An Inflammatory Myopathy in the Dutch Kooiker Dog
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Measurements
2.3. EMG and Muscle Biopsies
2.4. Muscle Histopathology
2.5. Pedigree Analysis
2.6. Statistical Analysis
3. Results
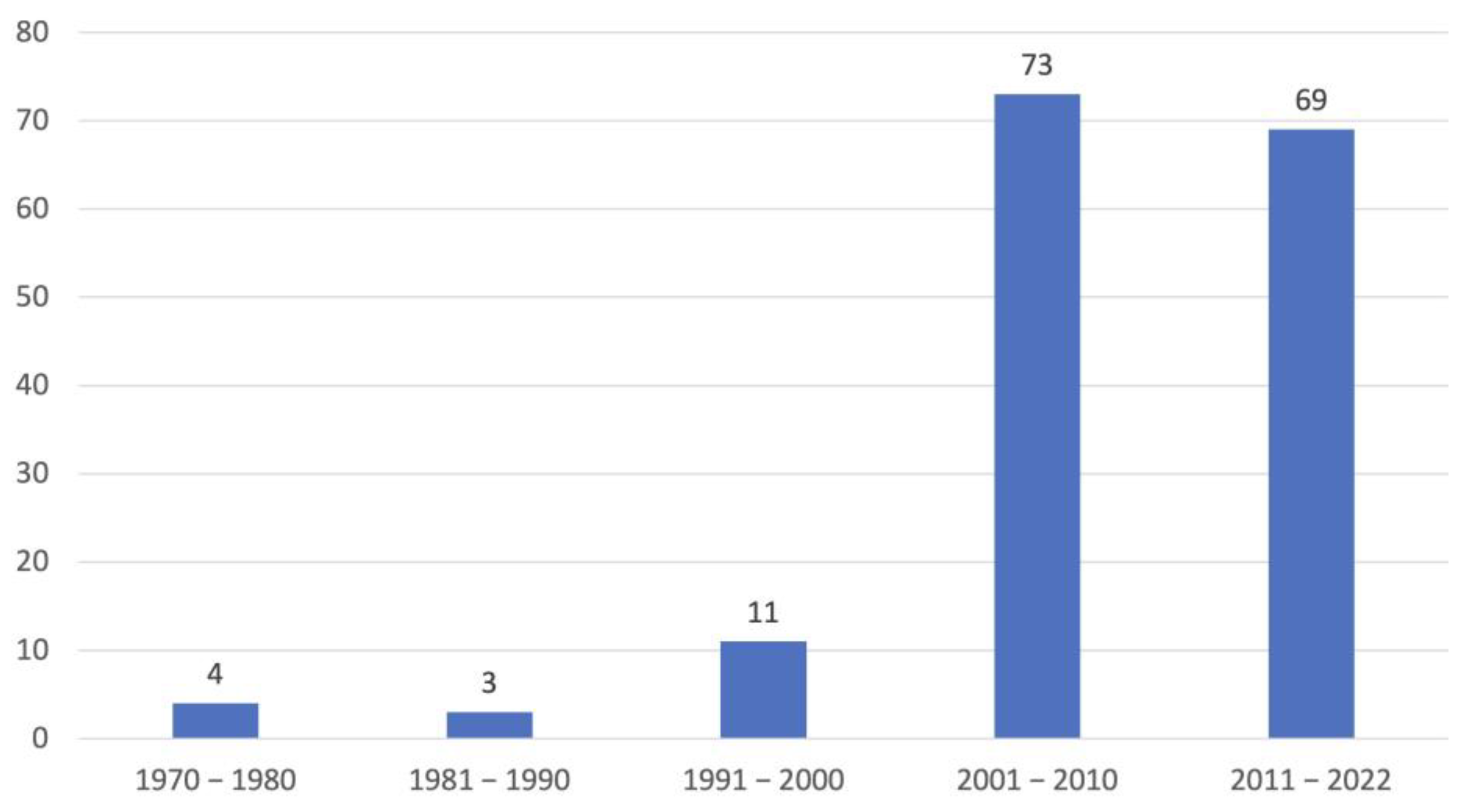
3.1. Dogs
3.2. Analysis of the Three Levels of Diagnostic Confidence
3.3. Clinical Signs
3.3.1. Laboratory Findings
3.3.2. Additional Diagnostics—Imaging
3.3.3. Additional Diagnostics—EMG’s
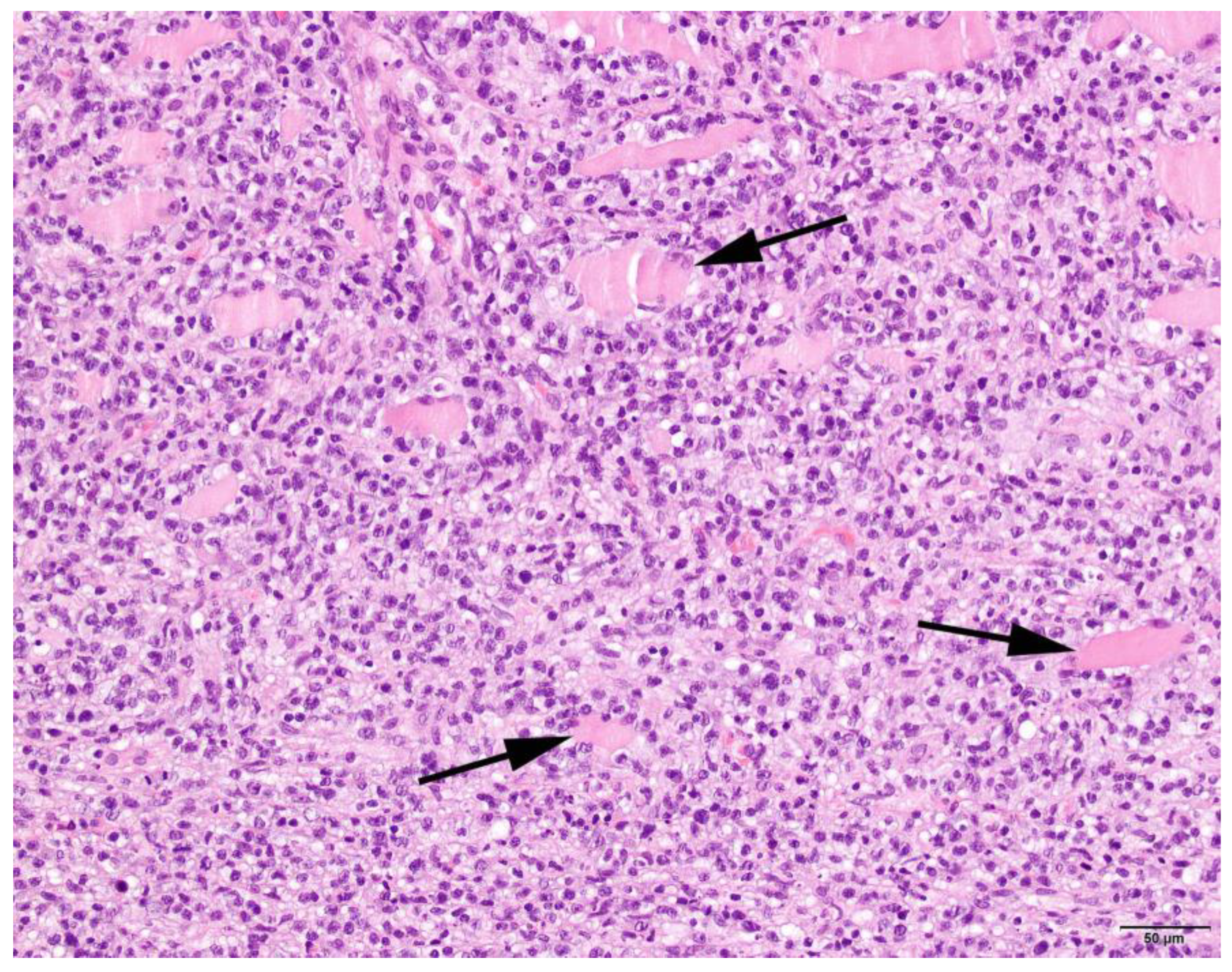
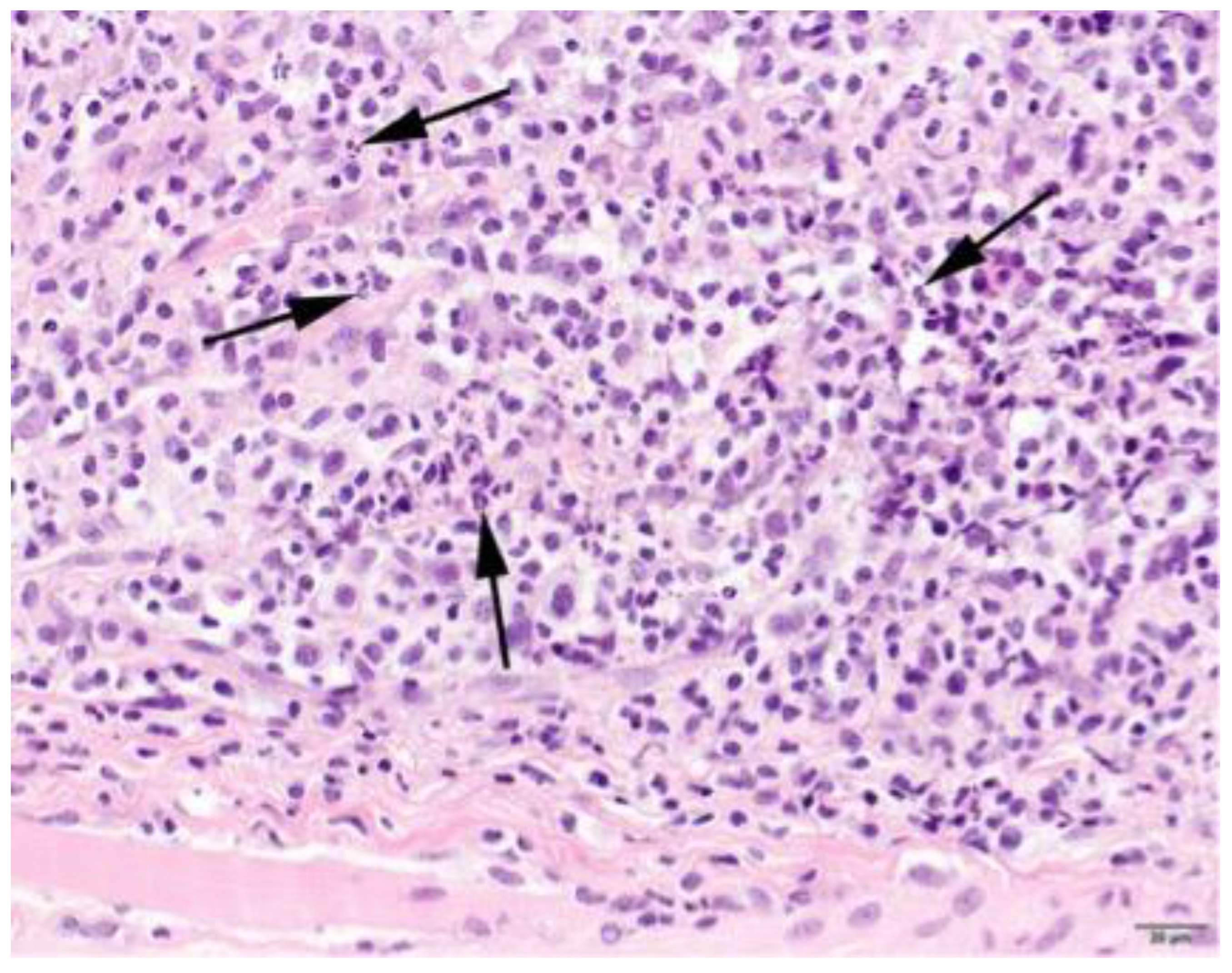
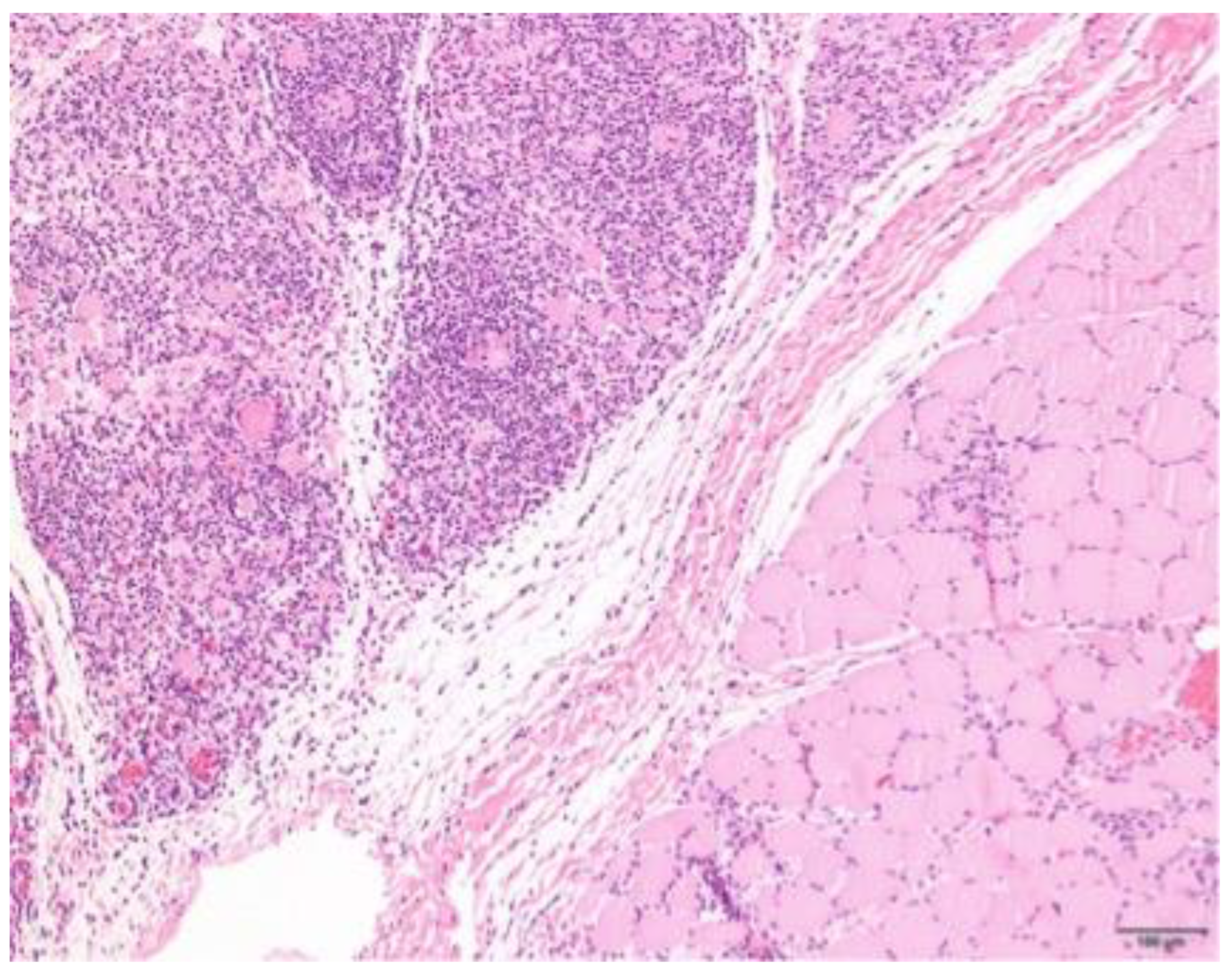
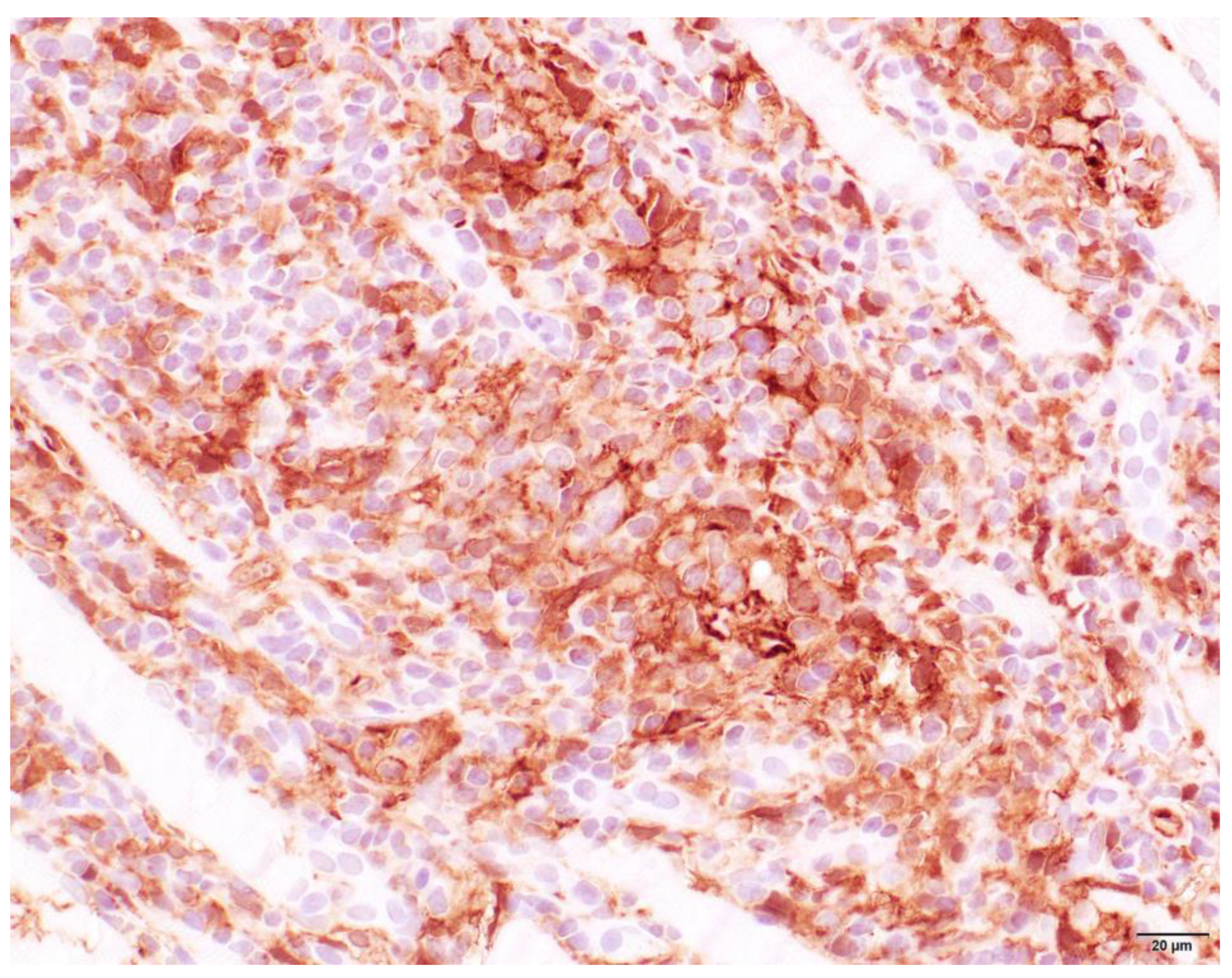
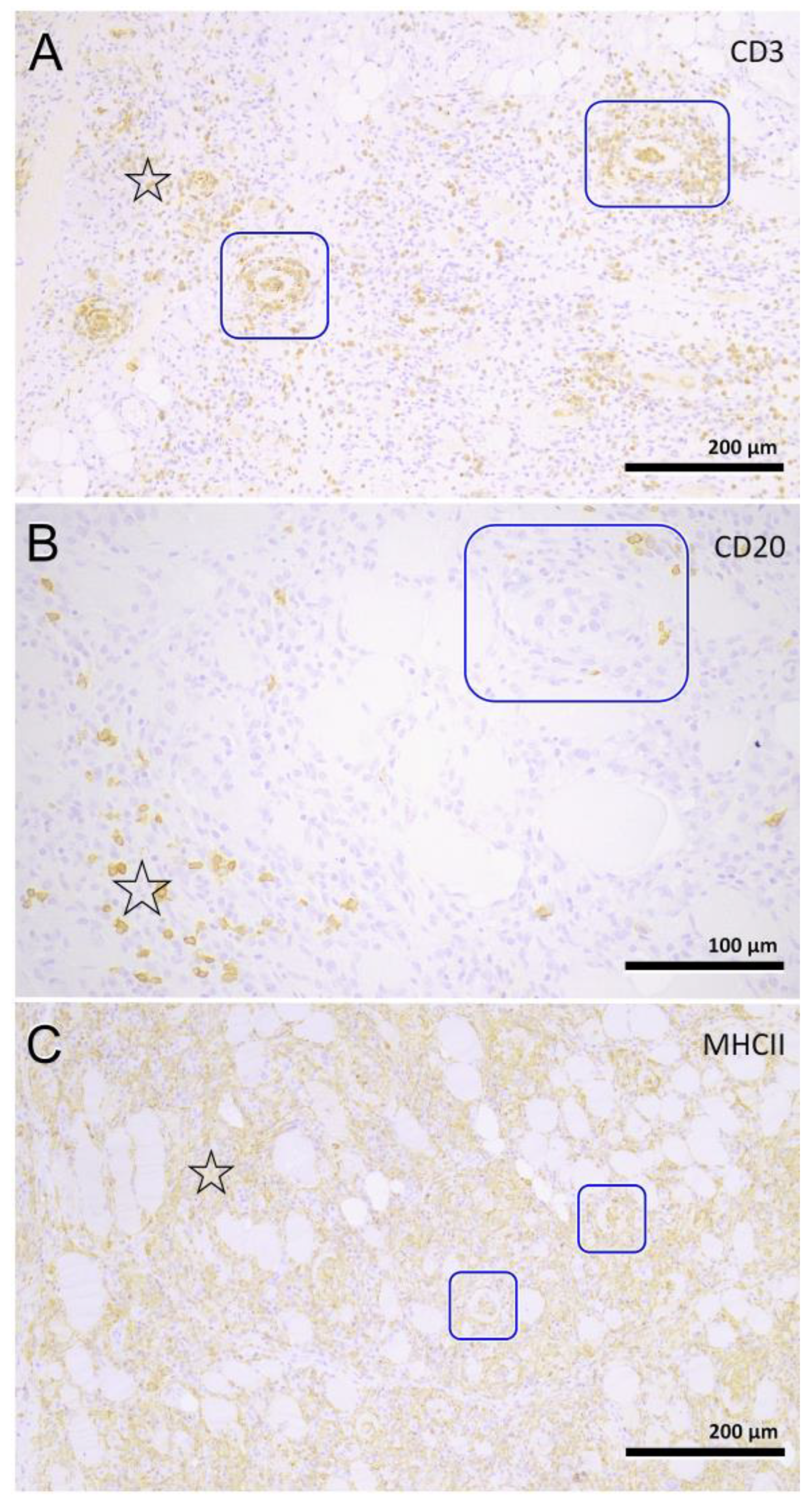
3.4. Histopathology
3.5. Treatment/Outcome
3.6. Pedigree Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AoO | Age of Onset |
| AaD | Age at Death |
| BF | Biceps muscle |
| CK | Creatine Kinase |
| DoB | Date of Birth |
| ECVN | European College of Veterinary Neurology |
| EMG | Electromyography |
| IM | Inflammatory Myopathy |
| PM | Polymyositis |
| TRI | Triceps muscle |
| TS | Time Sick |
| UVDL | University Veterinary Diagnostic Laboratory |
| VHNK | Vereniging Het Nederlandse Kooikerhondje |
References
