Psychometric Testing and Validation of the Italian Version of the Helsinki Chronic Pain Index (I-HCPI) in Dogs with Pain Related to Osteoarthritis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Translation and Back-Translation
2.2. The Italian Helsinki Chronic Pain Index (I-HCPI)
2.3. Animals and Study Design
2.4. Psychometric Tests and Statistical Analysis
2.4.1. Validity
2.4.2. Responsiveness
2.4.3. Reliability
2.4.4. Diagnostic Accuracy and Cut-Off Point
3. Results
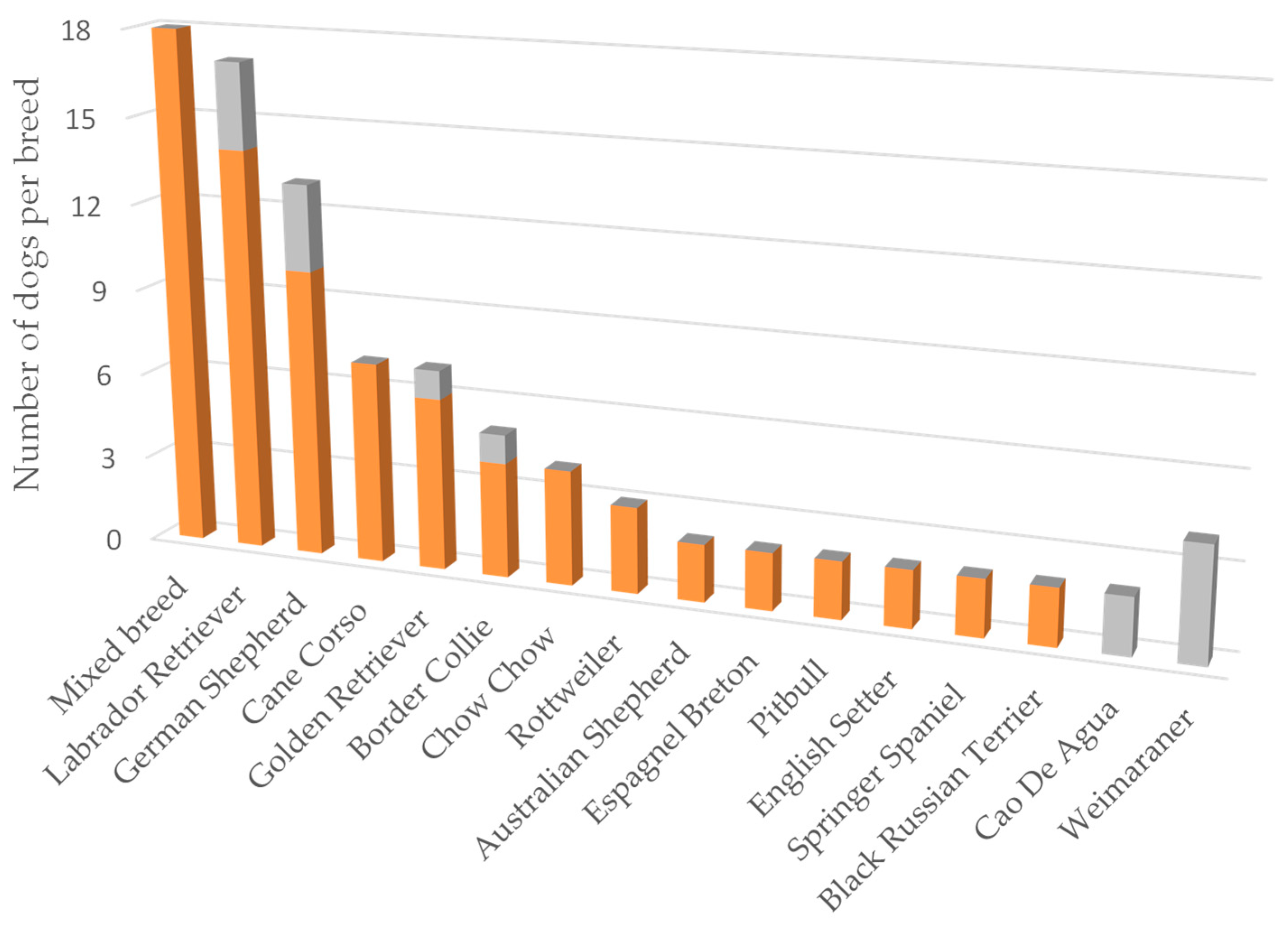
3.1. Demographics
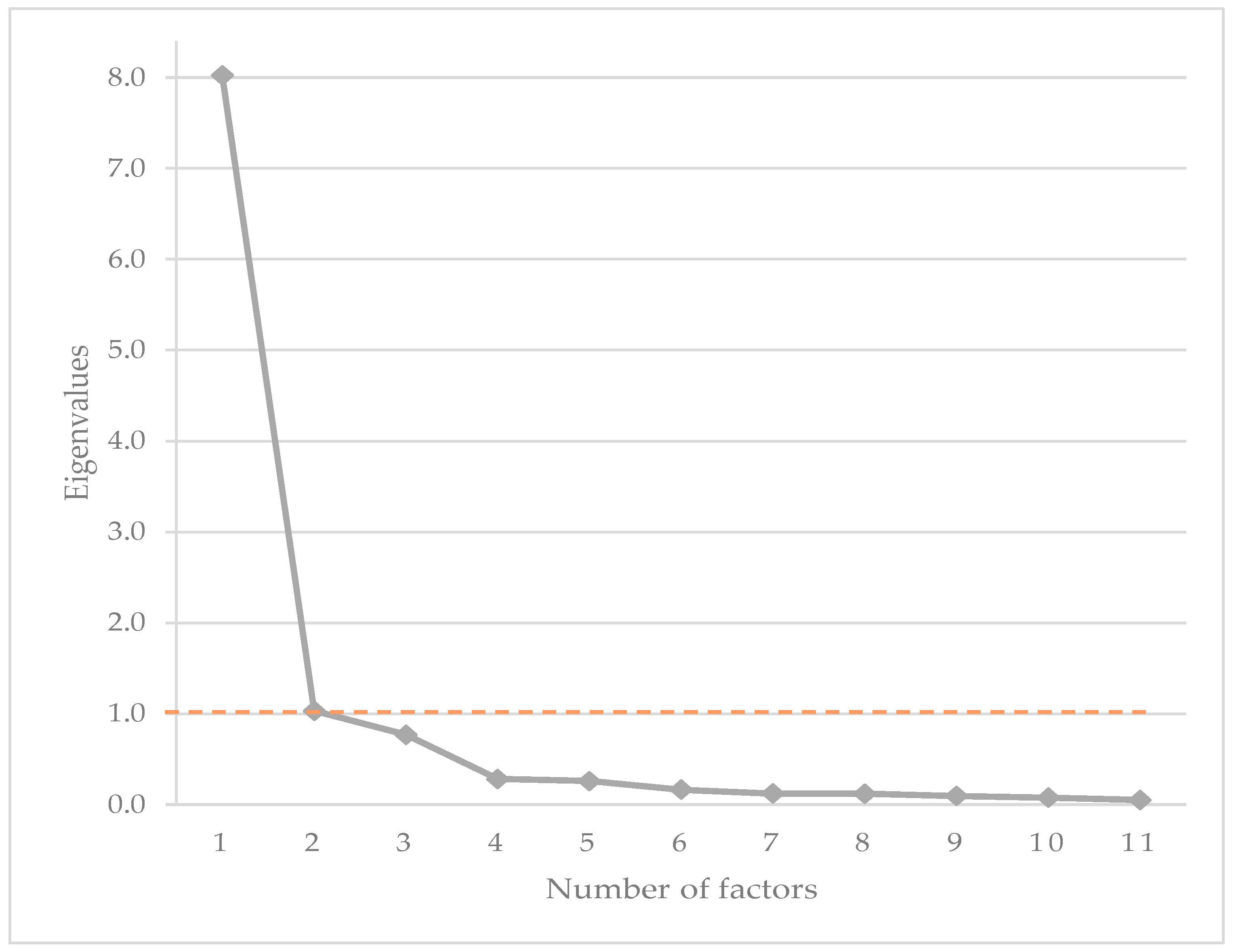
3.2. Construct Validity
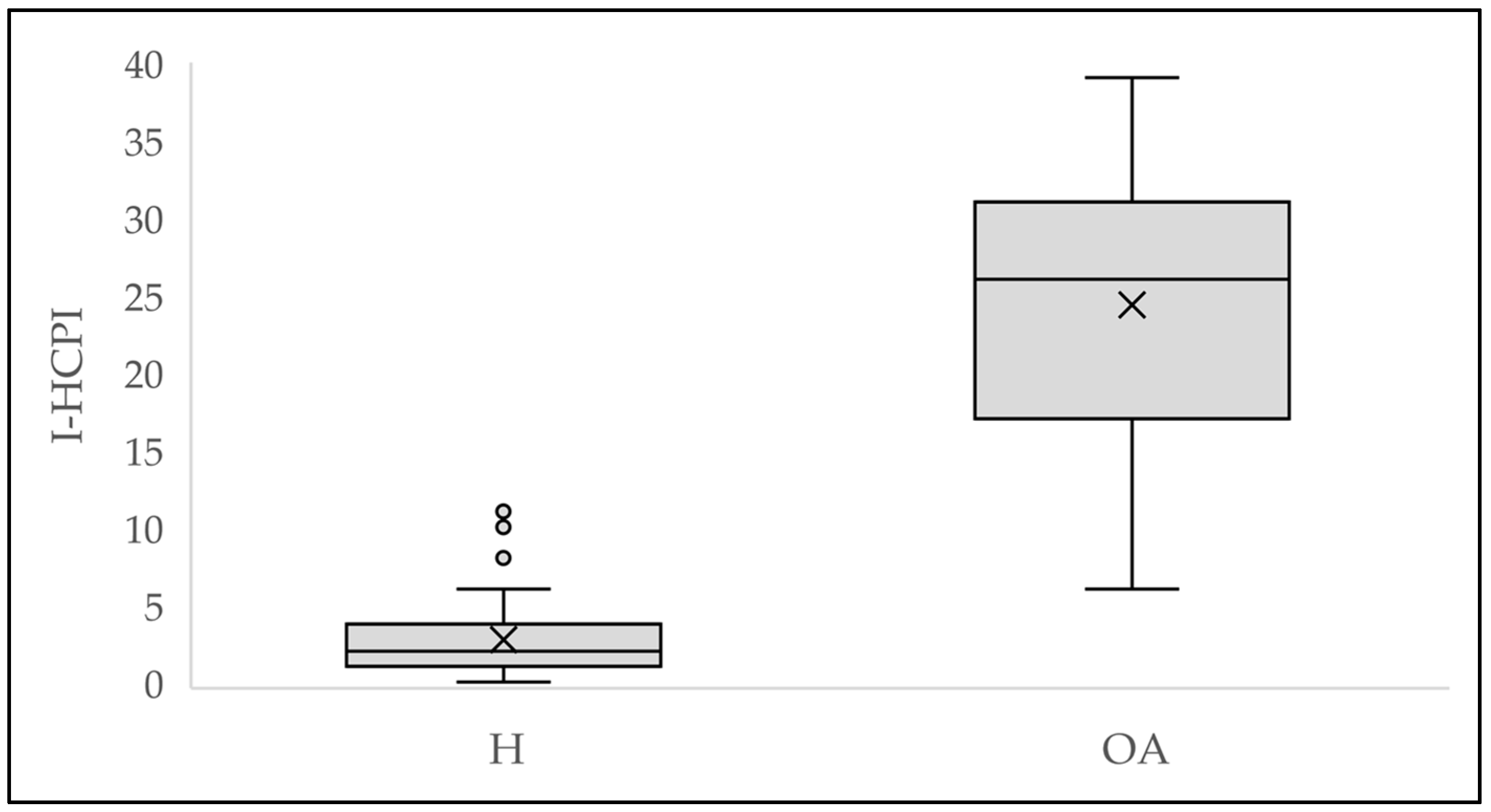
3.3. Convergent Validity
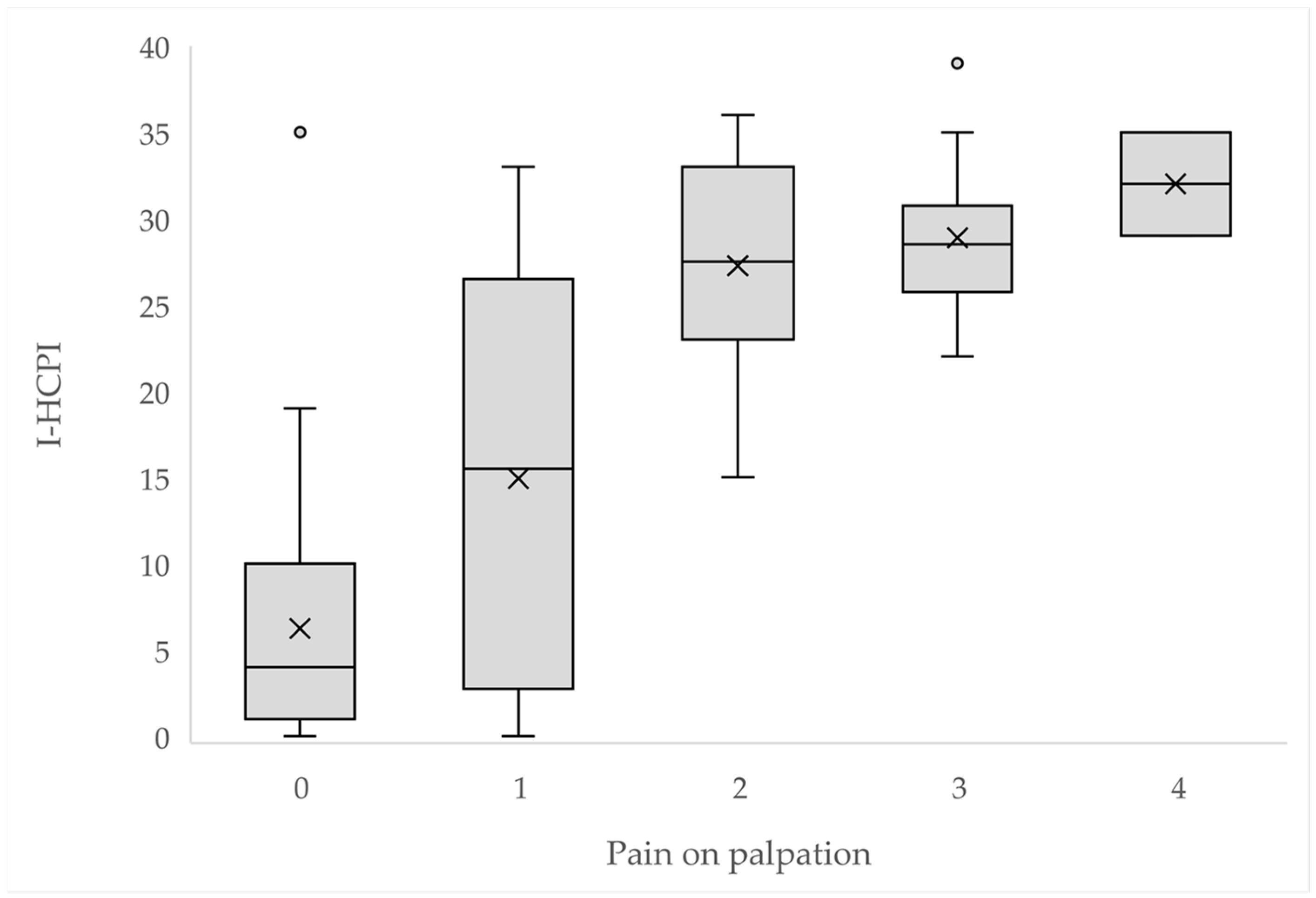
3.4. Criterion Validity
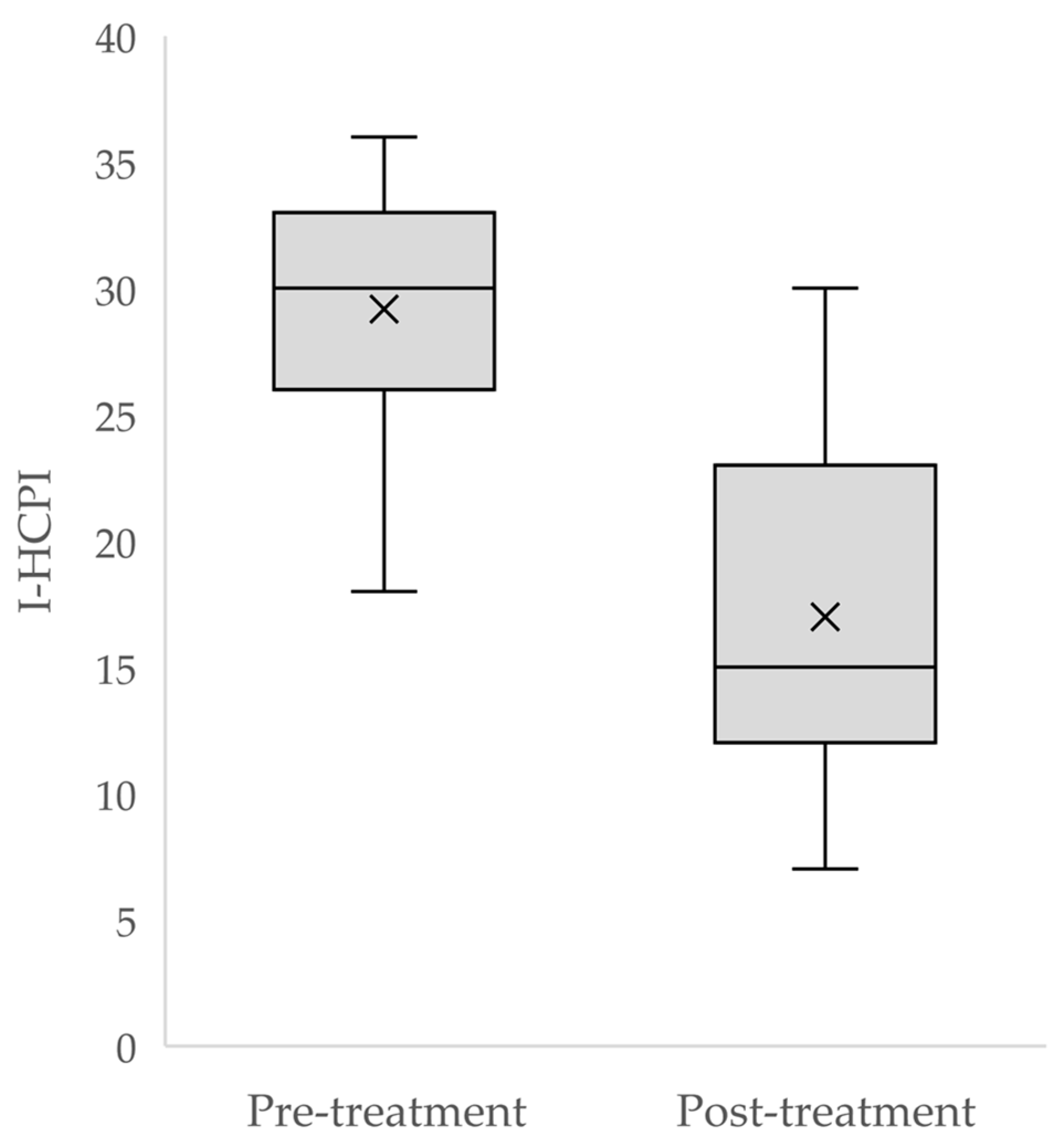
3.5. Responsiveness
3.6. Reliability
