. The Canine Erythrocyte Sedimentation Rate (ESR): Evaluation of a Point-of-Care Testing Device (MINIPET DIESSE). Vet. Med. Int. 2020, 2020, 3146845. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, C.; Grassi, A. Analytical Validation of MINI-PET as Point-of-Care for Erythrocyte Sedimentation Rate Measure in Horses. Vet. Med. Int. 2023, 2023, 9965095. [Google Scholar] [CrossRef]
- Higuchi, M.; Watanabe, N. Determination of the erythrocyte sedimentation rate using the hematocrit-corrected aggregation index and mean corpuscular volume. J. Clin. Lab. Anal. 2023, 37, e24877. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.L.; Wautier, M.P. Old and New Blood Markers in Human Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 12968. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Polzin, D.J.; Rendahl, A.; Granick, J.L. Urinary neutrophil gelatinase-associated lipocalin in dogs with stable or progressive kidney disease. J. Vet. Intern. Med. 2019, 33, 654–661. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Silva-Carvalho, R. A Comparative Approach of Tumor-Associated Inflammation in Mammary Cancer between Humans and Dogs. Biomed. Res. Int 2016, 2016, 4917387. [Google Scholar] [CrossRef]
- Nentwig, A.; Schweighauser, A. Assessment of the expression of biomarkers of uremic inflammation in dogs with renal disease. Am. J. Vet. Res. 2016, 77, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kadatane, S.P.; Satariano, M. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.E.; Sigurdsson, T. Erythrocyte Sedimentation Rate as a Tumor Marker in Human Prostatic Cancer: An Analysis of Prognostic Factors in 300 Population-Based Consecutive Cases. Cancer 1992, 70, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Bien, E.; Balcerska, A. Serum soluble interleukin-2 receptor, beta2-microglobulin, lactate dehydrogenase and erythrocyte sedimentation rate in children with hodgkin’s lymphoma. Scand. J. Immunol. 2009, 70, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Song, S.Y. The pretreatment erythrocyte sedimentation rate predicts survival outcomes after surgery and adjuvant radiotherapy for extremity soft tissue sarcoma. Radiat. Oncol. 2019, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Zhu, Y. Erythrocyte sedimentation rate kinetics as a marker of treatment response and predictor of prognosis in Chinese metastatic renal cell carcinoma patients treated with sorafenib. Int. J. Urol. 2011, 18, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, K.R.; Harr, K.E. ASVCP reference interval guidelines: Determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 2012, 41, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Geffré, A.; Concordet, D.; Braun, J.P.; Trumel, C. Reference Value Advisor: A new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet. Clin. Pathol. 2011, 40, 107–112. [Google Scholar] [CrossRef]
- Segev, G.; Vaden, S. ; Vaden, S. Urinary cystatin B differentiates progressive versus stable IRIS Stage 1 chronic kidney disease in dogs. J. Vet. Intern. Med. 2023, 37, 2251–2260. [Google Scholar] [CrossRef]