- Mandigers, P.J.; van Nes, J.J.; Knol, B.W.; Ubbink, G.J.; Gruys, E. Hereditary Kooiker dog ataxia. Tijdschr. Voor Diergeneeskd. 1993, 118 (Suppl. S1), 65S. [Google Scholar]
- Stipriaan, R. De Zwijger. Het Leven van Willem van Oranje; Querido: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Snels, C. Clubregister van de Vereniging Het Nederlandse Kooikerhondje; VHNK: Westendorp, The Netherlands, 2022; 1533p. [Google Scholar]
- Mandigers, P.J.; Ubbink, G.J.; Broek, J.V.; Bouw, J. Relationship between litter size and other reproductive traits in the Dutch Kooiker dog. Vet. Q. 1994, 16, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Slappendel, R.J.; Beijer, E.G.; van Leeuwen, M. Type III von Willebrand’s disease in Dutch kooiker dogs. Vet. Q. 1998, 20, 93–97. [Google Scholar] [CrossRef]
- van Oost, B.A.; Versteeg, S.A.; Slappendel, R.J. DNA testing for type III von Willebrand disease in Dutch Kooiker dogs. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 2004, 18, 282–288. [Google Scholar] [CrossRef]
- Kornegay, J.N.; Gorgacz, E.J.; Dawe, D.L.; Bowen, J.M.; White, N.A.; DeBuysscher, E.V. Polymyositis in dogs. J. Am. Vet. Med. Assoc. 1980, 176, 431–438. [Google Scholar]
- Podell, M. Inflammatory myopathies. Vet. Clin. N. Am. Small Anim. Pract. 2002, 32, 147–167. [Google Scholar] [CrossRef]