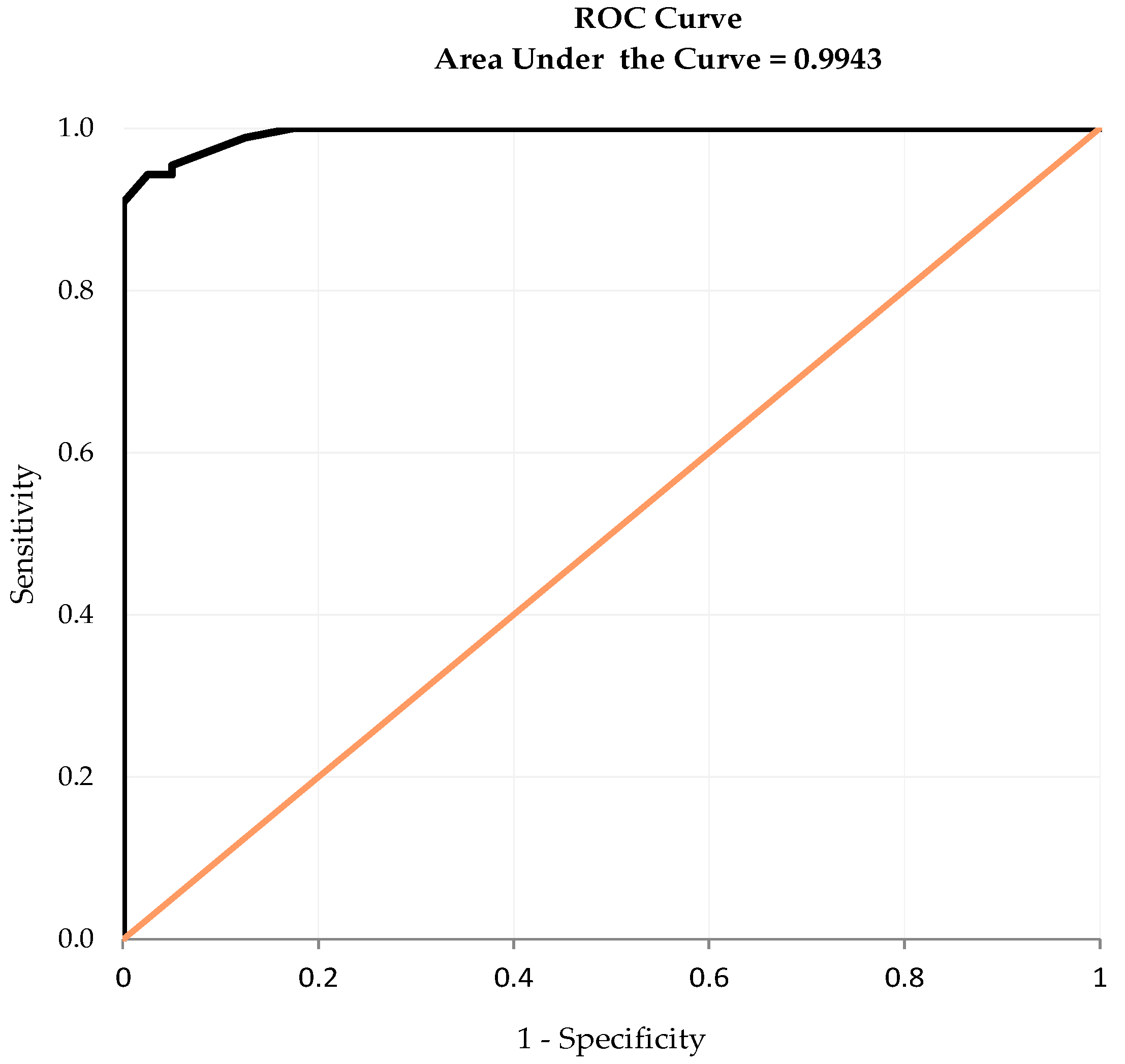
3.7. Diagnostic Accuracy and Cut-Off Point
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharkey, M. The Challenges of Assessing Osteoarthritis and Postoperative Pain in Dogs. AAPS J. 2013, 15, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Vezzoni, A.; Miolo, A. Il dolore ortopedico del cane: Indagine tra i medici veterinari italiani. Veterinaria 2015, 29, 45–53. [Google Scholar]
- Anderson, K.L.; Zulch, H.; O’Neill, D.G.; Meeson, R.L.; Collins, L.M. Risk Factors for Canine Osteoarthritis and Its Predisposing Arthropathies: A Systematic Review. Front. Vet. Sci. 2020, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, M. 2022 ACVS Surgery Summit. Available online: https://www.eventscribe.net/2022/ACVS/fsPopup.asp?Mode=presInfo&PresentationID=1098164 (accessed on 8 November 2023).
