Transversus Abdominis Plane (TAP) Block in Rabbit Cadavers: Anatomical Description and Measurements of Injectate Spread Using One- and Two-Point Approaches
Abstract
Simple Summary
Abstract
1. Introduction
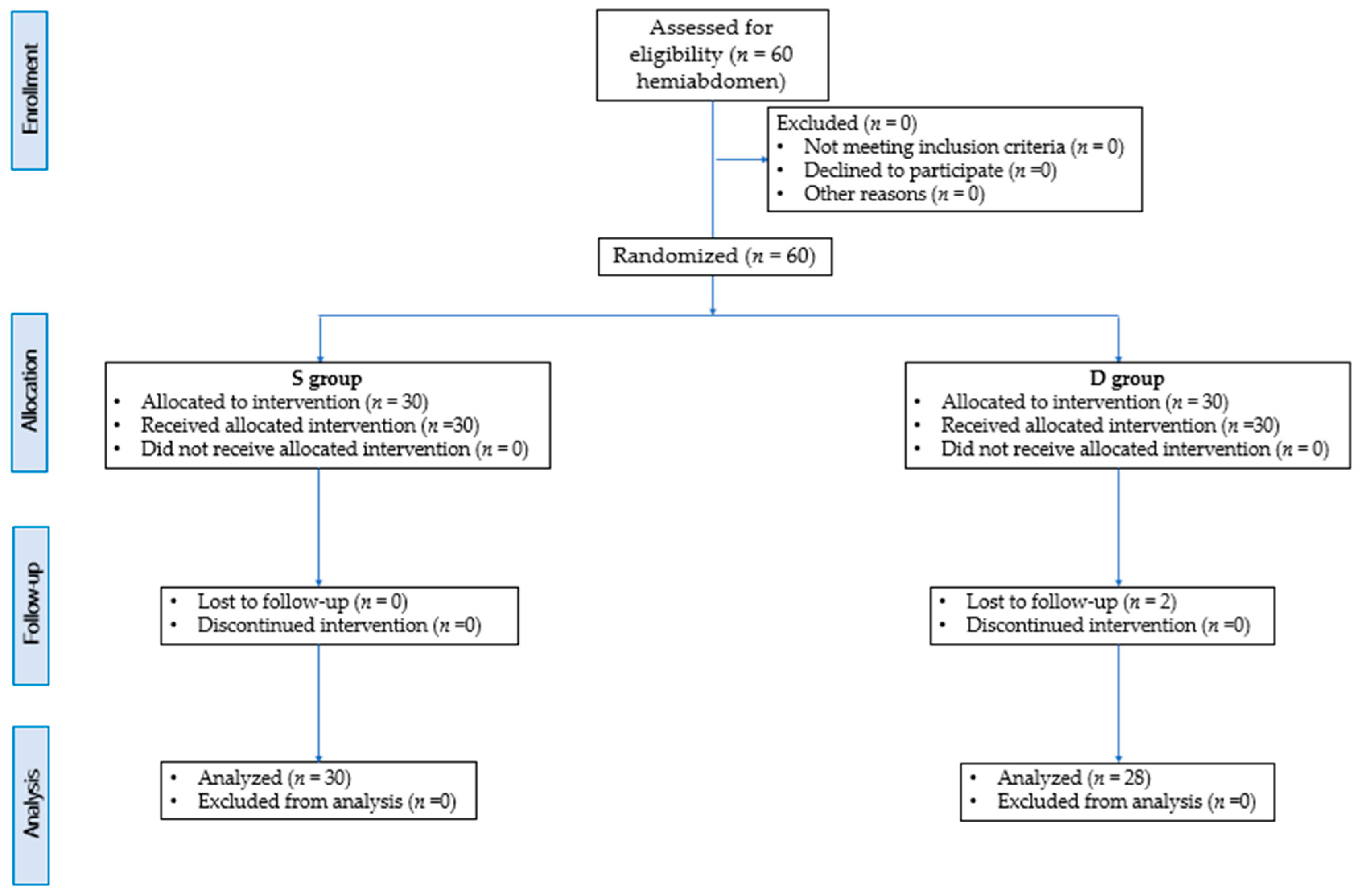
2. Materials and Methods
- S group (15 rabbits; 30 hemiabdomen) in which the one-point (preiliac) approach was carried out;
- D group = (15 rabbits; 30 hemiabdomen) in which the two-point approach was carried out (preiliac and retrocostal).