- Shelton, G.D. From dog to man: The broad spectrum of inflammatory myopathies. Neuromuscul. Disord. 2007, 17, 663–670. [Google Scholar] [CrossRef]
- Paciello, O.; Shelton, G.D.; Papparella, S. Expression of major histocompatibility complex class I and class II antigens in canine masticatory muscle myositis. Neuromuscul. Disord. 2007, 17, 313–320. [Google Scholar] [CrossRef]
- Shelton, G.D.; Cardinet, G.H., 3rd; Bandman, E. Canine masticatory muscle disorders: A study of 29 cases. Muscle Nerve 1987, 10, 753–766. [Google Scholar] [CrossRef]
- Carpenter, J.L.; Schmidt, G.M.; Moore, F.M.; Albert, D.M.; Abrams, K.L.; Elner, V.M. Canine bilateral extraocular polymyositis. Vet. Pathol. 1989, 26, 510–512. [Google Scholar] [CrossRef]
- Haupt, K.H.; Prieur, D.J.; Moore, M.P.; Hargis, A.M.; Hegreberg, G.A.; Gavin, P.R.; Johnson, R.S. Familial canine dermatomyositis: Clinical, electrodiagnostic, and genetic studies. Am. J. Vet. Res. 1985, 46, 1861–1869. [Google Scholar]
- Ferguson, E.A.; Cerundolo, R.; Lloyd, D.H.; Rest, J.; Cappello, R. Dermatomyositis in five Shetland sheepdogs in the United Kingdom. Vet. Rec. 2000, 146, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Pumarola, M.; Moore, P.F.; Shelton, G.D. Canine inflammatory myopathy: Analysis of cellular infiltrates. Muscle Nerve 2004, 29, 782–789. [Google Scholar] [CrossRef]
