Clinical Evaluation of a New Surgical Augmentation Technique for Transarticular Atlantoaxial Fixation for Treatment of Atlantoaxial Instability
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
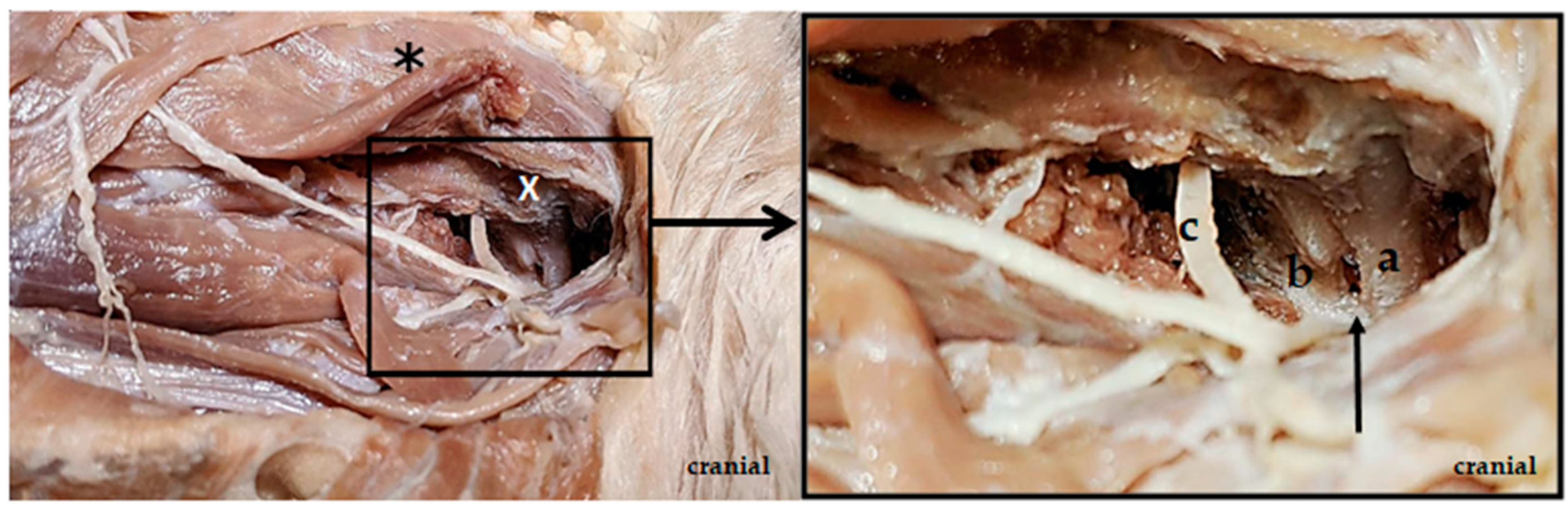
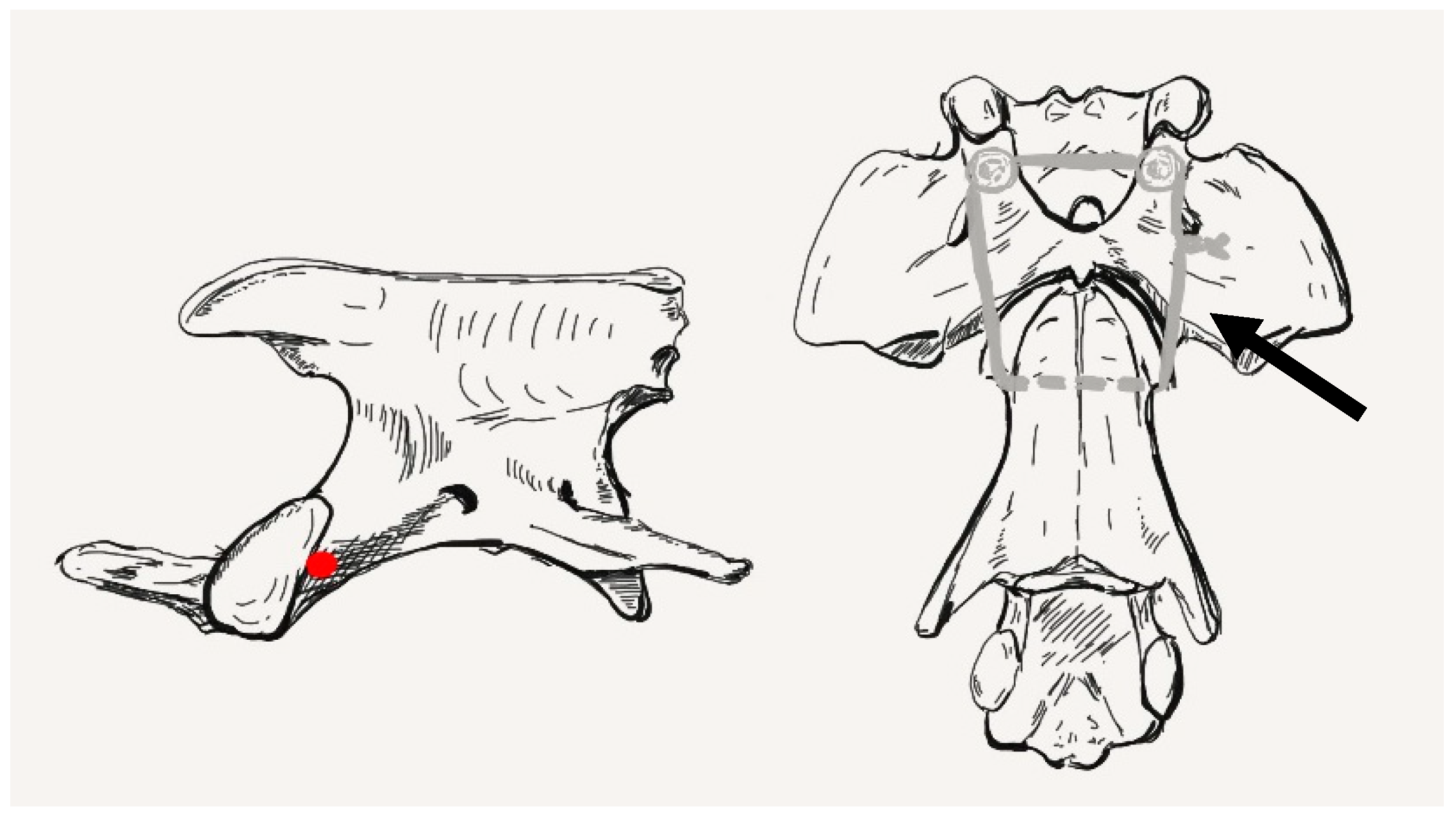
2.1. Anatomical Study
2.2. Case Selection
2.3. Anesthesia
2.4. Diagnostic Imaging
2.4.1. Radiographs
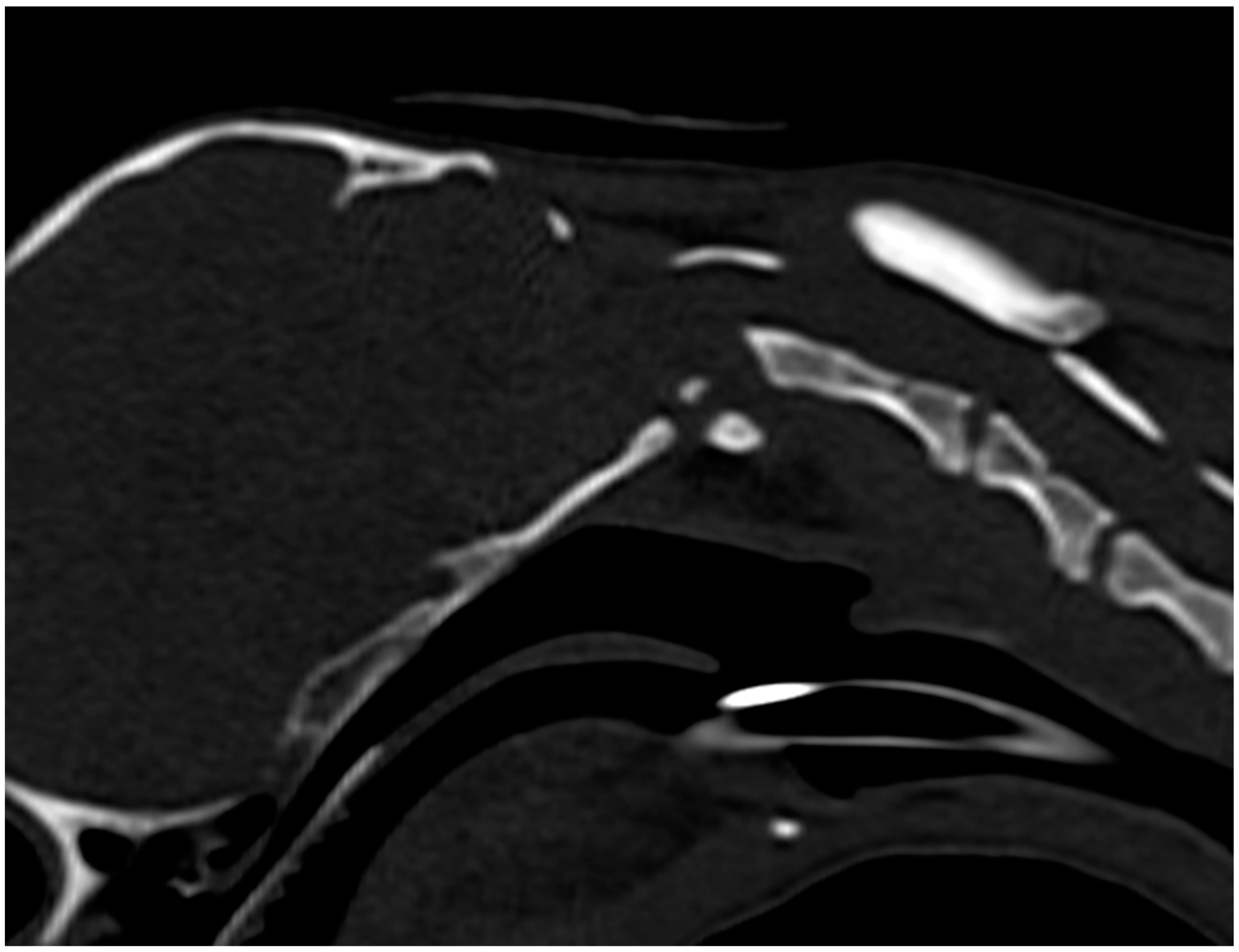
2.4.2. Computed Tomography
2.5. Magnetic Resonance Imaging
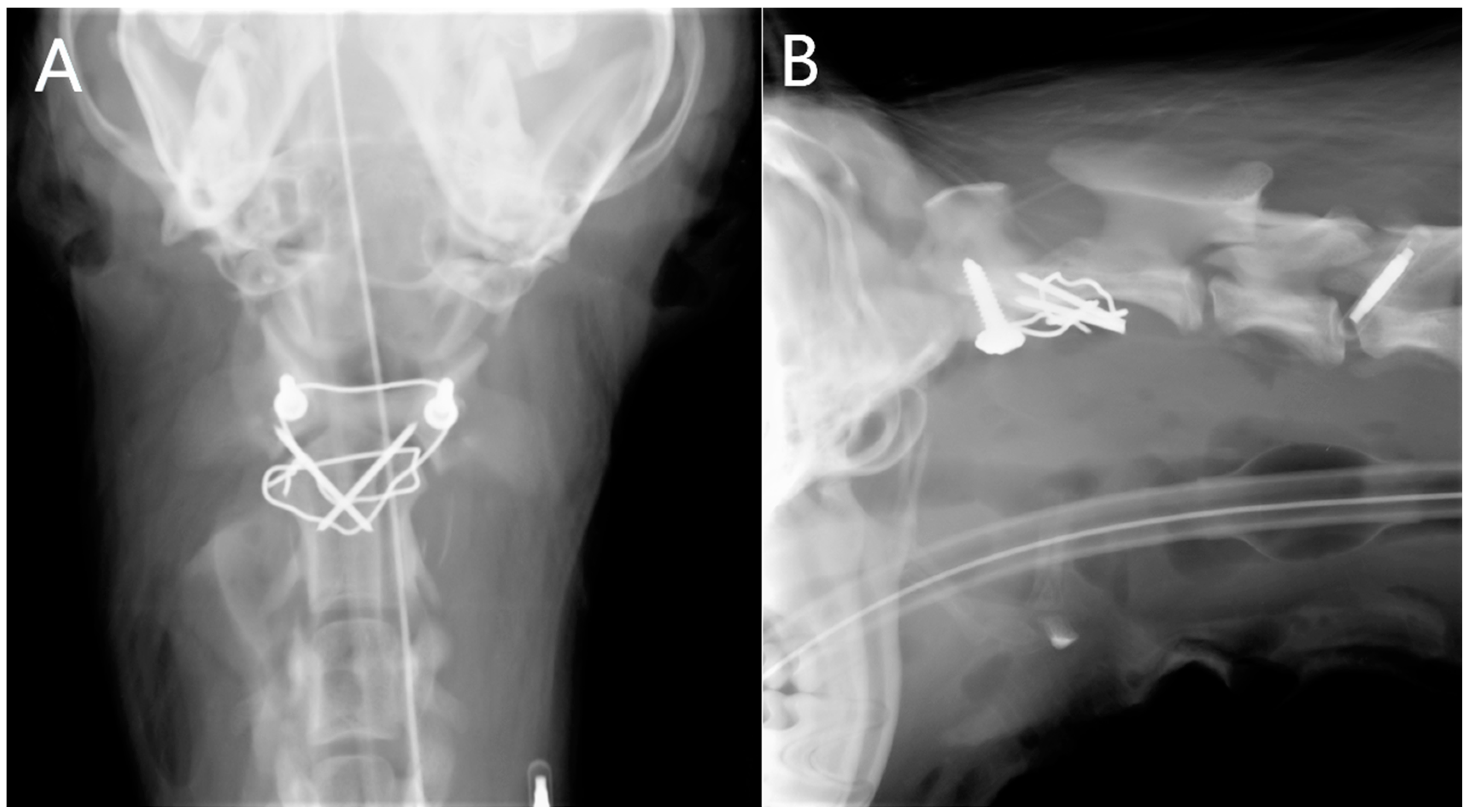
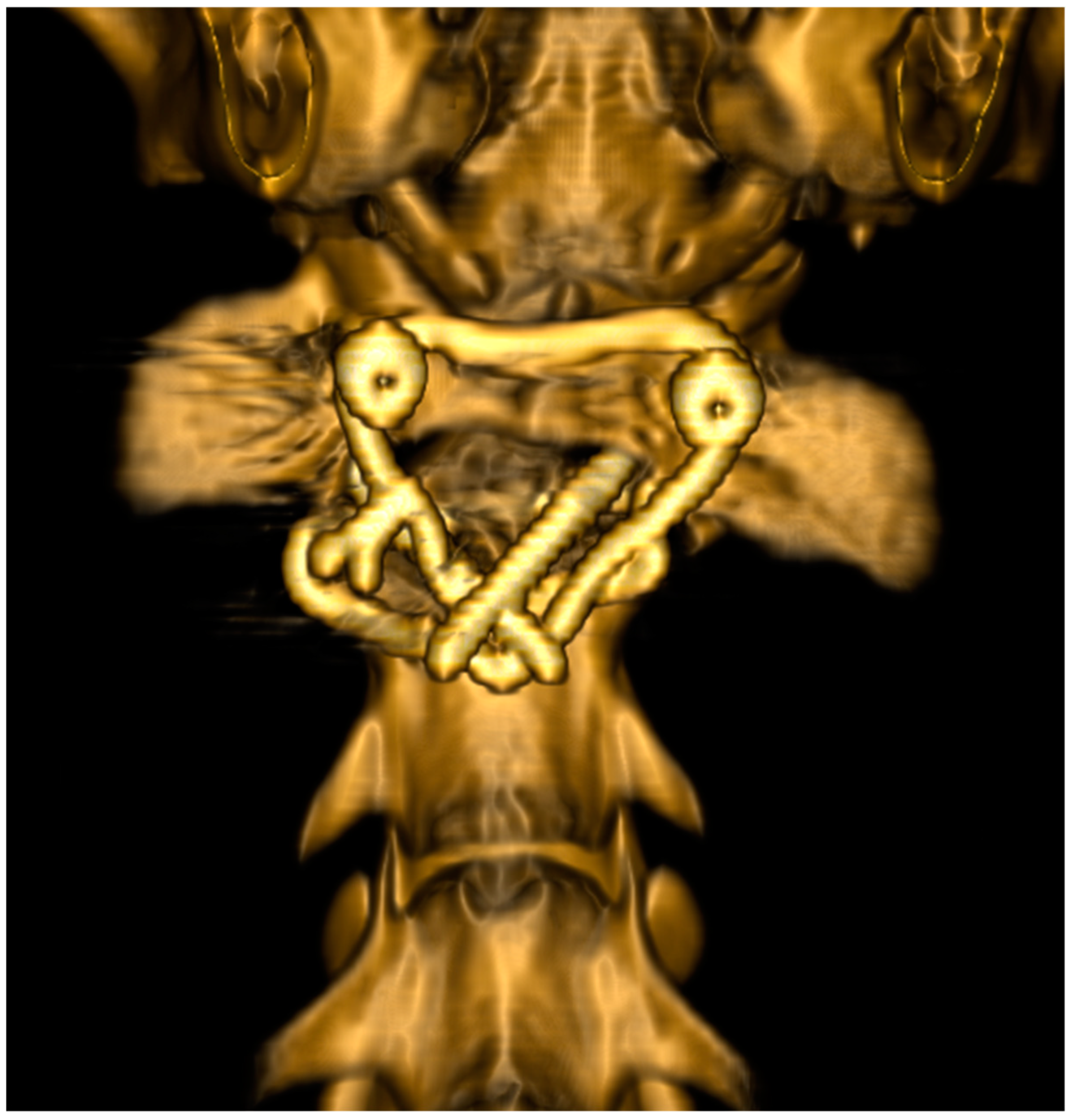
2.6. Surgical Technique
2.7. Post Operative Care
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCarthy, R.J.; Lewis, D.D.; Hodgood, G. Atlantoaxial subluxation in dogs. Compend. Contin. Educ. Pract. Vet. 1995, 17, 215–226. [Google Scholar]
- Denny, H.G.; Gibbs, C.; Waterman, A. Atlanto-axial subluxation in the dog: A review of 30 cases and an evaluation of treatment by lag screw fixation. J. Small Anim. Pract. 1988, 29, 37–47. [Google Scholar] [CrossRef]
- Thomas, W.B.; Sorjonen, D.C.; Simpson, S.T. Surgical management of atlantoaxial subluxation in 23 dogs. Vet. Surg. 1991, 20, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Beaver, D.P.; Ellison, G.W.; Lewis, D.D.; Goring, R.L.; Kubilis, P.S.; Barchard, C. Risk factors affecting the outcome of surgery for atlantoaxial subluxation in dogs: 46 cases (1978–1998). J. Am. Vet. Med. Assoc. 