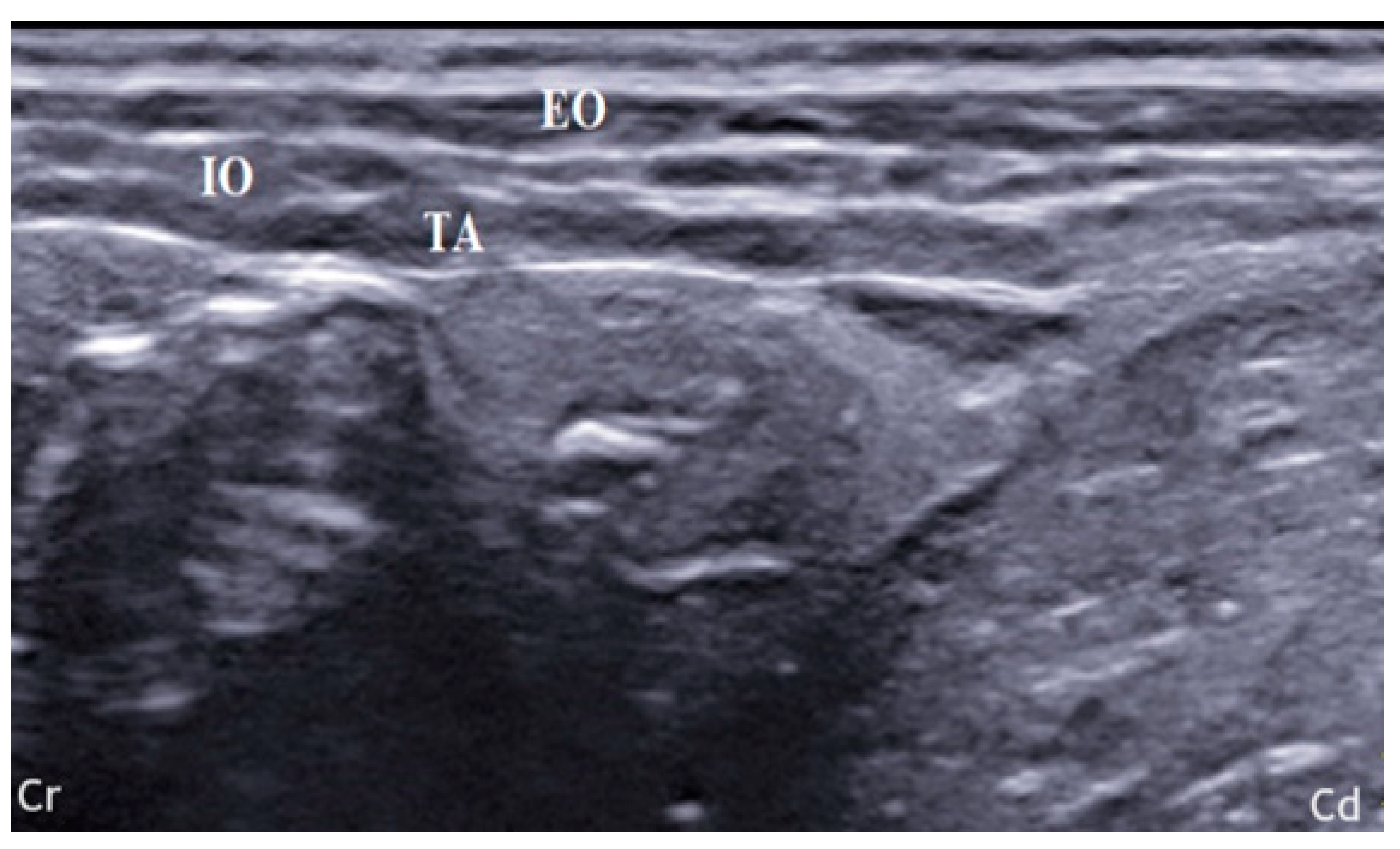
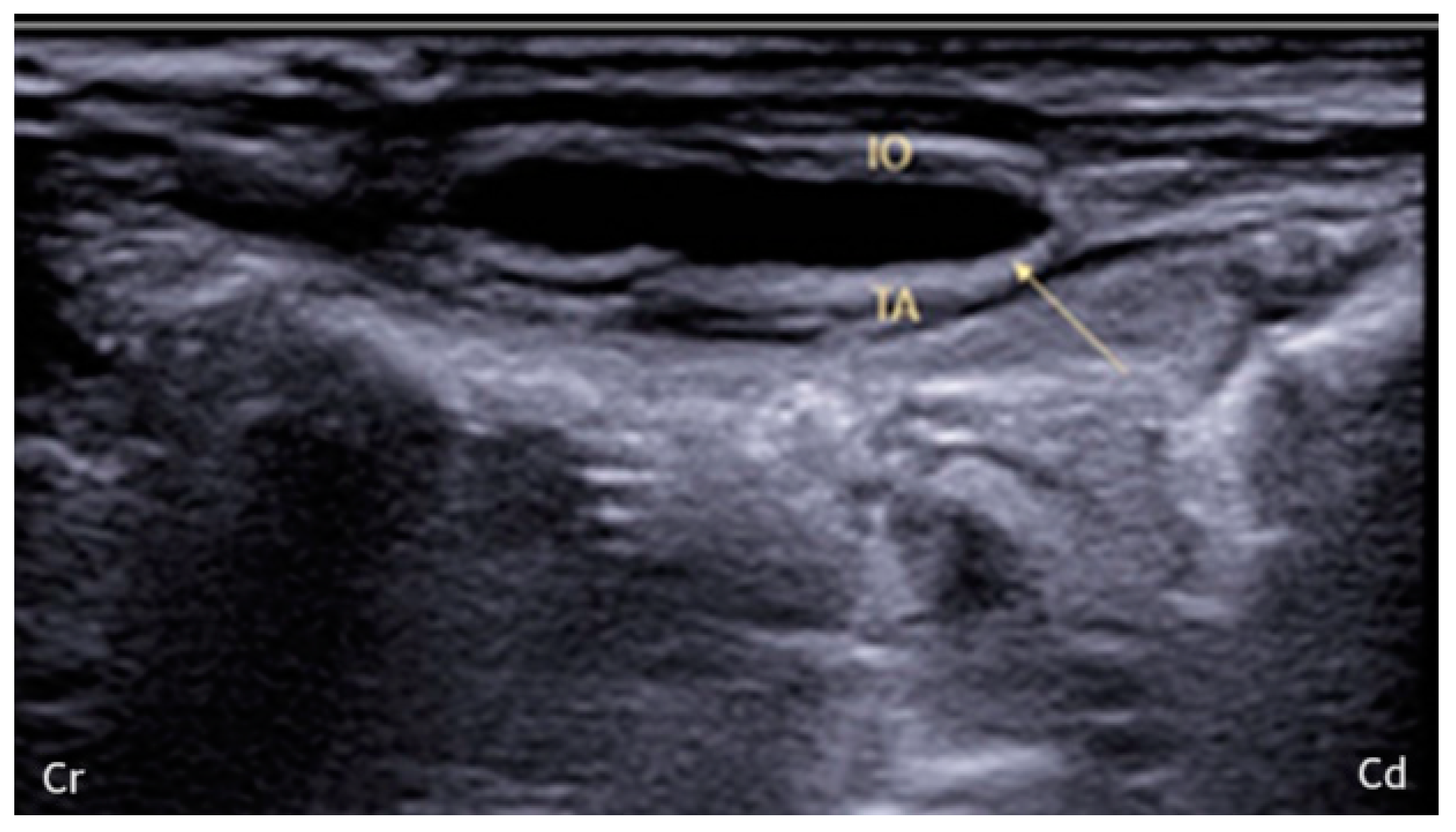
2.1. US-Guided TAP Injection
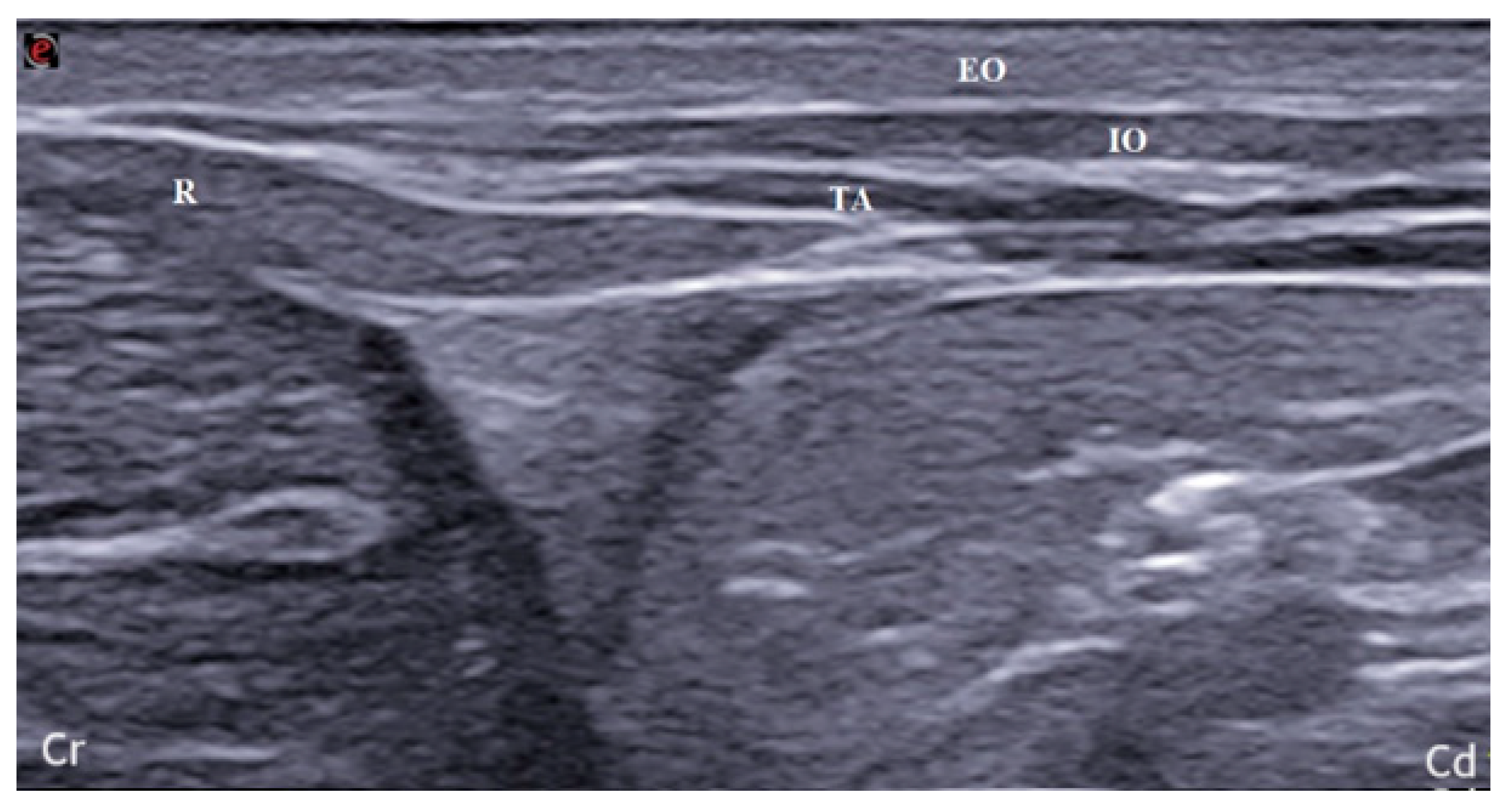
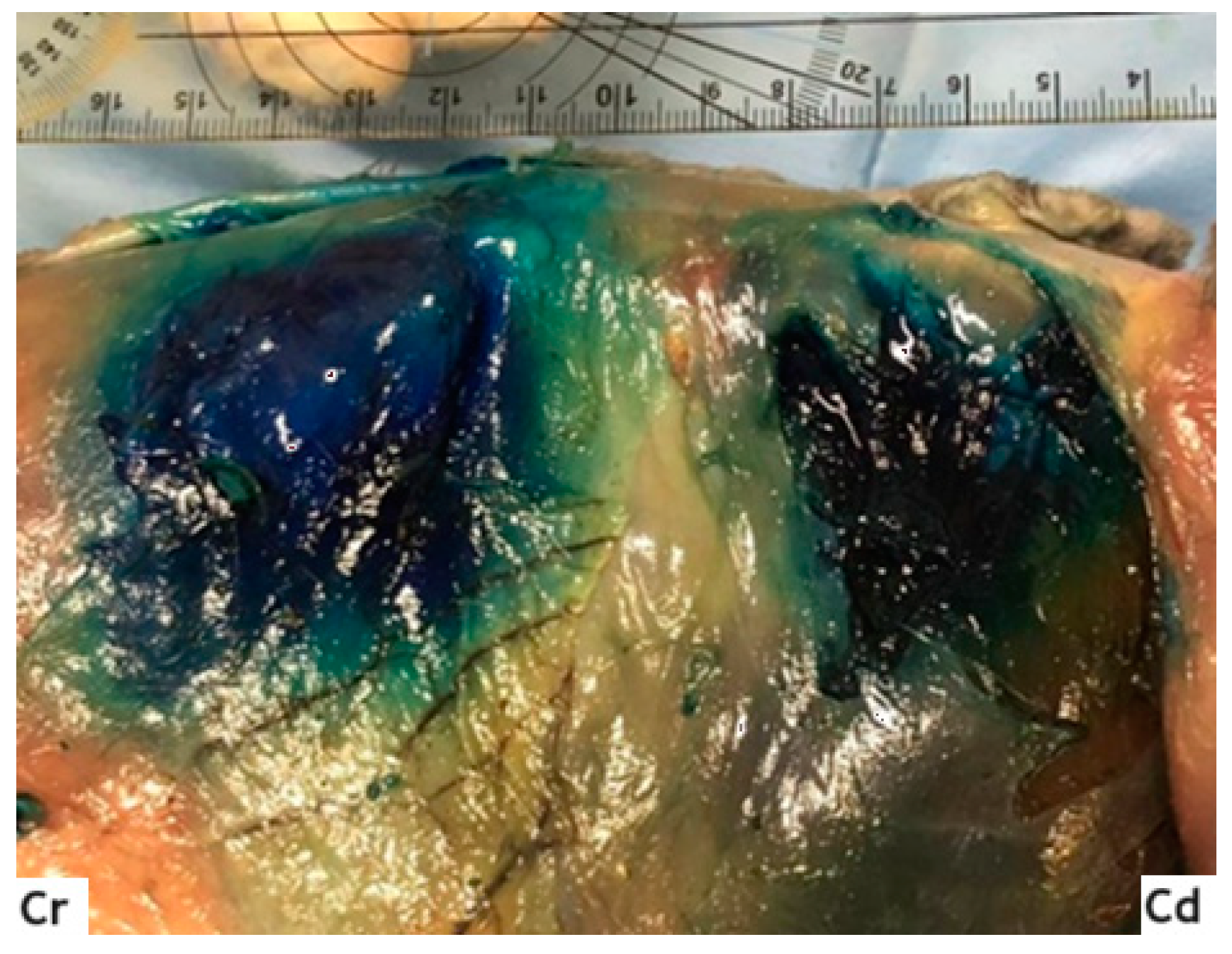
2.2. Anatomical Dissection and Misurements
2.3. Statistical Analysis
3. Results
3.1. US-Guided TAP Injection
3.2. Anatomical Dissection and Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brodbelt, D.; Blissit, K.J.; Hammond, R.A.; Neath, P.J.; Young, L.E.; Pfeiffer, D.U.; Wood, J.L.N. The risk of death: The Confidential Enquiry into Perioperative Small Animal Fatalities. Vet. Anaest. Anal. 2008, 35, 365–373. [Google Scholar] [CrossRef]
- Bersanetti, G.; Martorelli, M. Anesthesia in rabbits. Veterinaria 2021, 35, 221–230. [Google Scholar]
- Lee, H.W.; Machin, H.; Adami, C. Peri-anaesthetic mortality and non-fatal gastrointestinal complications in pet rabbits: A retrospective study on 210 cases. Vet. Anaest. Anal. 2018, 45, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Benato, L.; Rooney, N.J.; Murrell, J.C. Pain and analgesia in pet rabbits within the veterinary environment: A review. Vet. Anaesth. Analg. 2019, 46, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.N.; Pavone, K.J.; Naranjo, M. Multimodal general anesthesia: Theory and practice. Anesth. Analg. 2018, 127, 1246–1258. [Google Scholar] [CrossRef]