- Mills, D.S.; Demontigny-Bédard, I.; Gruen, M.; Klinck, M.P.; McPeake, K.J.; Barcelos, A.M.; Hewison, L.; Van Haevermaet, H.; Denenberg, S.; Hauser, H.; et al. Pain and Problem Behavior in Cats and Dogs. Animals 2020, 10, 318. [Google Scholar] [CrossRef]
- Gruen, M.E.; Samson, D.R.; Lascelles, B.D.X. Functional Linear Modeling of Activity Data Shows Analgesic-Mediated Improved Sleep in Dogs with Spontaneous Osteoarthritis Pain. Sci. Rep. 2019, 9, 14192. [Google Scholar] [CrossRef]
- Sordo, L.; Gunn-Moore, D.A. Cognitive Dysfunction in Cats: Update on Neuropathological and Behavioural Changes Plus Clinical Management. Vet. Rec. 2021, 188, e3. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Reid, J.; Nolan, A.M.; Scott, E.M. Measuring Pain in Dogs and Cats Using Structured Behavioural Observation. Vet. J. 2018, 236, 72–79. [Google Scholar] [CrossRef]
- Streiner, D.L.; Norman, G.R.; Cairney, J. Health Measurement Scales: A Practical Guide to Their Development and Use; Oxford University Press: New York, NY, USA, 2015; ISBN 0-19-968521-5. [Google Scholar]
- Radke, H.; Joeris, A.; Chen, M. Evidence-Based Evaluation of Owner-Reported Outcome Measures for Canine Orthopedic Care—A COSMIN Evaluation of 6 Instruments. Vet. Surg. 2022, 51, 244–253. [Google Scholar] [CrossRef]
- Hielm-Björkman, A.K.; Kuusela, E.; Liman, A.; Markkola, A.; Saarto, E.; Huttunen, P.; Leppäluoto, J.; Tulamo, R.-M.; Raekallio, M. Evaluation of Methods for Assessment of Pain Associated with Chronic Osteoarthritis in Dogs. J. Am. Vet. Med. Assoc. 2003, 222, 1552–1558. [Google Scholar] [CrossRef]
- Hielm-Björkman, A.K.; Rita, H.; Tulamo, R.-M. Psychometric Testing of the Helsinki Chronic Pain Index by Completion of a Questionnaire in Finnish by Owners of Dogs with Chronic Signs of Pain Caused by Osteoarthritis. Am. J. Vet. Res. 2009, 70, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Mölsä, S.H.; Hielm-Björkman, A.K.; Laitinen-Vapaavuori, O.M. Use of an Owner Questionnaire to Evaluate Long-Term Surgical Outcome and Chronic Pain after Cranial Cruciate Ligament Repair in Dogs: 253 Cases (2004–2006). J. Am. Vet. Med. Assoc. 2013, 243, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Essner, A.; Högberg, H.; Zetterberg, L.; Hellström, K.; Sjöström, R.; Gustås, P. Investigating the Probability of Response Bias in Owner-Perceived Pain Assessment in Dogs with Osteoarthritis. Top. Companion Anim. Med. 2020, 39, 100407. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Amodie, D.M.; Cernicchiaro, N.; Lascelles, B.D.X.; Pavlock, A.M.; Roberts, C.; Bartram, D.J. Identification of Canine Osteoarthritis Using an Owner-Reported Questionnaire and Treatment Monitoring Using Functional Mobility Tests. J. Small Anim. Pract. 2022, 63, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Wernham, B.G.J.; Trumpatori, B.; Hash, J.; Lipsett, J.; Davidson, G.; Wackerow, P.; Thomson, A.; Lascelles, B.D.X. Dose Reduction of Meloxicam in Dogs with Osteoarthritis-Associated Pain and Impaired Mobility. J. Vet. Intern. Med. 2011, 25, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.V.; Liska, W.D.; Hyytiäinen, H.K.; Hielm-Björkman, A. Canine Total Knee Replacement Performed Due to Osteoarthritis Subsequent to Distal Femur Fracture Osteosynthesis: Two-Year Objective Outcome. Vet. Comp. Orthop. Traumatol. 2012, 25, 427–432. [Google Scholar] [CrossRef]
- Carapeba, G.O.L.; Cavaleti, P.; Nicácio, G.M.; Brinholi, R.B.; Giuffrida, R.; Cassu, R.N. Intra-Articular Hyaluronic Acid Compared to Traditional Conservative Treatment in Dogs with Osteoarthritis Associated with Hip Dysplasia. Evid.-Based Complement. Altern. Med. 2016, 2016, 2076921. [Google Scholar] [CrossRef]
- Canapp, S.O.; Leasure, C.S.; Cox, C.; Ibrahim, V.; Carr, B.J. Partial Cranial Cruciate Ligament Tears Treated with Stem Cell and Platelet-Rich Plasma Combination Therapy in 36 Dogs: A Retrospective Study. Front. Vet. Sci. 2016, 3, 112. [Google Scholar] [CrossRef]
- Teixeira, L.R.; Luna, S.P.L.; Matsubara, L.M.; Cápua, M.L.B.; Santos, B.P.C.R.; Mesquita, L.R.; Faria, L.G.; Agostinho, F.S.; Hielm-Björkman, A. Owner Assessment of Chronic Pain Intensity and Results of Gait Analysis of Dogs with Hip Dysplasia Treated with Acupuncture. J. Am. Vet. Med. Assoc. 2016, 249, 1031–1039. [Google Scholar] [CrossRef]