- Hankel, S.; Shelton, G.D.; Engvall, E. Sarcolemma-specific autoantibodies in canine inflammatory myopathy. Vet. Immunol. Immunopathol. 2006, 113, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Levesque, D.; Shelton, G.D. Canine inflammatory myopathies: A clinicopathologic review of 200 cases. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 2004, 18, 679–691. [Google Scholar] [CrossRef]
- Hong, H.P.; Thomovsky, S.A.; Lewis, M.J.; Bentley, R.T.; Shelton, G.D. Clinical characteristics of non-infectious inflammatory myopathy in the boxer dog. J. Small Anim. Pract. 2021, 62, 765–774. [Google Scholar] [CrossRef]
- Haley, A.C.; Platt, S.R.; Kent, M.; Schatzberg, S.J.; Durham, A.; Cochrane, S.; Westworth, D.; Shelton, G.D. Breed-specific polymyositis in Hungarian Vizsla dogs. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 2011, 25, 393–397. [Google Scholar] [CrossRef]
- Tauro, A.; Addicott, D.; Foale, R.D.; Bowman, C.; Hahn, C.; Long, S.; Massey, J.; Haley, A.C.; Knowler, S.P.; Day, M.J.; et al. Clinical features of idiopathic inflammatory polymyopathy in the Hungarian Vizsla. BMC Vet. Res. 2015, 11, 97. [Google Scholar] [CrossRef]