- Portela, D.A.; Romano, M.; Briganti, A. Retrospective clinical evaluation of ultrasound guided transverse abdominis plane block in dogs undergoing mastectomy. Vet. Anaesth. Analg. 2014, 41, 319–324. [Google Scholar] [CrossRef]
- Cicirelli, V.; Burgio, M.; Lacalandra, G.; Aiudi, G. Local and Regional Anesthetic Technique in Canine Ovariectomy: A Review of the Literature and Technique Description. Animals 2022, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- Küls, N.; Trujanovic, R.; Otero, P.E.; Larenza-Menzies, M.P. Ultrasound-Guided Transversus Abdominis Plane Block in Shetland Ponies: A Description of a Three-Point Injection Technique and Evaluation of Potential Analgesic Effects. J. Equine Vet. Sci. 2020, 90, 102994. [Google Scholar] [CrossRef]
- Skouropoulou, D.; Lacitignola, L.; Centonze, P.; Simone, A.; Crovace, A.M.; Staffieri, F. Perioperative analgesic effects of an ultrasound-guided transversus abdominis plane block with a mixture of bupivacaine and lidocaine in cats undergoing ovariectomy. Vet. Anaesth. Analg. 2018, 45, 374–383. [Google Scholar] [CrossRef]
- Schroeder, C.A.; Schroeder, K.M.; Johnson, R.A. Transversus abdominis plane block for exploratory laparotomy in a Canadian lynx (Lynx canadensis). JZWM 2010, 41, 338–341. [Google Scholar] [CrossRef]
- Freitag, F.V.; Muehlbauera, E.; Das Gaio, T.; Dos Santosa, A.; Machadoc, M.; Sanchezb, A.; Duque, C.J. Evaluation of injection volumes for the transversus abdominis plane block in dog cadavers: A preliminary trial. Vet. Anaesth. Analg. 2021, 48, 142–146. [Google Scholar] [CrossRef]