- Silva, N.E.O.F.; Luna, S.P.L.; Joaquim, J.G.F.; Coutinho, H.D.; Possebon, F.S. Effect of Acupuncture on Pain and Quality of Life in Canine Neurological and Musculoskeletal Diseases. Can. Vet. J. 2017, 58, 941–951. [Google Scholar]
- Beths, T.; Munn, R.; Bauquier, S.H.; Mitchell, P.; Whittem, T. A Pilot Study of 4CYTETM Epiitalis® Forte, a Novel Nutraceutical, in the Management of Naturally Occurring Osteoarthritis in Dogs. Aust. Vet. J. 2020, 98, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Pownall, W.; Rytz, U.; Schuepbach, G.; Spadavecchia, C.; Rohrbach, H. The Influence of the Choice of Preemptive Analgesia on Long-Term Postsurgical Pain after Tibial Plateau Leveling Osteotomy in Dogs. Vet. Surg. 2021, 50, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Okamoto-Okubo, C.E.; Cassu, R.N.; Joaquim, J.G.F.; Reis Mesquita, L.D.; Rahal, S.C.; Oliveira, H.S.S.; Takahira, R.; Arruda, I.; Maia, L.; da Cruz Landim, F.; et al. Chronic Pain and Gait Analysis in Dogs with Degenerative Hip Joint Disease Treated with Repeated Intra-Articular Injections of Platelet-Rich Plasma or Allogeneic Adipose-Derived Stem Cells. J. Vet. Med. Sci. 2021, 83, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Piras, L.A.; Mancusi, D.; Olimpo, M.; Gastaldi, L.; Rosso, V.; Panero, E.; Staffieri, F.; Peirone, B. Post-Operative Analgesia Following TPLO Surgery: A Comparison between Cimicoxib and Tramadol. Res. Vet. Sci. 2021, 136, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Martello, E.; Bigliati, M.; Adami, R.; Biasibetti, E.; Dosio, F.; Pastorino, D.; Bruni, N. Evaluation of the Efficacy of a Dietary Supplement in Alleviating Symptoms in Dogs with Osteoarthritis. J. Food Nutr. 2018, 4, 1–8. [Google Scholar]
- Martello, E.; Biasibetti, E.; Bigliati, M.; Meineri, G.; Bruni, N. Preliminary Results on the Efficacy of a Dietary Supplement Combined with Physiotherapy in Dogs with Osteoarthritis on Biomarkers of Oxidative Stress and Inflammation. Ital. J. Anim. Sci. 2021, 20, 2131–2133. [Google Scholar] [CrossRef]
- Martello, E.; Bigliati, M.; Adami, R.; Biasibetti, E.; Bisanzio, D.; Meineri, G.; Bruni, N. Efficacy of a Dietary Supplement in Dogs with Osteoarthritis: A Randomized Placebo-Controlled, Double-Blind Clinical Trial. PLoS ONE 2022, 17, e0263971. [Google Scholar] [CrossRef]
- Corbee, R.J. The Efficacy of a Nutritional Supplement Containing Green-Lipped Mussel, Curcumin and Blackcurrant Leaf Extract in Dogs and Cats with Osteoarthritis. Vet. Med. Sci. 2022, 8, 1025–1035. [Google Scholar] [CrossRef]
- Della Rocca, G.; Schievano, C.; Di Salvo, A.; Conti, M.B.; Della Valle, M.F. Palmitoyl-Glucosamine Co-Micronized with Curcumin for Maintenance of Meloxicam-Induced Pain Relief in Dogs with Osteoarthritis Pain. BMC Vet. Res. 2023, 19, 37. [Google Scholar] [CrossRef]
- Zeira, O.; Scaccia, S.; Pettinari, L.; Ghezzi, E.; Asiag, N.; Martinelli, L.; Zahirpour, D.; Dumas, M.P.; Konar, M.; Lupi, D.M.; et al. Intra-Articular Administration of Autologous Micro-Fragmented Adipose Tissue in Dogs with Spontaneous Osteoarthritis: Safety, Feasibility, and Clinical Outcomes. Stem Cells Transl. Med. 2018, 7, 819–828. [Google Scholar] [CrossRef]
- Huggard, R.; Wicks, G.; Corfield, G. Short-Term Clinical Assessment of Hip Hemi-Arthroplasty in 11 Dogs. Vet. Comp. Orthop. Traumatol. 2022, 35, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kwananocha, I.; Magré, J.; Willemsen, K.; Weinans, H.; Sakkers, R.J.B.; How, T.; Verseijden, F.; Tryfonidou, M.A.; van der Wal, B.C.H.; Meij, B.P. Acetabular Rim Extension Using a Personalized Titanium Implant for Treatment of Hip Dysplasia in Dogs: Short-Term Results. Front. Vet. Sci. 2023, 10, 1160177. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Cho, J.-H.; Kim, C.-H.; Lee, D. Effect of Rehabilitation in a Dog with Delayed Recovery Following TPLO: A Case Report. Animals 2023, 13, 2778. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.B.; Cowderoy, E.; Lascelles, D.; Innes, J.F. Evaluation of Construct and Criterion Validity for the “Liverpool Osteoarthritis in Dogs” (LOAD) Clinical Metrology Instrument and Comparison to Two Other Instruments. PLoS ONE 2013, 8, e58125. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, F.; Bombardier, C.; Beaton, D. Cross-Cultural Adaptation of Health-Related Quality of Life Measures: Literature Review and Proposed Guidelines. J. Clin. Epidemiol. 1993, 46, 1417–1432. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.D.; Rojjanasrirat, W. Translation, Adaptation and Validation of Instruments or Scales for Use in Cross-Cultural Health Care Research: A Clear and User-Friendly Guideline. J. Eval. Clin. Pract. 2011, 17, 268–274. [Google Scholar] [CrossRef]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the Process of Cross-Cultural Adaptation of Self-Report Measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef]