- Massey, J.; Rothwell, S.; Rusbridge, C.; Tauro, A.; Addicott, D.; Chinoy, H.; Cooper, R.G.; Ollier, W.E.; Kennedy, L.J. Association of an MHC class II haplotype with increased risk of polymyositis in Hungarian Vizsla dogs. PLoS ONE 2013, 8, e56490. [Google Scholar] [CrossRef]
- Ryckman, L.R.; Krahwinkel, D.J.; Sims, M.H.; Donnell, R.L.; Moore, P.F.; Shelton, G.D. Dysphagia as the primary clinical abnormality in two dogs with inflammatory myopathy. J. Am. Vet. Med. Assoc. 2005, 226, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C.; Hohlfeld, R. Polymyositis and dermatomyositis. Lancet 2003, 362, 971–982. [Google Scholar] [CrossRef]
- Pearce, J.M.; Pennington, R.J.; Walton, J.N. Serum Enzyme Studies in Muscle Disease. I. Variations in Serum Creatine Kinase Activity in Normal Individuals. J. Neurol. Neurosurg. Psychiatry 1964, 27, 181. [Google Scholar] [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 1975, 292, 344–347. [Google Scholar] [CrossRef]
- Ostrander, E.A.; Galibert, F.; Patterson, D.F. Canine genetics comes of age. Trends Genet. 2000, 16, 117–124. [Google Scholar] [CrossRef]
- Patterson, D.F.; Aguirre, G.A.; Fyfe, J.C.; Giger, U.; Green, P.L.; Haskins, M.E.; Jezyk, P.F.; Meyers-Wallen, V.N. Is this a genetic disease? J. Small Anim. Pract. 1989, 30, 127–139. [Google Scholar] [CrossRef]