- Lee, T.H.W.; Barrington, M.J.; Tran, T.M.N.; Wong, D.; Hebbard, P.D. Comparison of extent of sensory block following posterior and subcostal approaches to ultrasound-guided transversus abdominis plane block. Anaesth. Intensive Care 2010, 38, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Portela, D.A.; Thomson, A.; Otero, P.E. Comparison between two approaches for the transversus abdominis plane block in canine cadavers. Vet. Anaesth. Analg. 2020, 48, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.E.; Romano, M.; Zaccagnini, A.S.; Fuensalida, S.E.; Verdier, N.; Sanchez, F.; Portela, D.A. Transversus abdominis plane block in cat cadavers: Anatomical description and comparison of injectate spread using two-and three-point approaches. Vet. Anaesth. Analg. 2021, 48, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Mirra, A.; Von Rotz, A.; Schmidhalter, M.; Moser, L.; Casoni, D.; Spadavecchia, C. Ultrasound-guided lateral and subcostal transversus abdominis plane block in calves: A cadaveric study. Vet. Anaesth. Anal. 2018, 45, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, C.; Pennasilico, L.; Staffieri, F.; Serino, F.; Palumbo Piccionello, A. Ultrasound-Guided Lateral Transversus Abdominis Plane (TAP) Block in Rabbits: A Cadaveric Study. Animals 2021, 11, 1953. [Google Scholar] [CrossRef] [PubMed]