- Sperber, A.D. Translation and Validation of Study Instruments for Cross-Cultural Research. Gastroenterology 2004, 126, S124–S128. [Google Scholar] [CrossRef]
- Matsubara, L.M.; Luna, S.P.L.; Teixeira, L.R.; Castilho, M.S.; Björkman, A.H.; Oliveira, H.S.; Anunciação, L.F.C. Avaliação Psicométrica Em Português Do Indicador de Dor Crônica de Helsinki Em Cães Com Sinais Crônicos de Osteoartrite. Arq. Bras. Med. Veterinária Zootec. 2019, 71, 109–118. [Google Scholar] [CrossRef]
- Costello, A.; Osborne, J. Best Practices in Exploratory Factor Analysis: Four Recommendations for Getting the Most from Your Analysis. Pract. Assess. Res. Eval. 2005, 10, 1–9. [Google Scholar]
- Della Rocca, G.; Di Salvo, A.; Medori, C.; Della Valle, M.F.; Cimino Brown, D. Initial Psychometric Testing and Validation of the Italian Version of the Canine Brief Pain Inventory in Dogs with Pain Related to Osteoarthritis. Front. Vet. Sci. 2021, 8, 736458. [Google Scholar] [CrossRef] [PubMed]
- Harman, R.; Carlson, K.; Gaynor, J.; Gustafson, S.; Dhupa, S.; Clement, K.; Hoelzler, M.; McCarthy, T.; Schwartz, P.; Adams, C. A Prospective, Randomized, Masked, and Placebo-Controlled Efficacy Study of Intraarticular Allogeneic Adipose Stem Cells for the Treatment of Osteoarthritis in Dogs. Front. Vet. Sci. 2016, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Crellin, D.; Sullivan, T.P.; Babl, F.E.; O’Sullivan, R.; Hutchinson, A. Analysis of the Validation of Existing Behavioral Pain and Distress Scales for Use in the Procedural Setting. Pediatr. Anesth. 2007, 17, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.A.; Beckman, T.J. Current Concepts in Validity and Reliability for Psychometric Instruments: Theory and Application. Am. J. Med. 2006, 119, 166.e7–166.e16. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.F. The Application of Electronic Computers to Factor Analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 7th ed.; Pearson: Boston, MA, USA, 2019; ISBN 0-13-479054-5. [Google Scholar]
- Iantovics, L.B.; Rotar, C.; Morar, F. Survey on Establishing the Optimal Number of Factors in Exploratory Factor Analysis Applied to Data Mining. WIREs Data Min. Knowl. Discov. 2019, 9, e1294. [Google Scholar] [CrossRef]
- Krabbe, P. The Measurement of Health and Health Status: Concepts, Methods and Applications from A Multidisciplinary Perspective; Academic Press: Cambridge, MA, USA, 2016; ISBN 0-12-801720-1. [Google Scholar]
- Taylor, R. Interpretation of the Correlation Coefficient: A Basic Review. J. Diagn. Med. Sonogr. 1990, 6, 35–39. [Google Scholar] [CrossRef]
- Sousa, F.; Da Silva, J.A. A Métrica Da Dor (Dormetria): Problemas Teóricos e Metodológicos. Rev. Dor. 2005, 6, 469–513. [Google Scholar]
- Von Baeyer, C.L.; Spagrud, L.J. Systematic Review of Observational (Behavioral) Measures of Pain for Children and Adolescents Aged 3 to 18 Years. Pain 2007, 127, 140–150. [Google Scholar] [CrossRef]
- Norman, G.R.; Wyrwich, K.W.; Patrick, D.L. The Mathematical Relationship among Different Forms of Responsiveness Coefficients. Qual. Life Res. 2007, 16, 815–822. [Google Scholar] [CrossRef]
- Streiner, D.L.; Cairney, J. What’s under the ROC? An Introduction to Receiver Operating Characteristics Curves. Can. J. Psychiatry 2007, 52, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Diehr, P.; Patrick, D.L. Reproducibility and Responsiveness of Health Status Measures. Statistics and Strategies for Evaluation. Control. Clin. Trials 1991, 12, S142–S158. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Mutiso, F.; Tian, L. Joint Hypothesis Testing of the Area under the Receiver Operating Characteristic Curve and the Youden Index. Pharm. Stat. 2021, 20, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Osteoarthritis and Obesity in Pets Go Hand in Hand (Slowly). Available online: https://www.aaha.org/publications/newstat/articles/2019-06/osteoarthritis-and-obesity-in-pets-go-hand-in-hand-slowly/ (accessed on 15 November 2023).