- Kroll, M.; Otis, J.; Kagen, L. Serum enzyme, myoglobin and muscle strength relationships in polymyositis and dermatomyositis. J. Rheumatol. 1986, 13, 349–355. [Google Scholar] [PubMed]
- Lu, T.Y.; Ng, K.P.; Isenberg, D.A. Inflammatory muscle disease assessment. Curr. Rheumatol. Rep. 2008, 10, 328–332. [Google Scholar] [CrossRef]
- Shelton, G.D.; Minor, K.M.; Li, K.; Naviaux, J.C.; Monk, J.; Wang, L.; Guzik, E.; Guo, L.T.; Porcelli, V.; Gorgoglione, R.; et al. A Mutation in the Mitochondrial Aspartate/Glutamate Carrier Leads to a More Oxidizing Intramitochondrial Environment and an Inflammatory Myopathy in Dutch Shepherd Dogs. J. Neuromuscul. Dis. 2019, 6, 485–501. [Google Scholar] [CrossRef] [PubMed]


| Tier I | Tier II | Tier III | ||||
|---|---|---|---|---|---|---|
| n | n | n | ||||
| Age of onset (years) | 4.1 ± 3.7 | 10 | 3.8 ± 1.8 | 27 | 4.2 ± 2.2 | 85 |
| Age at death (years) | 5.9 ± 3.1 | 26 | 5.5 ± 2.1 | 24 | 5.4 ± 2.4 | 70 |
| Duration of illness (years) | 2.5 ± 3.6 | 8 | 1.3 ± 1.4 | 21 | 1.3 ± 1.4 | 69 |
| CK activity (U/L) | - | 1726 ± 1099 | 33 | 2204 ± 1767 | 87 | |
| Locomotion | Dysphagia | General | |||