- Bruggink, S.M.; Schroeder, K.M.; Baker-Herman, T.L.; Schroeder, C.A. Weight-based volume of injection influences cranial to caudal spread of local anesthetic solution in ultrasound-guided transversus abdominis plane blocks in canine cadavers. Vet. Surg. 2012, 41, 455–457. [Google Scholar] [CrossRef]
- IToh, T.; Kawabe, M.; Nagase, T.; KoIKe, T.; MIyoshI, M.; MIyahara, K. Measurements of body surface area and volume in laboratory rabbits (New Zealand White rabbits) using a computed tomography scanner. Exp. Anim. 2018, 67, 527–534. [Google Scholar] [CrossRef]
- Raymond, S.A.; Steffensen, S.C.; Gugino, L.D.; Strichartz, G.R. The role of length of nerve exposed to local anesthetics in impulse blocking action. Anesth. Analg. 1989, 68, 563–570. [Google Scholar] [CrossRef]
- Aragón-Sánchez, J.; Quintana-Marrero, Y.; Aragón-Hernández, C.; Hernández-Herero, M.J. ImageJ: A Free, Easy, and Reliable Method to Measure Leg Ulcers Using Digital Pictures. Int. J. Low Extrem. Wounds 2017, 16, 269–273. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gotzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, K.; Xia, Y.; Zhang, X.; Papadimos, T.J.; Xu, X.; Wang, Q. Sensory assessment and regression rate of bilateral oblique subcostal transversus abdominis plane block in volunteers. RAMP J. 2018, 43, 174–179. [Google Scholar] [CrossRef]
- Nicoletti, A.; Di Girolamo, N.; Zeyen, U.; Selleri, P.; Masi, M.; Fonti, P. Ultrasound morphology of cecal appendix in pet rabbits. J. Ultrasound 2018, 21, 287–291. [Google Scholar] [CrossRef]