2000, 216, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.C.; Bacek, L.M.; Kuo, K.; Taylor, A.R. Traumatic atlantoaxial subluxation in dogs: 8 cases (2009–2016). J. Vet. Emerg. Crit. Care 2019, 29, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Havig, M.E.; Cornell, K.K.; Hawthorne, J.C.; McDonnell, J.J.; Selcer, B.A. Evaluation of nonsurgical treatment of atlantoaxial subluxation in dogs: 19 cases (1992–2001). J. Am. Vet. Med. Assoc. 2005, 227, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Sorjonen, D.C.; Shires, P.K. Atlantoaxial instability: A ventral surgical technique for decompression, fixation and fusion. Vet. Surg. 1981, 10, 22–29. [Google Scholar] [CrossRef]
- Sanders, S.G.; Bagley, R.S.; Silver, G.M.; Moore, M.; Tucker, R.L. Outcomes and complications associated with ventral screws, pins, and polymethylmethacrylate for atlantoaxial instability in 12 dogs. J. Am. Anim. Hosp. Assoc. 2004, 40, 204–210. [Google Scholar] [CrossRef]
- Alves, L.; Pekarkova, M.; Gorgas, D.; Forterre, F.; Dickomeit, M. Use of a 1.5 mm butterfly locking plate for stabilization of atlantoaxial pathology in three toy breed dogs. Vet. Comp. Orthop. Traumatol. 2011, 24, 246–251. [Google Scholar] [CrossRef]