|---|---|---|---|---|---|
| Difficulty getting up | 18% | Difficulty eating | 40% | Voice change | 12% |
| Difficulty standing | 14% | Unable to swallow food | 18% | Dyspnea | 22% |
| Difficulty walking | 92% | Gagging while eating/after eating | 7% | Panting | 36% |
| Stiff gait | 51% | Difficulty drinking | 37% | Coughing | 17% |
| Weakness/paresis | 18% | Unable to swallow water | 17% | Depression | 12% |
| Muscle atrophy limbs | 7% | Gagging while/after drinking | 4% | Anorexia | 49% |
| Muscle atrophy head | 16% | Drooling all day | 23% | Fever | 5% |
| Pain while walking | 6% | Drooling while walking | 8% | Myalgia | 14% |
| Drooling while eating/drinking | 34% | ||||
| Vomiting/regurgitation | 6% | ||||
| Pattern | Expanse | Phase | Location Infiltrate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Focal | 2 | Mild | 5 | Subacute | 4 | Degeneration | 63 | Endomysium | 27 |
| Multifocal | 63 | Moderate | 13 | Chronic | 29 | Regeneration | 38 | Endomysium and perimysium | 17 |
| Severe | 9 | Atrophic | 25 | Perimysium | 5 | ||||
| Necrosis | 31 | ||||||||
| Fibrosis | 35 | ||||||||
| Characterization of Infiltrate and Severity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lymphocytes | Neutrophils | Eosinophils | Histiocytes | Plasmacells | |||||