- Tran, D.Q.; Bravo, D.; Leurcharusmee, P.; Neal, J.M. Transversus abdominis plane block: A narrative review. Anesthesia 2019, 131, 1166–1190. [Google Scholar] [CrossRef] [PubMed]
- Drozdzynska, M.; Monticelli, P.; Neilson, D.; Viscasillas, J. Ultrasound-guided subcostal oblique transversus abdominis plane block in canine cadavers. Vet. Anaesth. Analg. 2017, 44, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Zoff, A.; Laborda-Vidal, P.; Mortier, J.; Amengual, M.; Rioja, E. Comparison of the spread of two different volumes of contrast medium when performing ultrasound-guided transversus abdominis plane injection in dog cadavers. J. Small Anim. Pract. 2017, 58, 269–275. [Google Scholar] [CrossRef]
- Barrington, M.J.; Ivanusic, J.J.; Rozen, W.M.; Hebbard, P. Spread of injectate after ultrasound-guided subcostal transversus abdominis plane block: A cadaveric study. Anaesthesia 2009, 64, 745–750. [Google Scholar] [CrossRef]
- Johnson, E.K.; Bauquier, S.H.; Carter, J.E.; Whittem, T.; Beths, T. Two-point ultrasound-guided transversus abdominis plane injection in canine cadavers—A pilot study. Vet. Anaesth. Analg. 2018, 45, 871–875. [Google Scholar] [CrossRef]
- Baldo, C.F.; Almeida, D.; Wendt-Hornickle, E.; Guedes, A. Transversus abdominis plane block in ponies: A preliminary anatomical study. Vet. Anaesth. Analg. 2018, 45, 392–396. [Google Scholar] [CrossRef]