- Schulz, K.S.; Waldron, D.R.; Fahie, M. Application of ventral pins and polymethylmethacrylate for the management of atlantoaxial instability: Results in nine dogs. Vet. Surg. 1997, 26, 317–325. [Google Scholar] [CrossRef]
- Aikawa, T.; Shibata, M.; Fujita, H. Modified ventral stabilization using positively threaded profile pins and polymethylmethacrylate for atlantoaxial instability in 49 dogs. Vet. Surg. 2013, 42, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Platt, S.R.; Chambers, J.N.; Cross, A. A modified ventral fixation for surgical management of atlantoaxial subluxation in 19 dogs. Vet. Surg. 2004, 33, 349–354. [Google Scholar] [CrossRef]
- Progin, A.; Voumard, B.; Friker, B.; Forterre, F. Biomechanical evaluation of two dorsal and two ventral stabilization techniques for atlantoaxial joint instability in toy-breed dogs. Am. J. Vet. Res. 2021, 82, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Quintana, R.; Faller, K.; Guevar, J.; Yeamans, C.; Penderis, J.; Stalin, C. A review of canine atlantoaxial joint subluxation. Vet. Comp. Orthop. Traumatol. 2015, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Luján-Feliu-Pascual, A.; Font, C.; Mascort, J.; Sánchez-Masian, D. Dorsal stabilization of atlantoaxial subluxation using non-absorbable sutures in toy breed dogs. Vet. Comp. Orthop. Traumatol. 2014, 27, 62–67. [Google Scholar] [CrossRef]