| Age (years) | Body Weight (kg) | |||||
|---|---|---|---|---|---|---|
| OA (N = 87) | Healthy (N = 40) | All (N = 127) | OA (N = 87) | Healthy (N = 40) | All (N = 127) | |
| Min | 0.5 | 1.2 | 0.5 | 8.3 | 8 | 8 |
| Q1 | 6.8 | 1.7 | 4.0 | 22.9 | 12 | 19.8 |
| Median | 9.0 | 4.0 | 8.0 | 32.8 | 20.5 | 28 |
| Q3 | 11.5 | 6.9 | 11.0 | 38.7 | 28 | 37 |
| Max | 16.0 | 14.5 | 16.0 | 60 | 52 | 60 |
| Mean | 9.0 | 4.8 | 7.7 | 31.0 | 21.6 | 28.1 |
| Std | 3.6 | 3.3 | 4.0 | 11.6 | 10.8 | 12.1 |
| OA | Healthy | All | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Fe | 6 | 6.9 | 10 | 25.0 | 16 | 12.6 |
| Fs | 35 | 40.2 | 10 | 25.0 | 45 | 35.4 |
| Me | 35 | 40.2 | 17 | 42.5 | 52 | 40.9 |
| Mn | 11 | 12.6 | 3 | 7.5 | 14 | 11.0 |
| Total | 87 | 100 | 40 | 100 | 127 | 100 |
| OA | Healthy | All | |
|---|---|---|---|
| N | 87 | 40 | 127 |
| Min | 6 | 0 | 0 |
| Q1 | 17 | 1 | 5 |
| Median | 26 | 2 | 18 |
| Q3 | 31 | 3.5 | 28 |
| Max | 39 | 11 | 39 |
| Mean | 24.4 | 2.7 | 17.5 |
| StdErr | 0.88 | 0.47 | 1.09 |
| PCA Loading | |||
|---|---|---|---|
| Item | Description | Factor 1 | Factor 2 |
| I-HCPI01 | Mood | 0.20 | 0.87 |
| I-HCPI02 | Play | 0.28 | 0.83 |
| I-HCPI03 | Vocalization | 0.65 | −0.21 |
| I-HCPI04 | Walking | 0.82 | 0.45 |
| I-HCPI05 | Trotting | 0.83 | 0.45 |
| I-HCPI06 | Galloping | 0.80 | 0.48 |
| I-HCPI07 | Jumping | 0.80 | 0.50 |
| I-HCPI08 | Lying down | 0.83 | 0.37 |
| I-HCPI09 | Getting up | 0.86 | 0.38 |
| I-HCPI10 | Movement after rest | 0.84 | 0.44 |
| I-HCPI11 | Movement after major exercise | 0.82 | 0.46 |
| Item | Description | Mean | SD | PCA Loading |
|---|---|---|---|---|
| I-HCPI | Items totaled | 17.54 | 12.28 | NA |
| I-HCPI01 | Mood | 1.26 | 1.03 | 0.64 |
| I-HCPI02 | Play | 1.14 | 1.04 | 0.68 |
| I-HCPI03 | Vocalization | 0.69 | 0.89 | 0.43 |
| I-HCPI04 | Walking | 1.51 | 1.28 | 0.93 |
| I-HCPI05 | Trotting | 1.86 | 1.51 | 0.95 |
| I-HCPI06 | Galloping | 1.95 | 1.46 | 0.93 |
| I-HCPI07 | Jumping | 2.10 | 1.57 | 0.95 |
| I-HCPI08 | Lying down | 1.39 | 1.24 | 0.89 |
| I-HCPI09 | Getting up | 1.78 | 1.42 | 0.92 |
| I-HCPI10 | Movement after rest | 1.87 | 1.38 | 0.95 |
| I-HCPI11 | Movement after major exercise | 1.99 | 1.41 | 0.94 |
| Item | Description | Communalities | Item-Total Correlations | Cronbach α |
|---|---|---|---|---|
| I-HCPI | Items totaled | NA | NA | 0.96 |
| I-HCPI01 | Mood | 0.80 | 0.64 | 0.96 |
| I-HCPI02 | Play | 0.77 | 0.68 | 0.96 |
| I-HCPI03 | Vocalization | 0.47 | 0.45 | 0.97 |
| I-HCPI04 | Walking | 0.87 | 0.93 | 0.96 |
| I-HCPI05 | Trotting | 0.90 | 0.95 | 0.95 |
| I-HCPI06 | Galloping | 0.87 | 0.93 | 0.96 |
| I-HCPI07 | Jumping | 0.90 | 0.95 | 0.95 |
| I-HCPI08 | Lying down | 0.82 | 0.89 | 0.96 |
| I-HCPI09 | Getting up | 0.88 | 0.92 | 0.96 |
| I-HCPI10 | Movement after rest | 0.90 | 0.95 | 0.95 |
| I-HCPI11 | Movement after major exercise | 0.88 | 0.94 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
della Rocca, G.; Schievano, C.; Di Salvo, A.; Hielm-Björkman, A.K.; della Valle, M.F. Psychometric Testing and Validation of the Italian Version of the Helsinki Chronic Pain Index (I-HCPI) in Dogs with Pain Related to Osteoarthritis. Animals 2024, 14, 83. https://doi.org/10.3390/ani14010083
della Rocca G, Schievano C, Di Salvo A, Hielm-Björkman AK, della Valle MF. Psychometric Testing and Validation of the Italian Version of the Helsinki Chronic Pain Index (I-HCPI) in Dogs with Pain Related to Osteoarthritis. Animals. 2024; 14(1):83. https://doi.org/10.3390/ani14010083
Chicago/Turabian Styledella Rocca, Giorgia, Carlo Schievano, Alessandra Di Salvo, Anna K. Hielm-Björkman, and Maria Federica della Valle. 2024. "Psychometric Testing and Validation of the Italian Version of the Helsinki Chronic Pain Index (I-HCPI) in Dogs with Pain Related to Osteoarthritis" Animals 14, no. 1: 83. https://doi.org/10.3390/ani14010083
APA Styledella Rocca, G., Schievano, C., Di Salvo, A., Hielm-Björkman, A. K., & della Valle, M. F. (2024). Psychometric Testing and Validation of the Italian Version of the Helsinki Chronic Pain Index (I-HCPI) in Dogs with Pain Related to Osteoarthritis. Animals, 14(1), 83. https://doi.org/10.3390/ani14010083