- Schroeder, C.A.; Snyder, L.B.; Tearney, C.C.; Baker-Herman, T.L.; Schroeder, K.M. Ultrasound-guided transversus abdominis plane block in the dog: An anatomical evaluation. Vet. Anaesth. Analg. 2011, 38, 267–271. [Google Scholar] [CrossRef] [PubMed]
- İpek, C.B.; Kara, D.; Yılmaz, S.; Yeşiltaş, S.; Esen, A.; Dooply, S.L.; Karaaslan, K.; Türköz, A. Comparison of ultrasound-guided transversus abdominis plane block, quadratus lumborum block, and caudal epidural block for perioperative analgesia in pediatric lower abdominal surgery. Turk. J. Med. Sci. 2019, 49, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Baeriswyl, M.; Kirkham, K.R.; Kern, C.; Albrecht, E. The Analgesic Efficacy of Ultrasound-Guided Transversus Abdominis Plane Block in Adult Patients: A Meta-Analysis. Anaest. Analg. 2015, 121, 1640–1654. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhang, J.J.; Xi, F.C.; Shi, J.L.; Lu, Y.; Tan, S.J.; Yu, W.K. Evaluation of Transversus Abdominis Plane (TAP) Block in Hernia Surgery: A Meta-analysis. Clin. J. Pain 2017, 33, 369–375. [Google Scholar] [CrossRef] [PubMed]

| Group | CCspread (mm) | DVspread (mm) | Area Spread (mm2) |
|---|---|---|---|
| S | 48.91 ± 7.24 | 54.98 ± 8.8 | 2265.5 ± 461.17 |
| D | 69.36 ± 10 * | 35.36 ± 7.12 * | 2306.4 ± 656.9 |
| Group | T10 | T11 | T12 | L1 | L2 | L3 | L4 |
|---|---|---|---|---|---|---|---|
| S | - | 52% | 75% | 95% | 100% | 60% | 40% |