- Salomon, F.H.; Geyer, H.; Gille, U. Anatomie für die Tiermedizin. Schweiz. Arch. Tierheilkd. 2005, 147, 405. [Google Scholar] [CrossRef]
- Cook, J.R.; Oliver, J.E. Atlantoaxial Luxation in the Dog. Compend. Contin. Educ. 1981, 242, 242–250. [Google Scholar]
- da Costa, R.C.; Poma, R.; Parent, J.M.; Partlow, G.; Monteith, G. Correlation of motor evoked potentials with magnetic resonance imaging and neurologic findings in Doberman Pinschers with and without signs of cervical spondylomyelopathy. Am. J. Vet. Res. 2006, 67, 1613–1620. [Google Scholar] [CrossRef]
- Fenn, J.; Olby, N.J. Classification of Intervertebral Disc Disease. Front. Vet. Sci. 2020, 7, 579025. [Google Scholar] [CrossRef]
- Shores, A.; Tepper, L.C. A modified ventral approach to the atlantoaxial junction in the dog. Vet. Surg. 2007, 36, 765–770. [Google Scholar] [CrossRef]
- Forterre, F.; Vizcaino Revés, N.; Stahl, C.; Gendron, K.; Spreng, D. An indirect reduction technique for ventral stabilization of atlantoaxial instability in miniature breed dogs. Vet. Comp. Orthop. Traumatol. 