- Cannon, J.G.; Angel, J.B. Hormonal Influences on Stress-Induced Neutrophil Mobilization in Health and Chronic Fatigue Syndrome. J. Clin. Immunol. 1998, 18, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Lapić, I.; Rogić, D. Erythrocyte sedimentation rate is associated with severe coronavirus disease 2019 (COVID-19): A pooled analysis. Clin. Chem. Lab. Med. 2020, 58, 1146–1148. [Google Scholar] [CrossRef] [PubMed]
- Triga, M.G.; Syrogiannopoulos, G.A. Correlation of Leucocyte Count and Erythrocyte Sedimentation Rate with the Day of Illness in Presumed Bacterial Pneumonia of Childhood. J. Infect. 1998, 36, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Park, K.K.; Kim, T.K. Normative temporal values of CRP and ESR in unilateral and staged bilateral TKA. Clin. Orthop. Relat. Res. 2008, 466, 179–188. [Google Scholar] [CrossRef] [PubMed]
| Group | N | Breed | Sex | Age |
|---|---|---|---|---|
| Controls | 53 | Crossbred (n = 13), Breed unknown (n = 7), Labrador retriever (n = 6), Chihuahua (n = 4), German shepherd (n = 3), Belgian shepherd, Cavalier King Charles spaniel, Bolognese, Poodle (n = 2), American bulldog, Dachshund, Beagle, Entlebucher mountain dog, Drahthaar, Pug, Swiss mountain dog, Jack Russell terrier, Kurzhaar, Abruzzese shepherd, Bergamasco shepherd, German pinscher (n = 1) | 17 F 8 Fs 24 M 2 Mc 2 Unk | 6.3 (0.4–16.6) 6 Unk |
| Mild chronic disorders | 53 | Crossbred (n = 9), Chihuahua (n = 6), Yorkshire terrier (n = 5), Breed unknown, German shepherd (n = 4), Golden retriever (n = 3), Poodle, Dachshund, Cavalier King Charles spaniel, English setter, French bouledogue (n = 2), Italian bracco, Chow chow, Corso, Dobermann pinscher, English Staffordshire terrier, Jack Russell terrier, Labrador retriever, Maltese, Pitbull terrier, Toy schnauzer, Shi tzu (n = 1) | 11 F 16 Fs 24 M 1 Mc 1 Unk | 9.3 (0.4–15.3) 4 Unk |
| Severe/acute diseases | 52 | Breed unknown (n = 11), Crossbred (n = 9), Golden retriever (n = 5), Dobermann pinscher (n = 4), American Staffordshire, (n = 3), Cavalier King Charles spaniel, Jack Russell terrier, Italian segugio, French bouledogue (n = 2), Australian shepherd, Dobermann pinscher, Dogo argentino, Drahthaar, Labrador retriever, German shepherd, German Pinscher, Pitbull terrier, Pointer, Rottweiler, Shar pei, Shiba inu, Whippet (n = 1) | 11 F 18 Fs 19 M 3 Mc 1 Unk | 8.0 (0.4–16.0) 7 Unk |
| Tumors | 92 | Crossbred (n = 14), Rottweiler, English setter (n = 10), Golden retriever (n = 8), German shepherd (n = 6), Boxer (n = 5), Beagle, French bouledogue, West highland white terrier (n = 4), Australian shepherd, Poodle (n = 3), Breed unknown, Bull terrier, Dogue de Bordeaux, German pinscher, Pitbull terrier (n = 2), Dachshund, Bernese mountain dog, Cavalier King Charles spaniel, Jack Russell terrier, Labrador retriever, Afghan sighthound, Abruzzese shepherd, Bergamasco shepherd, Tosa inu, Welsh Corgi Pembroke, Yorkshire terrier (n = 1) | 22 F 25 Fs 30 M 14 Mc 1 Unk | 9.4 (1.9–14.5) 8 Unk |
| CKD | 15 | Crossbred (n = 4); Boxer (n = 3), Shi-tzu (n = 2), Italian bracco, English bulldog, Cocker, Dogue de Bordeaux, Shiba inu, Pomeranian (n = 1) | 4 Fs 9 M 2 Mc | 8.1 (1.8–16.6) 0 Unk |
| Urinary disorders | 10 | Crossbred (n = 2), Dachshund, Bolognese, Bull terrier, Epagneul breton, Fox terrier, Golden retriever, German pinscher, Yorkshire terrier (n = 1) | 2 F 3 Fs 3 M 2 Mc | 12.8 (5.1–18.6) 2 Unk |