| D | 68% * | 100% * | 98% * | 88% | 100% | 62% | 31% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serino, F.; Pennasilico, L.; Galosi, M.; Palumbo Piccionello, A.; Tambella, A.M.; Di Bella, C. Transversus Abdominis Plane (TAP) Block in Rabbit Cadavers: Anatomical Description and Measurements of Injectate Spread Using One- and Two-Point Approaches. Animals 2024, 14, 684. https://doi.org/10.3390/ani14050684
Serino F, Pennasilico L, Galosi M, Palumbo Piccionello A, Tambella AM, Di Bella C. Transversus Abdominis Plane (TAP) Block in Rabbit Cadavers: Anatomical Description and Measurements of Injectate Spread Using One- and Two-Point Approaches. Animals. 2024; 14(5):684. https://doi.org/10.3390/ani14050684
Chicago/Turabian StyleSerino, Federica, Luca Pennasilico, Margherita Galosi, Angela Palumbo Piccionello, Adolfo Maria Tambella, and Caterina Di Bella. 2024. "Transversus Abdominis Plane (TAP) Block in Rabbit Cadavers: Anatomical Description and Measurements of Injectate Spread Using One- and Two-Point Approaches" Animals 14, no. 5: 684. https://doi.org/10.3390/ani14050684
APA StyleSerino, F., Pennasilico, L., Galosi, M., Palumbo Piccionello, A., Tambella, A. M., & Di Bella, C. (2024). Transversus Abdominis Plane (TAP) Block in Rabbit Cadavers: Anatomical Description and Measurements of Injectate Spread Using One- and Two-Point Approaches. Animals, 14(5), 684. https://doi.org/10.3390/ani14050684