| + | 18 | + | 25 | + | 22 | + | 16 | + | 22 |
| ++ | 29 | ++ | 10 | ++ | 8 | ++ | 28 | ++ | 14 |
| +++ | 22 | +++ | 4 | +++ | 6 | +++ | 31 | +++ | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opmeer, Y.; Grinwis, G.C.M.; Shelton, G.D.; Rosati, M.; Alf, V.; Fieten, H.; Leegwater, P.A.J.; Matiasek, K.; Mandigers, P.J.J. An Inflammatory Myopathy in the Dutch Kooiker Dog. Animals 2023, 13, 1508. https://doi.org/10.3390/ani13091508
Opmeer Y, Grinwis GCM, Shelton GD, Rosati M, Alf V, Fieten H, Leegwater PAJ, Matiasek K, Mandigers PJJ. An Inflammatory Myopathy in the Dutch Kooiker Dog. Animals. 2023; 13(9):1508. https://doi.org/10.3390/ani13091508
Chicago/Turabian StyleOpmeer, Yvet, Guy C. M. Grinwis, G. Diane Shelton, Marco Rosati, Vanessa Alf, Hille Fieten, Peter A. J. Leegwater, Kaspar Matiasek, and Paul J. J. Mandigers. 2023. "An Inflammatory Myopathy in the Dutch Kooiker Dog" Animals 13, no. 9: 1508. https://doi.org/10.3390/ani13091508
APA StyleOpmeer, Y., Grinwis, G. C. M., Shelton, G. D., Rosati, M., Alf, V., Fieten, H., Leegwater, P. A. J., Matiasek, K., & Mandigers, P. J. J. (2023). An Inflammatory Myopathy in the Dutch Kooiker Dog. Animals, 13(9), 1508. https://doi.org/10.3390/ani13091508