2012, 25, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Hakozaki, T.; Kanno, N.; Suzuki, S.; Harada, Y.; Soeta, S.; Nakamura, S.; Yamaguchi, S.; Hara, Y. Influence of ventral fixation techniques on atlantoaxial joint fusion in canine models with dens partial resection. J. Vet. Med. Sci. 2022, 84, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Renegar, W.R.; Stoll, S. The use of methyl methacrylate bone cement in the repair of atlantoaxial subluxation stabilization failures. Case report and discussion. J. Am. Anim. Hosp. Assoc. 1979, 15, 313–318. [Google Scholar]
| Breeds | Gender | Age | Body Weight | Duration of Clinical Signs until Presentation | Clinical Signs | Surgical Technique | Complications | Clinical Signs after Hospitalization |
|---|---|---|---|---|---|---|---|---|
| Havanese | female | 16 months | 5.2 kg | 2 weeks | Ambulatory tetraparetic | Bilateral plating and augmentation technique | Dysphagia and dyspnea post-operatively | Ambulatory with slight tetraparesis |
| Chihuahua | female | 11 months | 0.9 kg | 10 days | Ambulatory tetraparetic | Transarticular pins and augmentation technique | Dysphagia and dyspnea post-operatively. Death | |
| Miniature Spitz | female | 12 months | 2.6 kg | 3 weeks | Ambulatory tetraparetic | Transarticular screws and augmentation technique | Dysphagia and dyspnea post-operatively | Ambulatory with slight tetraparesis |