| Acute/subacute inflammation | 20 | Crossbred (n = 5), Unknown breed (n = 3), Rottweiler (n = 2), Poodle, Basset-hound, Cocker spaniel, Setter gordon, Labrador retriever, Belgian shepherd, German shepherd, Pekingese, Italian segugio, English setter (n = 1) | 10 F 2 Fs 7 M 1 Mc | 6.1 (0.9–14.2) 2 Unk |
| Group | Median (I–III IQR) | >URL | >URL + ERR |
|---|---|---|---|
| 1—Controls | 7.0 (2.7–10.0) 4,5,7 (p < 0.001); 3 (p = 0.016) | 0/53 (0.0%) | 0/53 (0.0%) |
| 2—Mild chronic disorders | 10.0 (5.4–11.0) 5,7 (p = 0.003); 4 (p = 0.039) | 4/50 (8.0%) | 7/53 (13.2%) |
| 3—Severe/acute diseases | 11.0 (5.2–26.8) 1 (p = 0.016) | 13/47 (27.7%) | 20/52 (38.5%) |
| 4—Tumors | 12.0 (10.0–23.7) 1 (p < 0.001), 2 (p = 0.039) | 32/89 (40.0%) | 35/92 (38.0%) |
| 5—Chronic Kidney Disease (CKD) | 15.0 (10.6–45.7) 1 (p < 0.001); 2 (p = 0.003) | 8/15 (53.3%) | 8/15 (53.3%) |
| 6—Urinary tract disorders | 11.0 (9.4–14.9) | 2/10 (20.0%) | 2/10 (20.0%) |
| 7—Acute/subacute inflammation | 24.0 (9.0–38.0) 1 (p < 0.001); 2 (p = 0.003) | 11/19 (57.8%) | 12/20 (60.0%) |
| Group | Ht (%) | WBC × 103/μL | Neutrophils × 103/μL |
|---|---|---|---|
| 1—Controls | 45.0 (41.3–49.9) 3 (p = 0.018); 4 (p < 0.001); 5 (p = 0.042); 7 (p = 0.024) | 9.8 (8.1–13.3) 3 (p = 0.018) | 5.9 (4.7–8.2) 3 (p < 0.001) |
| 2—Mild chronic disorders | 44.9 (37.7–50.6) 4 (p = 0.017) | 10.5 (8.2–13.8) | 7.3 (5.3–10.4) |
| 3—Severe/acute diseases | 43.4 (32.4–47.0) 1 (p = 0.018) | 14.8 (10.3–21.6) 1 (p = 0.018); 4 (p < 0.001) | 11.5 (7.2–17.5) 1 (p < 0.001); 4 (p < 0.001) |
| 4—Tumors | 39.6 (33.2–44.0) 1 (p < 0.001); 2 (p = 0.017) | 8.6 (5.8–12.5) 3 (p < 0.001) | 6.3 (3.5–9.2) 3 (p < 0.001) |
| 5—Chronic Kidney Disease (CKD) | 36.4 (35.4–41.7) 1 (p = 0.042) | 9.6 (8.6–12.9) | 6.2 (4.9–9.9) |
| 6—Urinary tract disorders | 45.6 (43.1–47.4) | 10.4 (7.5–14.9) | 6.4 (4.6–11.6) |
| 7—Acute/subacute inflammation | 37.5 (34.7–43.7) 1 (p = 0.024) | 13.0 (10.5–17.0) | 9.5 (7.2–15.2) |
| p | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paltrinieri, S.; Ferrari, R.; Scavone, D.; Pieroni, C.; Diamanti, D.; Tagliasacchi, F. Increased Erythrocyte Sedimentation Rate in Dogs: Frequency in Routine Clinical Practice and Association with Hematological Changes. Animals 2024, 14, 1409. https://doi.org/10.3390/ani14101409
Paltrinieri S, Ferrari R, Scavone D, Pieroni C, Diamanti D, Tagliasacchi F. Increased Erythrocyte Sedimentation Rate in Dogs: Frequency in Routine Clinical Practice and Association with Hematological Changes. Animals. 2024; 14(10):1409. https://doi.org/10.3390/ani14101409
Chicago/Turabian StylePaltrinieri, Saverio, Roberta Ferrari, Donatella Scavone, Carolina Pieroni, Daniela Diamanti, and Filippo Tagliasacchi. 2024. "Increased Erythrocyte Sedimentation Rate in Dogs: Frequency in Routine Clinical Practice and Association with Hematological Changes" Animals 14, no. 10: 1409. https://doi.org/10.3390/ani14101409
APA StylePaltrinieri, S., Ferrari, R., Scavone, D., Pieroni, C., Diamanti, D., & Tagliasacchi, F. (2024). Increased Erythrocyte Sedimentation Rate in Dogs: Frequency in Routine Clinical Practice and Association with Hematological Changes. Animals, 14(10), 1409. https://doi.org/10.3390/ani14101409