| Yorkshire Terrier | female | 16 months | 2.6 kg | 2 weeks | Ambulatory tetraparetic | Transarticular screws and augmentation technique | None | Ambulatory with slight tetraparesis |
| Chihuahua | male | 14 months | 1.8 kg | 2 weeks | Ambulatory tetraparetic | Transarticular pins and augmentation technique | None | Ambulatory with slight tetraparesis |
| Mix breed dog | female | 12 months | 11 kg | 4 days | Tetraparetic non ambulatory | Bilateral plating and augmentation technique | None | Tetraparetic non ambulatory |
| Chihuahua | female | 15 months | 2.1 kg | 2 weeks | Pain | Transarticular pins and augmentation technique | None | Ambulatory with slight tetraparesis |
| Bolonka Zwetka | male | 14 months | 3.1 kg | 10 days | Ambulatory tetraparetic | Bilateral plating and augmentation technique | None | Ambulatory with slight tetraparesis |
| Miniature Spitz | male | 11 months | 2.9 kg | 7 days | Ambulatory tetraparetic | Bilateral plating and augmentation technique | None | Ambulatory with slight tetraparesis |
| Mix breed dog | female | 6 months | 5.5 kg | 3 days | Tetraparetic non ambulatory | Bilateral plating and augmentation technique | None | Tetraparetic non ambulatory |
| Border collie | female | 3 months | 7 kg | 3 days | Tetraplegic with preserved deep pain | Bilateral plating and augmentation technique | None | Ambulatory with slight tetraparesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forterre, F.; Zorgevica-Pockevica, L.; Precht, C.; Haenssgen, K.; Stein, V.; Düver, P. Clinical Evaluation of a New Surgical Augmentation Technique for Transarticular Atlantoaxial Fixation for Treatment of Atlantoaxial Instability. Animals 2023, 13, 1780. https://doi.org/10.3390/ani13111780
Forterre F, Zorgevica-Pockevica L, Precht C, Haenssgen K, Stein V, Düver P. Clinical Evaluation of a New Surgical Augmentation Technique for Transarticular Atlantoaxial Fixation for Treatment of Atlantoaxial Instability. Animals. 2023; 13(11):1780. https://doi.org/10.3390/ani13111780
Chicago/Turabian StyleForterre, Franck, Ligita Zorgevica-Pockevica, Christina Precht, Kati Haenssgen, Veronika Stein, and Pia Düver. 2023. "Clinical Evaluation of a New Surgical Augmentation Technique for Transarticular Atlantoaxial Fixation for Treatment of Atlantoaxial Instability" Animals 13, no. 11: 1780. https://doi.org/10.3390/ani13111780
APA StyleForterre, F., Zorgevica-Pockevica, L., Precht, C., Haenssgen, K., Stein, V., & Düver, P. (2023). Clinical Evaluation of a New Surgical Augmentation Technique for Transarticular Atlantoaxial Fixation for Treatment of Atlantoaxial Instability. Animals, 13(11), 1780. https://doi.org/10.3390/ani13111780







